Abstract
Background and objective: The impairments and duration of PASC (post-acute sequelae of COVID-19) symptoms in mental health have, to date, not been comprehensively examined. Our objective is to provide longitudinal data on the mental health of Post-COVID patients and to identify risk and protective factors associated with a severe or prolonged course. Methods: The mental health of 265 Post-COVID patients of the outpatient Post-COVID centre of the University Hospital Erlangen was assessed 17.1 (T0) and 22.5 months after infection (T1). An online survey with validated questionnaires for Post-COVID symptoms (Post-COVID Syndrome Score), depression (Patient Health Questionnaire-9), somatic symptoms (Patient Health Questionnaire-15), anxiety (Generalized Anxiety Disorder-7), fatigue (Fatigue Severity Scale) and Post-Exertional Malaise (PEM) (DePaul Post-Exertional Malaise Screening) was conducted in the home environment. Results: In total, 80% of patients experienced severe PASC at follow-up. Clinically relevant symptoms of depression, persistent somatic symptoms, anxiety and fatigue were reported by 55.8%, 72.5%, 18.9% and 89.4% of patients, respectively. Depressive, anxiety and somatic symptom severity decreased significantly over time; fatigue and PEM remained at an unchanged high level. The risk factor for higher depression scores was older age; prior psychiatric illness treated with psychotherapy was associated with more severe depressive, somatic, anxiety and PASC symptoms. PEM symptoms were significantly associated with longer duration between acute infection and initial presentation in the Post-COVID centre. Conclusions: Our findings align with previous research, claiming severe mental health symptoms in PASC syndrome, lasting for months after infection. In-depth assessment of risk and protective factors for the mental health implications of PASC is needed for the planning of health services and disease prevention.
1. Introduction
1.1. Post-COVID Syndrome
According to the World Health Organization, around 10–20% of patients afflicted with the SARS-CoV-2 virus still suffer from symptoms 3 months after infection [1]. As long as there is no other explanation for these prolonged symptoms, this condition is referred to as Post-COVID or PASC (post-acute sequelae of COVID-19) [1,2,3].
While fatigue and dyspnoea are the most common symptoms [4,5], many patients also experience neuropsychiatric or psychological symptoms encompassing cognitive impairment, memory loss, brain fog, anxiety and depression [5,6,7,8].
The precise pathophysiology of PASC remains incompletely understood. Common hypotheses include autoimmunity and inflammation, potential viral tissue reservoirs or alterations in the gut microbiome [4,9,10]. What has become evident is that COVID-19 and PASC affect multiple organs, extending beyond the respiratory tract [11].
Risk factors for PASC include female sex, older age, pre-existing comorbidities, neuropsychiatric diseases, longer hospital stays and high BMI [4,5,12]. Some studies suggest that the severity of acute COVID-19 is not associated to PASC onset [13,14], while others postulate a higher PASC prevalence after severe illness [5,10].
1.2. Mental Health Impairments in PASC
PASC not only manifests in physical symptoms but also exerts an impact on the individual’s mental health. Several analyses have observed that 14.5% to one-fifth of individuals recovering from an infection exhibit mental health problems [15,16].
Common psychological symptoms in PASC include depression (12%; 4–31%), anxiety (23%; 6.5–63%), concentration difficulties (22%) and fatigue (37%; 28–87%) [6,17,18]. Other symptoms include Post-Exertional Malaise (PEM) (89%) [19], somatization (37.5%) [20] and Post-Traumatic Stress Disorder (12.1% to 46.9%) [17]. Due to the highly heterogeneous patient collectives, numbers vary significantly.
Several risk factors for the development of psychological symptoms have been identified, a prior mental disorder emerging as the most significant [4,12]. Further, females, patients admitted to ICU during acute infection and younger people exhibited a higher likelihood of experiencing mental health problems [6,8,17]. A potential general protective factor for psychiatric Post-COVID symptoms is vaccination [12].
Despite limited long-term longitudinal research, several studies support the hypothesis of a high symptomatic burden with minimal improvement within 3–12 months [21,22,23,24]. One cross-sectional meta-analysis found that patients still suffer from somatization (37.5%), fatigue (26.2%), anxiety (18%) and depression (12.6%) 12 months after infection [20]. Further, Guillen-Burgos conducted a comparative analysis among 1565 patients, assessing their status at 12 and 24 months, revealing only a slight decrease in depression from 33.6% to 21.79% and anxiety from 27.90% to 16.55% [25]. Studies with longer surveying periods show persisting mental health symptoms up to 24 months after infection [26,27].
This persisting impairment on everyday life results in 58% of PASC patients reporting a poorer quality of life than before the infection [15]. In particular, symptoms like fatigue and PEM were identified as severely impacting mental health [15]. Both PEM and fatigue persisted over a 12-month period without significant improvement, and some studies even showed a worsening over time [20,28,29,30].
The question of PASC duration and whether PASC follows a self-limiting course, like some other post-viral syndromes [31], remains unanswered, especially concerning the duration and alterations in the mental health and psychological trajectory.
1.3. Research Objectives
This study endeavours to offer longitudinal data concerning the mental health trajectory of Post-COVID patients, given the current scarcity of research therein. Our objectives were firstly to examine the trajectory of PASC symptoms and mental health outcomes, i.e., whether PASC follows a self-limiting course, and secondly to identify both risk and protective factors associated with a more severe symptomatology in PASC patients.
2. Material and Methods
2.1. Participants and Data Collection
This research took place in the interdisciplinary outpatient Post-COVID centre of the University Hospital Erlangen. Individuals experiencing ongoing symptoms after confirmed COVID-19 infection were referred by their general practitioners with the suspected diagnosis of PASC. These patients were consecutively recruited for this study between December 2022 and February 2024.
Inclusion criteria were a minimum age of 18 years, confirmed COVID-19 infection, and symptom persistence for 3 months without another explanation as observed by the general practitioners. Exclusion criteria encompassed Post-Vaccination Syndrome and a high symptomatic burden that prevented participation.
During their clinical appointment (T0), patients underwent psychological/psychosomatic evaluation via in-depth interviews with mental health professionals (physicians) and extensive neurocognitive/neuropsychological testing [7]. Additionally, participants completed different validated questionnaires (Patient Reported Outcomes Measures, PROMs) at home, using an online survey.
Using an online survey with PROMs allowed for a comprehensive assessment and monitoring of the diverse symptoms of PASC patients at T0 and T1, especially considering that some patients could not attend two appointments due to their physical impairments. Conducting the survey at home saved both time as well as medical capacity.
