Relative Health Risk Reduction from an Advanced Multi-Modal Air Purification System: Evaluation in a Post-Surgical Healthcare Setting
Abstract
1. Introduction
2. Materials and Methods
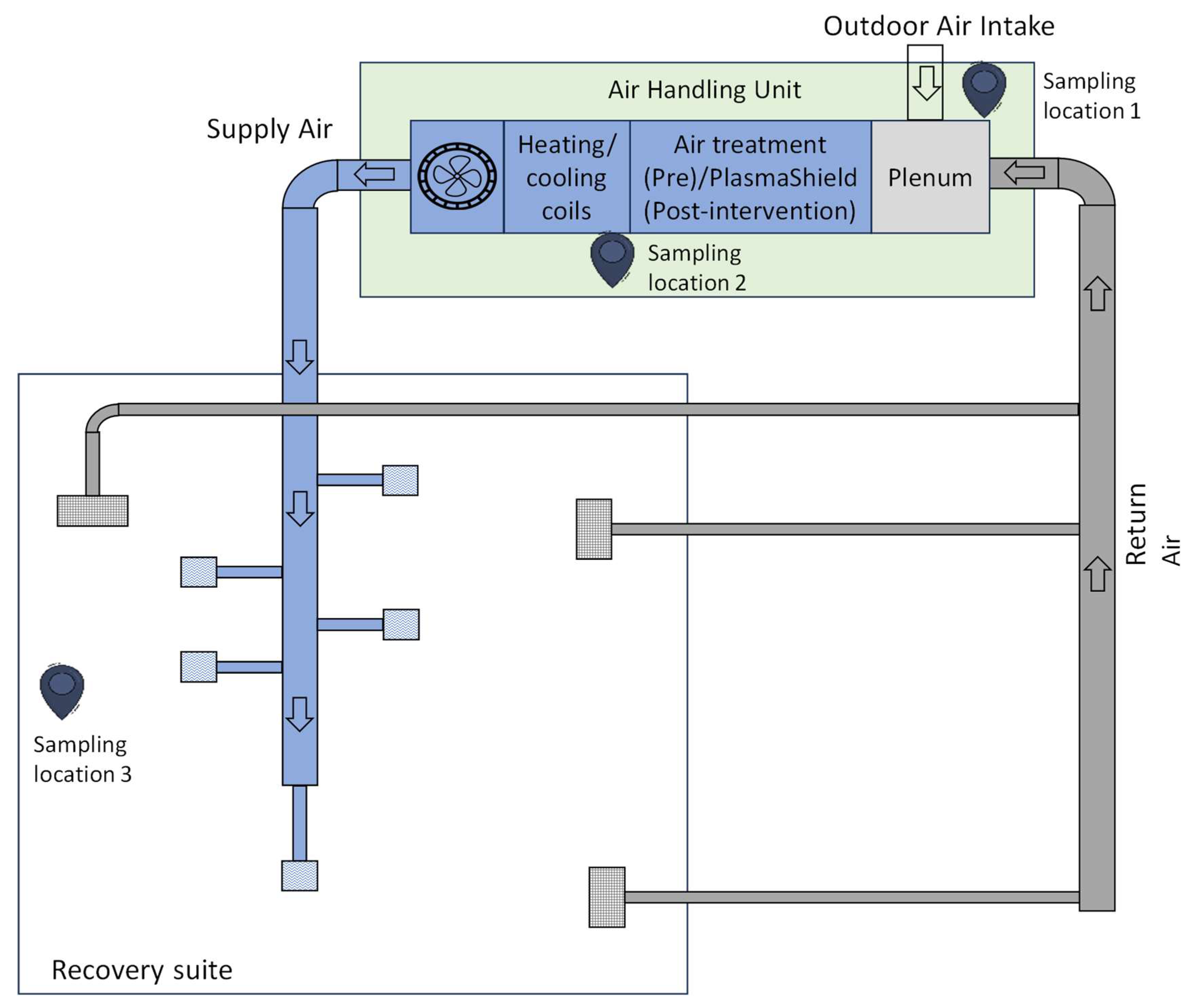
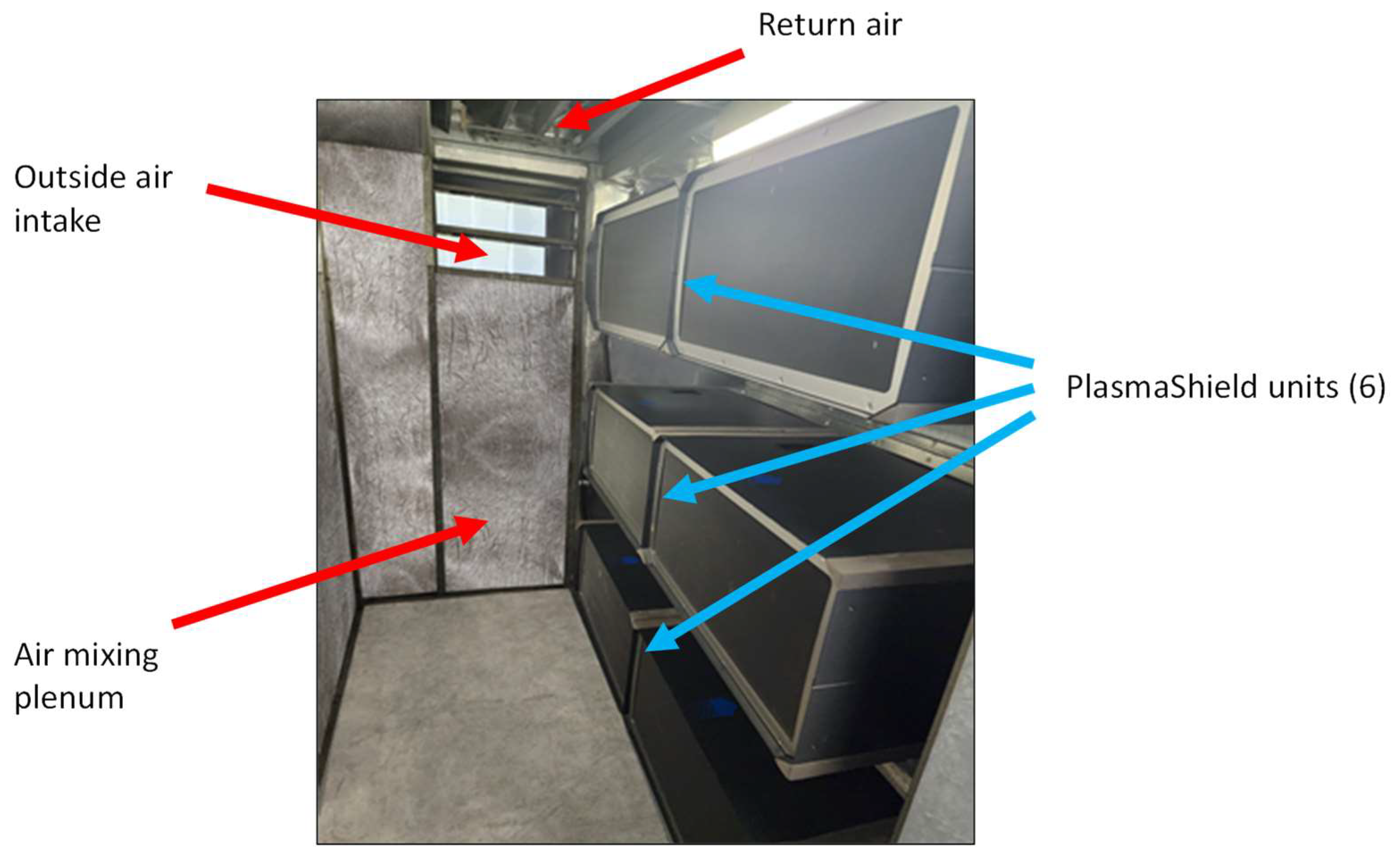
2.1. Study Location
2.2. Pre- and Post-Intervention Trial
2.3. Air Sampling
2.3.1. Sampling Methods