Moreover, data concerning sociodemographic, health behaviour, pre-existing (psychiatric/psychosomatic) diseases and pain were collected.
A specialist of internal medicine reviewed important findings, e.g., echocardiograms and current laboratory results, and examined the patients physically. This process helped rule out immunological diseases that could have been the cause of the patients’ complaints.
Based on these interdisciplinary diagnostics, patients received classification regarding PASC and their symptom severity and recommendations regarding possible treatment options.
The follow-up survey (T1) started in October 2023 and lasted until February 2024. It was conducted at least 3 months after first presentation to the centre (T0). Taking into consideration that the follow-up study (T1) started in October 2023, the maximum time span between T0, which started in December 2022, and T1 was 10 months.
The same questionnaires were employed at T0 and T1. The survey took approximately 45 min and was conducted in the home environment without clinical presentation.
Patients were invited to participate via letter and E-Mail. Throughout a four-week period, non-responders received one weekly reminder, via E-Mails (3 times) and one phone call.
This study was approved by the ethics committee of the Friedrich-Alexander-University Erlangen-Nürnberg, (T0: 22-443-B, T1: 22-444_3-B) and all participants gave their written informed consent.
2.2. Measures/Variables
2.2.1. Sociodemographic and Health-Related Data
The collected sociodemographic variables comprised sex, age, marital status, number of children, education level and current employment status at baseline and follow-up. Further, information regarding health behaviour, such as smoking habits, BMI and the presence of pre-existing illnesses was gathered.
The following PROMs were used at T0 and T1:
2.2.2. PASC Symptoms
PASC symptoms were assessed using Post-COVID Syndrome Score (PCS-S) [32], which consists of 12 dichotomised questions covering various symptoms: ageusia or anosmia, fatigue, lack of physical resilience, joint or muscle pain, throat/nose/ear discomfort, lung/breathing difficulties, cardiac symptoms, intestinal symptoms, neurological complaints, dermal problems, signs of infection and sleeping disorders. Each answer weighs differently, resulting in a scale from 0 to 59. Cut-off values are as follows: no/mild PCS ≤ 10.75, moderate PCS > 10.75 to ≤26.25 and severe/relevant PCS > 26.25. Cronbach’s alpha was 0.66 at T0 and 0.67 at T1.
Additionally, information regarding the acute COVID-19 infection was collected: the date of the acute infection and the course of infection (asymptomatic infection, at home or outpatient therapy, symptomatic infection requiring clinical therapy, symptomatic infection requiring ICU admission).
Further, information about vaccination and proof of infection by positive PCR was gathered.
2.2.3. Mental Health Symptoms
Generalized Anxiety Disorder-7 (GAD-7)
Symptoms of anxiety were assessed using the GAD-7, with total scores from 0 to 28. A score of ≥10 indicates clinically significant symptoms of anxiety [33]. Cronbach’s alpha was at T0 0.87 and at T1 0.89.
Patient Health Questionnaire-9 (PHQ-9)
Depressive symptoms were measured with the PHQ-9. Sum scores range from 0 to 27 points, whereas clinically significant depression can be assumed at ≥10 points [34]. Cronbach’s alpha at T0 was 0.78 and at T1 0.78.
Patient Health Questionnaire-15 (PHQ-15)
The PHQ-15 was used to assess the severity of somatic symptoms. A cut-off value of ≥10 points hints at relevant somatization [35]. Cronbach’s alpha at T0 was 0.80 and at T1 0.81.
The Fatigue Severity Scale (FSS)
This questionnaire consists of nine statements to assess fatigue with a Likert scale, between 1 and 7 (range: 9–63). A mean score of ≥4 indicates clinically relevant fatigue [36]. Cronbach’s alpha was 0.94 at T0 and 0.95 at T1.
DePaul Post-Exertional Malaise (PEM) Screening
Post-Exertional Malaise, meaning complete and disproportionate exhaustion induced by physical or mental activities, was assessed with this questionnaire. Patients answered questions regarding the severity of exhaustion after different activities, as well as the frequency of this occurring [37]. Cronbach’s alpha was 0.71 at T0 and 0.62 at T1.
Psychotherapeutic Treatment
The follow-up survey queried participants about psychotherapeutic treatments before developing PASC using the question, “Have you ever been in psychotherapy/psychosomatic therapy/psychiatric therapy before your Post-COVID symptoms?”
Cardiological Data
Patients presenting at the Post-COVID centre were required to provide echocardiography results carried out after their COVID-19 infection. These echocardiograms were analysed to assess their physical health and detect potential cardiac damage. The analysis was performed with an algorithm displayed in Supplement S1.
Other Data
Additional data were collected through validated questionnaires and are reserved for upcoming publications. These include assessments of social support, loneliness, insomnia, stress, pain, quality of life and coping with illness.
Moreover, patients were asked about the various treatments they had utilized since T0 and how these treatments affected their symptoms.
2.3. Data Analysis
SPSS V. 28 (IBM Corporation, Armonk, NY, USA) was utilized to analyse the data. Descriptive statistics including means, standard deviations, ranges and frequencies were computed. The prevalence of mental health conditions was determined using established cut-off values. The paired t-test was employed to assess differences in examined variables between T0 and T1. The one sample t-tests were performed to compare the study cohort to the general population. The McNemar test was used to establish the prevalence of mental health impairments over validated cut-off values. We examined the relationship between independent variables at T0: sex, age, dichotomized BMI, smoking, echocardiography, occupation, psychological condition, relationship, time interval, education, course of disease, number of vaccinations and time point, and the dependent variables PHQ-9, PHQ-15, GAD-7, DSQ-PEM, FSS and PSC-S, using mixed linear regression models with a random intercept using the program R V4.3.3. We provided coefficients, their 95% confidence intervals and p-values. The significance level was set at p = 0.05.
2.4. Missings
At T0, three patients did not reply to the GAD-7, PHQ-9, PHQ-15, FSS and PCS-S; 14 did not reply to PEM; one patient missed two answers in the FSS; and two patients missed one answer in the PCS-S. At T1, two patients missed one answer in the PHQ-9. Missing values were imputed using the random forest imputation with the missForest package.
3. Results
n = 328 patients, who attended the Post-COVID centre, were invited consecutively for the follow-up. Four patients rejected participation at T1 and 10 patients had to be excluded because their condition was attributed to Post-Vaccination Syndrome. A total of 49 patients did not complete the questionnaires at T1, leading to a response rate of 83.8% and a total number of n = 265 included patients.
3.1. Sociodemographic and Health-Related Data
The mean age was 45.5 (SD = 12.1) years and the majority of participants were female (n = 187, 70.6%) (Table 1). Most participants had graduated from high school (n = 128, 48.3%), while 97 (36.6%) had a higher educational certificate. Table 1 only presents baseline data (T0) because they remained very similar at T1 with the exception of employment status. At T0, 88 patients (33.2%) worked full-time and 74 (27.9%) part-time. Upon follow-up (T1), 67 (25.3%) were found to be working full-time and 60 (22.6%) engaged in part-time work. Both the unemployment rate and sick leave/incapacitated for work rate increased over the surveying period from 5.3% to 7.9% and from 15% to 21.9%, respectively.

Table 1.
Sociodemographic and health-related data.
3.2. Post-COVID-Related and Cardiac Variables
The mean time since SARS-CoV-2 infection at follow-up was 22.5 (SD = 8.2) months, with the follow-up surveying period being, on average, 5.0 (SD = 2.4) months. The majority of the sample had suffered a symptomatic COVID-19 infection that did not require inpatient therapy (n = 224, 84.5%). Over one-half of the sample had received the vaccination at least three times (n = 154, 58.1%) (Table 2).

Table 2.
Post-COVID-Related and cardiac data.
3.3. Mental Health
3.3.1. Severity of Symptoms and Development at Follow-Up (T1)
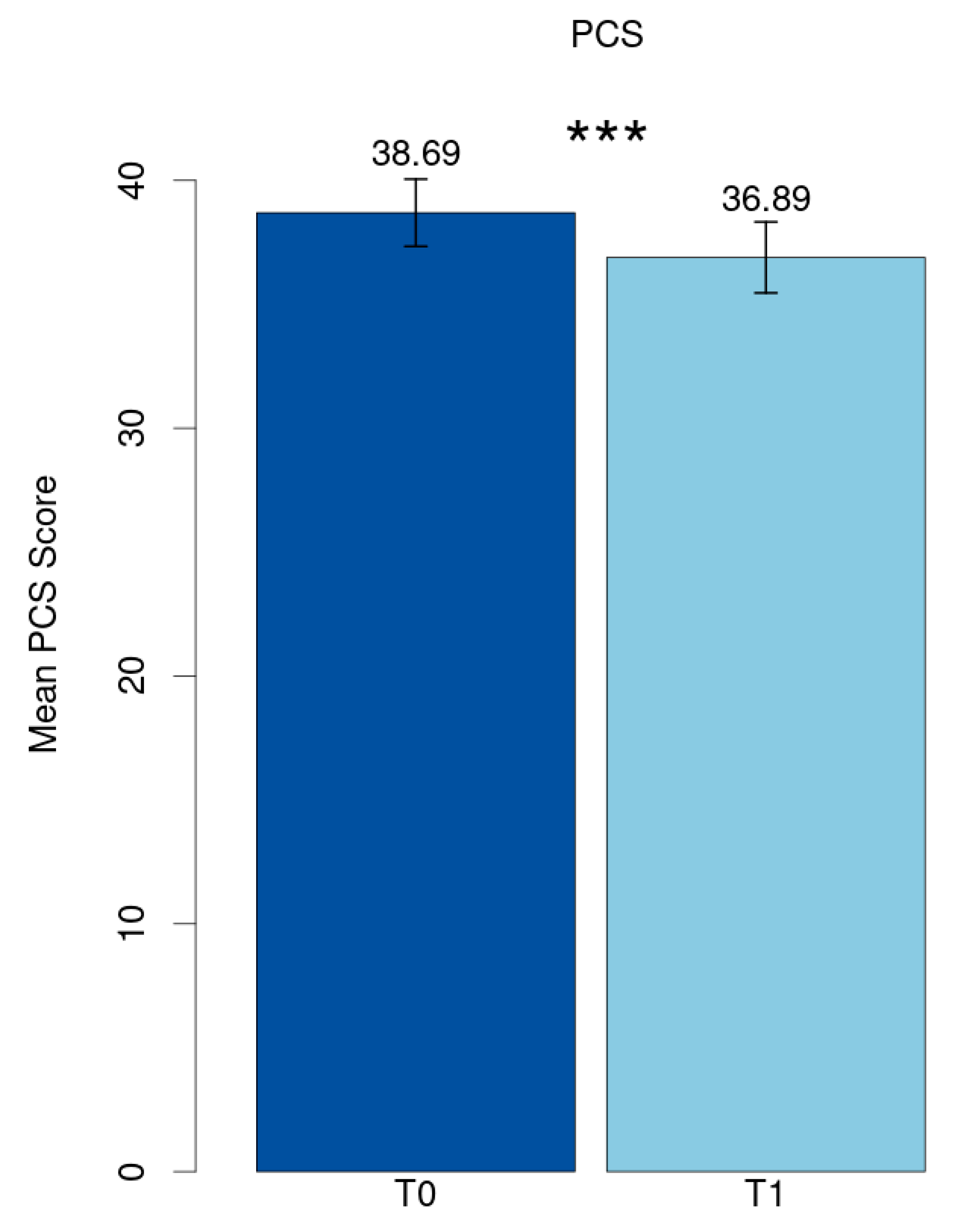
The mean value of the Post-COVID Syndrome score at baseline (T0) was 38.69 (SD = 11.21) and reduced significantly to 36.89 (SD = 11.90) at follow-up (T1) (p < 0.001, Cohen’s d = 0.21) (Figure 1).
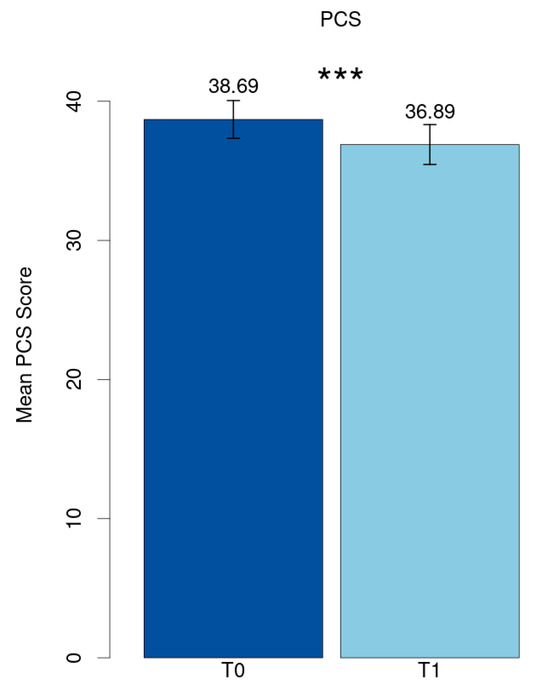
Figure 1.
Mean PCS score. *** p < 0.001.
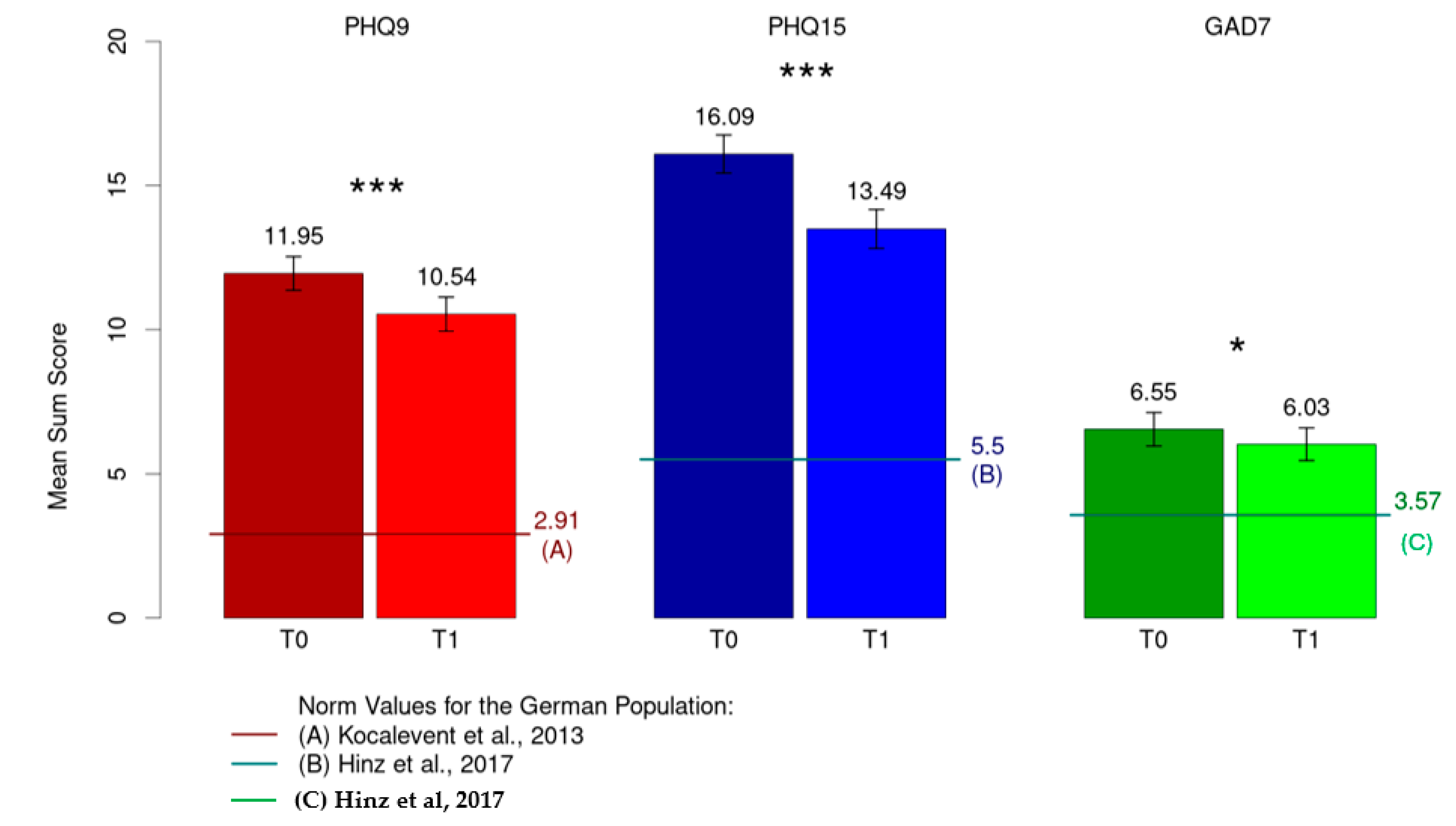
A significant decrease in severity was observed from T0 to T1 for depressive symptoms (M = 11.95, SD = 4.82 to M = 10.54, SD = 4.92; p < 0.001, d = 0.34), persisting somatic symptoms (M = 16.09, SD = 5.46 to M = 13.49, SD = 5.60; p < 0.001, d = 0.73) and anxiety symptoms (M = 6.55, SD = 4.78 to M = 6.03, SD = 4.69; p = 0.038, d = 0.13) (Figure 2).
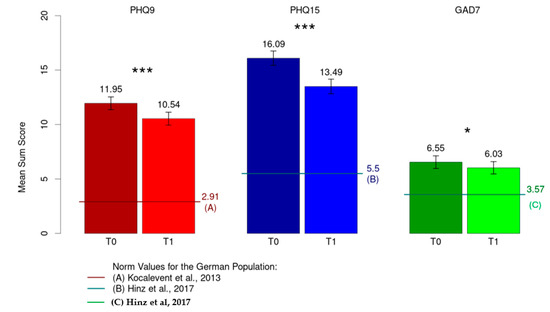
Figure 2.
Mean sum score for depression (PHQ-9), somatization (PHQ-15) and anxiety (GAD-7). * p < 0.05; *** p < 0.001; [38,39,40].
When compared with the normal population in terms of depression (M = 2.91, SD = 3.52), persistent somatic symptoms (M = 5.5 SD = 3.93) and anxiety (M = 3.57, SD = 3.38) [38,39,40], all three constructs showed a significantly higher mean value in the study sample (p < 0.001).
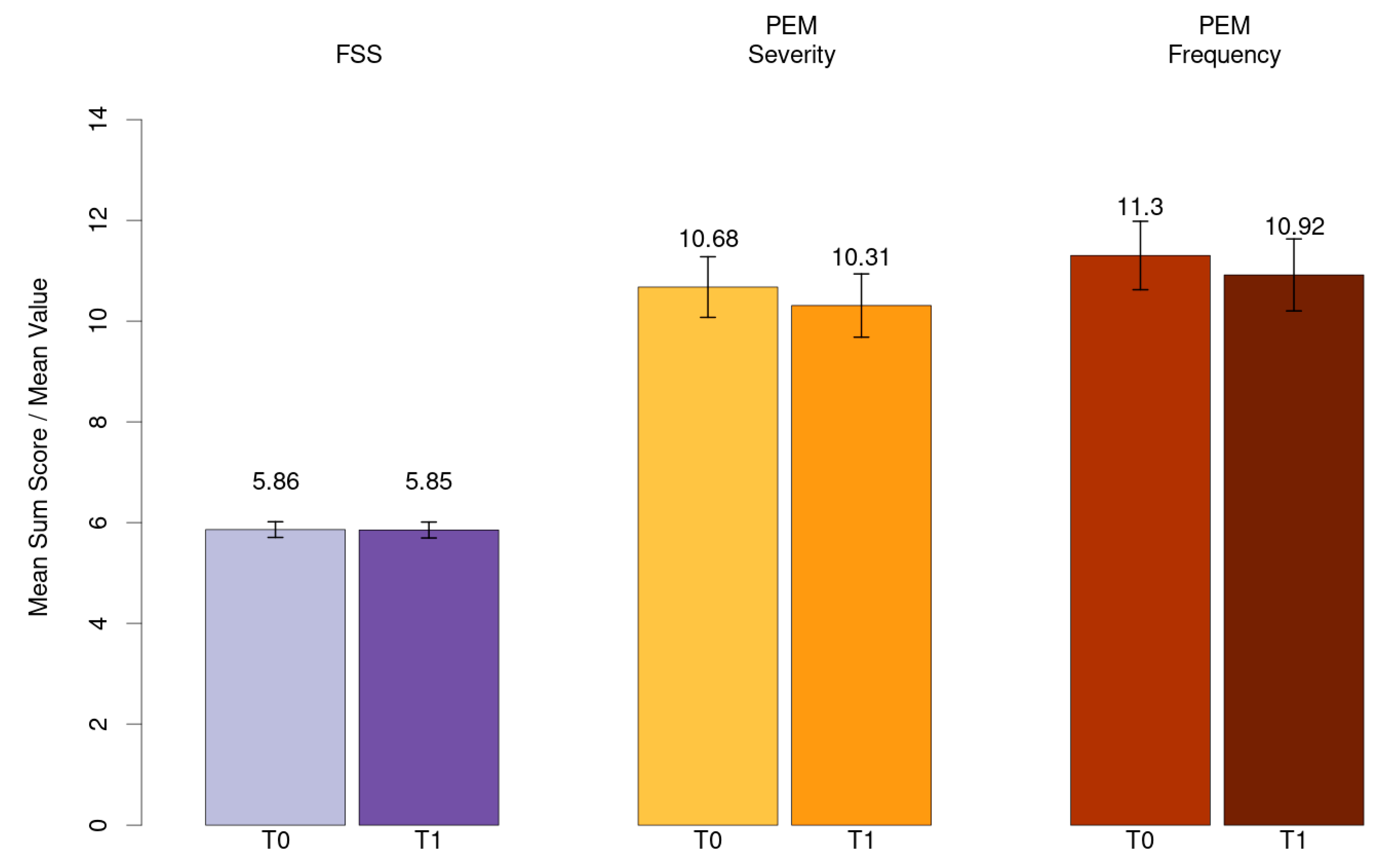
Mean value for fatigue (FSS) and mean sum score for PEM severity and frequency has been shown in Figure 3.
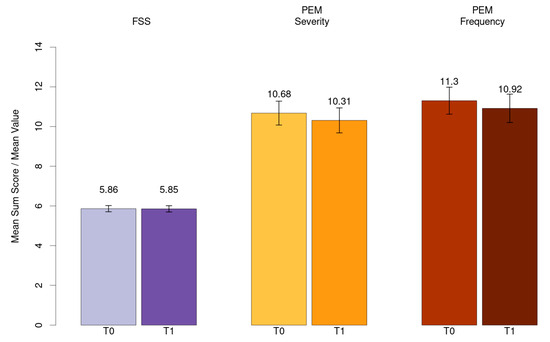
Figure 3.
Mean value for fatigue (FSS) and mean sum score for PEM severity and frequency.
3.3.2. Frequency of Symptoms
The frequency of symptoms above the cut-off was calculated at T0 and T1 and is displayed in Table 3. PEM frequency was not calculated due to the absence of a validated cut-off score. The frequency of PASC symptoms, using the PCS-S, is shown in Supplement S2.

Table 3.
Frequency of symptoms.
3.4. Utilization of Psychotherapeutic Treatment
Approximately 30% (n = 78) of the patients reported utilization of psychotherapeutic treatment prior to the onset of their PASC condition.
3.5. Mixed Model Analysis
Advanced age was associated with higher PHQ-9 values at T1 (p < 0.001) (Table 4). A significant reduction in mean scores over time was seen in PHQ-9, PHQ-15, GAD-7 and the PCS-S (pPHQ.9; PHQ-15, PCS-s < 0.001; pGAD-7 = 0.026). Additionally, a longer time span between the acute infection and baseline was associated with both higher PEM severity and frequency scores at T1 (p = 0.038; p = 0.017). Other variables such as sex, the course of the acute infection or a pathologic echocardiogram did not reach the level of significance in the analyses.

Table 4.
Mixed model analysis.
Patients with mental illness and prior psychotherapy had significantly higher scores in depression, anxiety, somatic and PASC symptoms at T1 compared to those without (pPHQ-9 = 0.035, pPHQ-15 = 0.006, pGAD-7; PCS-s = 0.038).
4. Discussion
To the best of our knowledge, the course of the mental health of PASC patients has, to date, not been comprehensively examined longitudinally. In our study, we present data of participants at our outpatient clinic, using six validated questionnaires (PROMs). Our data provide important insights on the trajectory of the mental health of patients with PASC, as well as on risk and protective factors.
4.1. Sociodemographic and Health-Related Variables
Our study sample’s demographics align with prior studies, with mostly female participants averaging 46 years of age [6,8,23]. Education levels were medium to high, and BMI and comorbidity rates resembled previous studies as well [5,23,28,32].
Employment rates at baseline resembled those of Peter’s [28], and, as observed in other studies, our cohort exhibited an increase in sick leave and incapacitation as well as unemployment rates due to PASC [19,28,41].
4.2. Post-COVID-Related Variables and Post-COVID Syndrome Score
The mean duration between acute infection and admission to our centre was 17 months extending to 22.5 months at follow-up, longer than in other studies [32]. The surveying period between baseline and follow-up spanned five months, similar to the existing literature [22,23]. However, the standard deviations cover a comparably large time span, which must be considered when comparing mental health changes to other studies.
Most patients received the vaccine three times and suffered a symptomatic course that did not require hospitalization, consistent with the other literature [5,28]. This substantiates the hypothesis that a severe and prolonged course of PASC can be seen regardless of the acute infection course [13,14].
In our sample, severe PASC was seen in 80.8% of the patients at follow-up. A Swedish study [42] revealed a similar prevalence of at least one symptom of PASC after 24 months; another study showed a lower prevalence of 62% [43].
It can be assumed that our patients experienced multiple symptoms, indicating an even higher symptomatic burden than in other studies.
The severity of PASC symptoms reduced significantly over time. However, the mean score was still 36.89, much higher and for a longer period than in other studies. Lemhöfer, for instance, found a mean PCS-S of 23.5 506 days post-infection, significantly lower than in our study cohort [44]. Considering that a score >26.25 indicates severe PASC, it is striking that our patients still experienced profound symptoms 22.5 months after the acute infection. Despite the significant decrease in severity, it remains on a very high level.
It is important to acknowledge that the Post-COVID centre in Erlangen was established specifically for patients already diagnosed with severe PASC. This particular patient population may account for the persistent severity of the symptoms and the heightened symptomatic burden observed in our study compared to other research findings.
4.3. Mental Health
4.3.1. Depression, Anxiety, Persisting Somatic Symptoms
Upon follow-up (T1), clinically relevant levels of depression, persisting somatic symptoms and anxiety were prevalent in 55.8%, 72.5% and 18.9% of patients, respectively. These percentages exceed those reported in several other longitudinal studies, particularly considering that many of these studies had follow-up periods of twelve months or less [8,20,28,45]. Guillen-Burgos, surveying patients over 24 months, revealed a lower depression frequency, whereas anxiety stood at a comparable level [25]. The prevalence of these symptoms is much higher in our cohort than reported in previous studies, where the frequency was approximately 20% [26,27,46].
The severity of depressive, anxiety and somatic symptoms exhibited a statistically relevant decrease over the surveying period, which aligns with two recent reviews [16,47]. However, when compared to the normal population, our sample values still significantly surpassed the established cut-off values, even after 22.5 months after infection [38,39,40]. This finding is substantiated in several studies all claiming persistent high symptomatic burdens of depression and anxiety and a general decline in the mental health of PASC patients [15,20,47,48].
Comparing our study’s values to those of other studies with similar sociodemographics reveals comparable to somewhat higher scores [49,50,51].
4.3.2. Fatigue and Post-Exertional Malaise
Fatigue emerges as the most common Post-COVID symptom of our cohort, consistent with numerous studies [4,5,9]. However, severe fatigue’s prevalence in our cohort was much higher than in previous studies [9,26,52,53]. Moreover, fatigue severity did not decrease, supporting the hypothesis that fatigue does not show a self-limiting course and remains on a clinically high level over an extended period. In some cases, it may even worsen over time [10,14,20,48]. Contradictory to our findings, Poole-Wright’s meta-analysis revealed a significant decrease over time [52].
In line with the existing literature, the severity and frequency of PEM showed a similar pattern, lacking a significant decrease over the study’s duration [29,54,55]. It can be assumed that therapy as usual, encompassing the diagnostic process and therapeutic recommendations, did not lead to an alleviation of fatigue and PEM symptoms.
Fatigue and PEM demonstrated a significant correlation in another study, suggesting that most patients suffering from PEM also suffer from ongoing fatigue [55]. It can be assumed that this observation holds true in our study as well, given that both constructs stayed on high levels without improvement over time. Bearing in mind that these symptoms are particularly debilitating for patients in their daily life, it is not striking that the unemployment rates, alongside sick rates, increased over the survey period, underscoring the urgency for special therapeutic options for PASC patients suffering from PEM and fatigue, with the objective of preventing a permanent inability to work and a worsening of the patient’s health over time.
4.3.3. Persistence of Symptoms
The persistence and severity of mental health impairments can potentially be attributed to the mental load caused by severe PASC on individuals striving to return to their pre-infection activity levels. Fatigue and PEM, in particular, pose obstacles to this return, a phenomenon reported in numerous studies and observed within our cohort [19,41,55].
With limited specific therapeutic options available, and no known causal therapy for fatigue and PEM [56], patients may struggle to adapt to ongoing challenges posed by PASC, such as difficulties in resuming activities of daily life. This ongoing incapacitation can lead to feelings of helplessness and patients may feel limited in their ability to improve or seek professional help. This most likely creates a vicious circle that maintains the impairments and the psychological strain of the patients.
Therefore, the slight reduction in symptom severity in some outcomes might result from the diagnostic process and medical attention provided by the Post-COVID centre. Putatively, similar to cancer and other chronic illnesses, confrontation with the new life situation may lead to new flexible forms of coping mechanisms over time, resulting in a reduction in the symptoms of mental health impairment.
The pathophysiological mechanisms of Post-COVID have to be taken into account as well, as systemic and central nervous system inflammation have been described as contributing to depression and anxiety even months after the acute infection [4,47,48]. We did not find a significant association between the course of the acute infection and elevated depressive and anxiety symptoms; however, this might be due to the high number of non-hospitalized patients in our cohort.
4.4. Risk and Protective Factors
4.4.1. Sociodemographic Data and Time
In our cohort, advanced age was associated with higher PHQ-9 values at follow-up, consistent with previous studies [4,5,6].
A longer duration between infection and baseline was associated with higher scores in both the severity and frequency of PEM at follow-up. One explanation might be that those patients did not receive guidance on symptom management, such as pacing protocols, early enough. PEM can be exacerbated even with activities of daily life [57], making early recommendations crucial to prevent sustained symptom elevation over time.
Over time, PHQ-9, PHQ-15, GAD-7 and the PCS-S fell significantly. This suggests the hypothesis that some symptoms of PASC may follow a self-limiting course, similar to other post-viral symptoms [31]. A missing association for fatigue and PEM may indicate that these symptoms only diminish slowly or not at all, as is reported in ME/CFS [31].
4.4.2. Psychotherapeutic Utilization before PASC
There was a significant association between higher scores in the PHQ-9, PHQ-15, GAD-7 and PCS-S in patients with a history of mental illness and psychotherapy before the onset of PASC.
This finding aligns with the existing literature indicating that prior psychiatric illnesses are a recognized risk factor for developing mental health symptoms in PASC [4,12,58], as well as more severe symptoms than patients without psychiatric history [59]. Contradictory to our findings, three studies found a protective factor of a prior psychiatric history, leading to lower GAD-7 and PHQ-9 scores in those individuals with previous psychiatric diagnoses than in those without [48,60,61].
4.5. Strengths and Limitations
To the best of our knowledge, this study is the first in-depth exploration of the mental health trajectory of Post-COVID patients after the first 22 months of the Post-COVID condition. Notably, this study boasts a substantial cohort size and a prospective design. We utilized six validated questionnaires covering a vast spectrum of possible symptoms and gathered crucial sociodemographic and clinical data as well as the information of prior psychotherapy.
Limitations are, primarily, the absence of a control group. Additionally, potential sex biases may exist, given that 70% of the cohort are females. Furthermore, there might be biases due to the absence of clinical structured interviews; instead, patients self-reported their symptoms and prior illnesses. Lastly, first presentation to the centre was relatively late after the acute infection; therefore, symptom development between the infection and first presentation is unknown.
5. Conclusions
In conclusion, this study emphasizes the need for further examination of the long-term mental health outcomes of PASC. The prevalence of severe Post-COVID syndrome was 80%, indicating a substantial symptomatic burden extending for the period of 22 months after infection. Depression, anxiety and somatic symptoms remain prevalent at elevated levels. While their severity exhibits a decrease over time, suggesting a self-limiting course, symptom manifestation is still significantly higher than in the normal population. Notably, symptoms of fatigue and PEM remained unchanged, emphasizing the need for specific therapeutic options for PASC patients and further studies herein. Our findings suggest, that therapy as usual does not lead to a sufficient alleviation of symptoms in fatigue and PEM, whereas other symptoms responded through a slight decrease in severity. Identified risk factors for a more severe disease course encompassed older age for depressive symptoms and a longer duration between infection and presentation at baseline for PEM. Additionally, mentally vulnerable patients, suffering from a prior psychiatric illness, were seen to be associated with more severe symptoms of PASC, depression, anxiety and somatic symptoms.
Further studies including control groups are warranted to assess more risk and potential protective factors and to identify individuals who are more vulnerable to PASC mental health symptoms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph21081076/s1, Supplement S1: Algorithm for echocardiograms; Supplement S2: PASC symptoms (PCS-S) at T0 and T1.
Author Contributions
I.C.S.: conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft and editing, visualization; J.K.: resources, methodology, data curation; W.A.: software, formal analysis; A.B.: conceptualization, methodology, software, data curation; R.H.: methodology, software, data curation; B.G.: methodology, data curation; M.R.: methodology, data curation; E.M.: conceptualization, methodology, validation, formal analysis, supervision; Y.E.: conceptualization, methodology, validation, resources, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the ethics committee of the Friedrich-Alexander-University Erlangen-Nürnberg (T0: 22-443-B, T1: 22-444_3-B).
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
The data used for the current study are not publicly available due to ethical and legal data protection restrictions.
Acknowledgments
The authors thank all participating patients as well as all colleagues working at the Post-COVID centre. Special thanks go to Michaela Lepschi, Julia Geyer and Aylin Wagner.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Coronavirus Disease (COVID-19). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-%28covid-19%29 (accessed on 7 May 2024).
- NICE Guideline. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. 2020. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 7 May 2024).
- German Guidelines for “Long/Post COVID Syndrome”. 2021. Available online: https://register.awmf.org/assets/ (accessed on 7 May 2024).
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Mei, B.; Wang, P.; Li, X.; Chen, X.; Wei, G.; Kuang, F.; Li, B.; Su, S. Prevalence and risk factors for persistent symptoms after COVID-19: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 30, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Morawa, E.; Krehbiel, J.; Borho, A.; Herold, R.; Lieb, M.; Schug, C.; Erim, Y. Cognitive impairments and mental health of patients with post-COVID-19: A cross-sectional study. J. Psychosom. Res. 2023, 173, 111441. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society statement on long COVID follow-up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Zhao, Y.M.; Yan, W.; Li, C.; Lu, Q.D.; Liu, L.; Ni, S.Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol. Psychiatry 2023, 28, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-Arimi, S. Physical and mental health complications post-COVID-19: Scoping review. J. Psychosom. Res. 2021, 147, 110525. [Google Scholar] [CrossRef]
- Marchi, M.; Grenzi, P.; Serafini, V.; Capoccia, F.; Rossi, F.; Marrino, P.; Pingani, L.; Galeazzi, G.M.; Ferrari, S. Psychiatric symptoms in Long-COVID patients: A systematic review. Front. Psychiatry 2023, 14, 1138389. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Yang, T.; Yan, M.Z.; Li, X.; Lau, E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: A systematic review and meta-analysis. Infection 2022, 50, 1067–1109. [Google Scholar] [CrossRef]
- Tracy, M.F.; Hagstrom, S.; Mathiason, M.; Wente, S.; Lindquist, R. Emotional, mental health and physical symptom experience of patients hospitalized with COVID-19 up to 3 months post-hospitalization: A longitudinal study. J. Clin. Nurs. 2024, 33, 591–605. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Lindner, A.; Kofler, M.; Ianosi, B.A.; Mahlknecht, P.; Heim, B.; Peball, M.; Carbone, F.; et al. Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study. Eur. J. Neurol. 2022, 29, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Purpura, L.J.; Shah, J.; Cantos, A.; Nordvig, A.S.; Yin, M.T. Anxiety, depression, insomnia, and trauma-related symptoms following COVID-19 infection at long-term follow-up. Brain Behav. Immun. Health 2021, 16, 100315. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Burgos, H.F.; Galvez-Florez, J.F.; Moreno-Lopez, S.; Gonzalez, I.; Guillen, M.; Anaya, J.M. Factors associated with mental health outcomes after COVID-19: A 24-month follow-up longitudinal study. Gen. Hosp. Psychiatry 2023, 84, 241–249. [Google Scholar] [CrossRef] [PubMed]
- van der Feltz-Cornelis, C.; Turk, F.; Sweetman, J.; Khunti, K.; Gabbay, M.; Shepherd, J.; Montgomery, H.; Strain, W.D.; Lip, G.Y.H.; Wootton, D.; et al. Prevalence of mental health conditions and brain fog in people with long COVID: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2024, 88, 10–22. [Google Scholar] [CrossRef]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; et al. A systematic review and meta-analysis of long COVID symptoms. Syst. Rev. 2023, 12, 88. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Kräusslich, H.G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V. Post-acute sequelae of covid-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef] [PubMed]
- Legler, F.; Meyer-Arndt, L.; Mödl, L.; Kedor, C.; Freitag, H.; Stein, E.; Hoppmann, U.; Rust, R.; Wittke, K.; Siebert, N.; et al. Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: Results from a prospective observational cohort. EClinicalMedicine 2023, 63, 102146. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. EClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.; Froehlich, L. DePaul Symptom Questionnaire—Short Form & Post Exertional Malaise (DSQ-SF & DSQ-PEM) German Translation; FernUniversität: Hagen, Germany, 2022. [Google Scholar]
- Kocalevent, R.D.; Hinz, A.; Brähler, E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry 2013, 35, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Ernst, J.; Glaesmer, H.; Brähler, E.; Rauscher, F.G.; Petrowski, K.; Kocalevent, R.D. Frequency of somatic symptoms in the general population: Normative values for the Patient Health Questionnaire-15 (PHQ-15). J. Psychosom. Res. 2017, 96, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Klein, A.M.; Brähler, E.; Glaesmer, H.; Luck, T.; Riedel-Heller, S.G.; Wirkner, K.; Hilbert, A. Psychometric evaluation of the Generalized Anxiety Disorder Screener GAD-7, based on a large German general population sample. J. Affect. Disord. 2017, 210, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Goodfellow, H.; Pookarnjanamorakot, P.; Murray, E.; Bindman, J.; Blandford, A.; Bradbury, K.; Cooper, B.; Hamilton, F.L.; Hurst, J.R.; et al. Impact of fatigue as the primary determinant of functional limitations among patients with post-COVID-19 syndrome: A cross-sectional observational study. BMJ Open 2023, 13, e069217. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.; Forsberg, G.; Divanoglou, A.; Balkhed, A.; Niward, K.; Berg, S.; Levi, R. Two-year follow-up of patients with post-COVID-19 condition in Sweden: A prospective cohort study. Lancet Reg. Health Eur. 2023, 28, 100595. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bae, S.; Chang, H.H.; Kim, S.W. Characteristics of long COVID and the impact of COVID-19 vaccination on long COVID 2 years following COVID-19 infection: Prospective cohort study. Sci. Rep. 2024, 14, 854. [Google Scholar] [CrossRef]
- Lemhofer, C.; Stallmach, A. Variations and Predictors of Post-COVID Syndrome Severity in Patients Attending a Post-COVID Outpatient Clinic. J. Clin. Med. 2023, 12, 4013. [Google Scholar] [CrossRef]
- Ahmed, G.K.; Khedr, E.M.; Hamad, D.A.; Meshref, T.S.; Hashem, M.M.; Aly, M.M. Long term impact of Covid-19 infection on sleep and mental health: A cross-sectional study. Psychiatry Res. 2021, 305, 114243. [Google Scholar] [CrossRef] [PubMed]
- Seighali, N.; Abdollahi, A.; Shafiee, A.; Amini, M.J.; Teymouri Athar, M.M.; Safari, O.; Faghfouri, P.; Eskandari, A.; Rostaii, O.; Salehi, A.H.; et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 105. [Google Scholar] [CrossRef]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; Villa, G.; Lorenzo, R.; Querini, P.; Benedetti, F. Prevalence, trajectory over time, and risk factor of post-COVID-19 fatigue. J. Psychiatr. Res. 2022, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.S.; Lubetkin, E.I.; Janssen, M.F.; Yfantopolous, J.; Bonsel, G.J.; Haagsma, J.A. Cross-sectional and longitudinal comparison of health-related quality of life and mental well-being between persons with and without post COVID-19 condition. Front. Epidemiol. 2023, 3, 1144162. [Google Scholar] [CrossRef] [PubMed]
- Bota, A.V.; Bogdan, I.; Razvan, D.V.; Ilie, A.C.; Tudor, R.; Indries, M.F.; Csep, A.N.; Marincu, I. A Three-Year Cross-Sectional Analysis of Depression, Anxiety, and Quality of Life in Patients with Post-COVID-19 Syndrome. Int. J. Gen. Med. 2024, 17, 751–762. [Google Scholar] [CrossRef]
- Lier, J.; Stoll, K.; Obrig, H.; Baum, P.; Deterding, L.; Bernsdorff, N.; Hermsdorf, F.; Kunis, I.; Bräsecke, A.; Herzig, S.; et al. Neuropsychiatric phenotype of post COVID-19 syndrome in non-hospitalized patients. Front. Neurol. 2022, 13, 988359. [Google Scholar] [CrossRef]
- Poole-Wright, K.; Guennouni, I.; Sterry, O.; Evans, R.A.; Gaughran, F.; Chalder, T. Fatigue outcomes following COVID-19: A systematic review and meta-analysis. BMJ Open 2023, 13, e063969. [Google Scholar] [CrossRef]
- Diem, L.; Fregolente-Gomes, L.; Warncke, J.D.; Hammer, H.; Friedli, C.; Kamber, N.; Jung, S.; Bigi, S.; Funke-Chambour, M.; Chan, A.; et al. Fatigue in Post-COVID-19 Syndrome: Clinical Phenomenology, Comorbidities and Association with Initial Course of COVID-19. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221102727. [Google Scholar] [CrossRef]
- Jason, L.A.; Dorri, J.A. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol. Int. 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Nehme, M.; Chappuis, F.; Kaiser, L.; Assal, F.; Guessous, I. The Prevalence, Severity, and Impact of Post-COVID Persistent Fatigue, Post-Exertional Malaise, and Chronic Fatigue Syndrome. J. Gen. Intern. Med. 2023, 38, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Boing, S.; Brinkmann, F.; Franke, C.; Glockl, R.; Gogoll, C.; Hummel, T.; et al. S1 Guideline Post-COVID/Long-COVID. Pneumologie 2021, 75, 869–900. [Google Scholar] [PubMed]
- Holtzman, C.S.; Bhatia, S.; Cotler, J.; Jason, L.A. Assessment of Post-Exertional Malaise (PEM) in Patients with Myalgic Encephalomyelitis (ME) and Chronic Fatigue Syndrome (CFS): A Patient-Driven Survey. Diagnostics 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Hazumi, M.; Usuda, K.; Okazaki, E.; Kataoka, M.; Nishi, D. Differences in the Course of Depression and Anxiety after COVID-19 Infection between Recovered Patients with and without a Psychiatric History: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 11316. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.C.; Barlow, P.B.; Comellas, A.P.; Garg, A. Anxiety and depression symptoms among patients with long COVID: A retrospective cohort study. Eur. Arch. Psychiatry Clin. Neurosci. 2024. [Google Scholar] [CrossRef]
- Rastogi, R.; Cerda, I.H.; Ibrahim, A.; Chen, J.A.; Stevens, C.; Liu, C.H. Long COVID and psychological distress in young adults: Potential protective effect of a prior mental health diagnosis. J. Affect. Disord. 2023, 340, 639–648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).