Air Quality Assessments (Non-Biological)
Microbial Assessments
2.4. Statistics
3. Results
3.1. General Observations
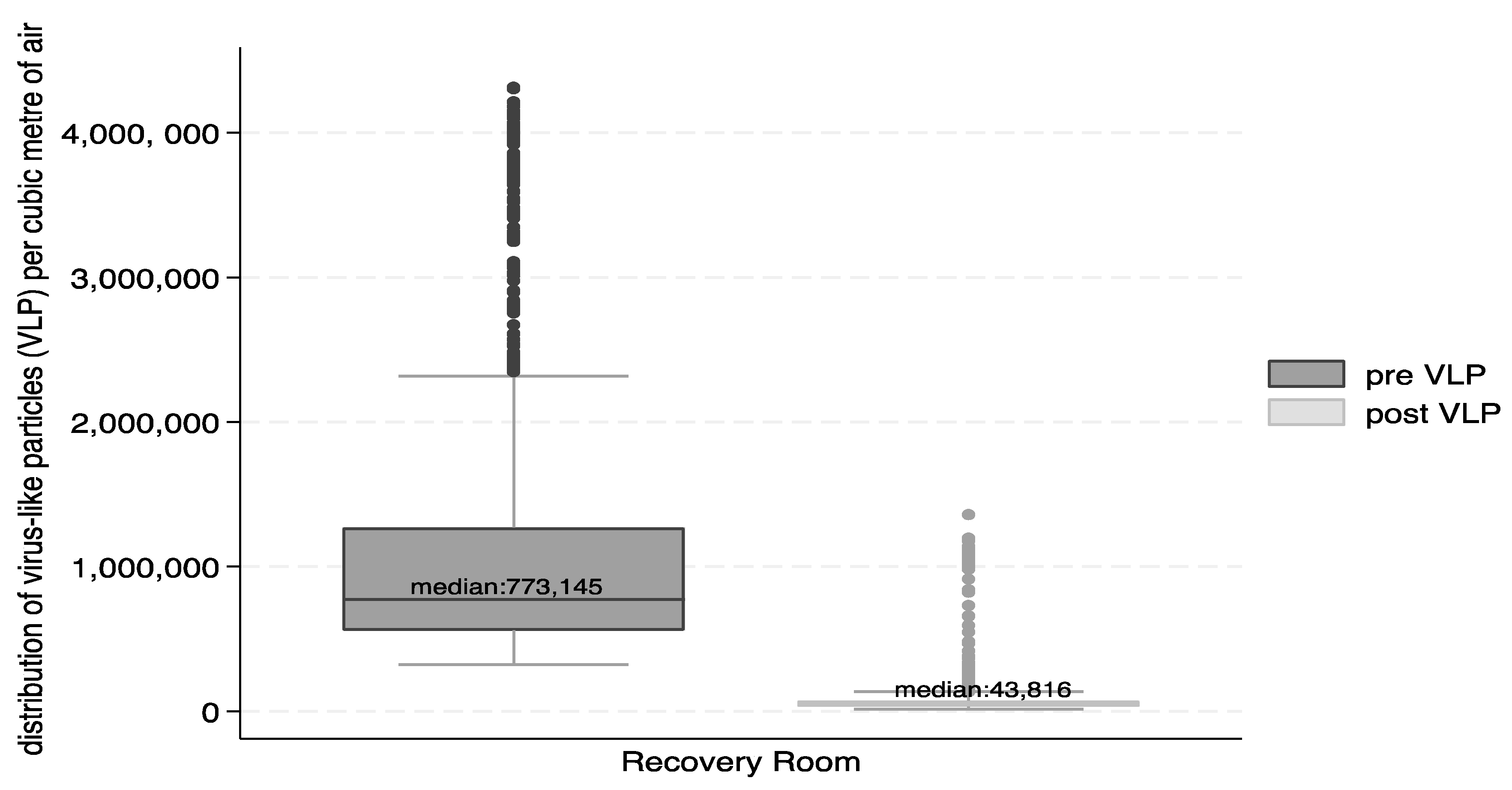
3.2. Airborne Particles
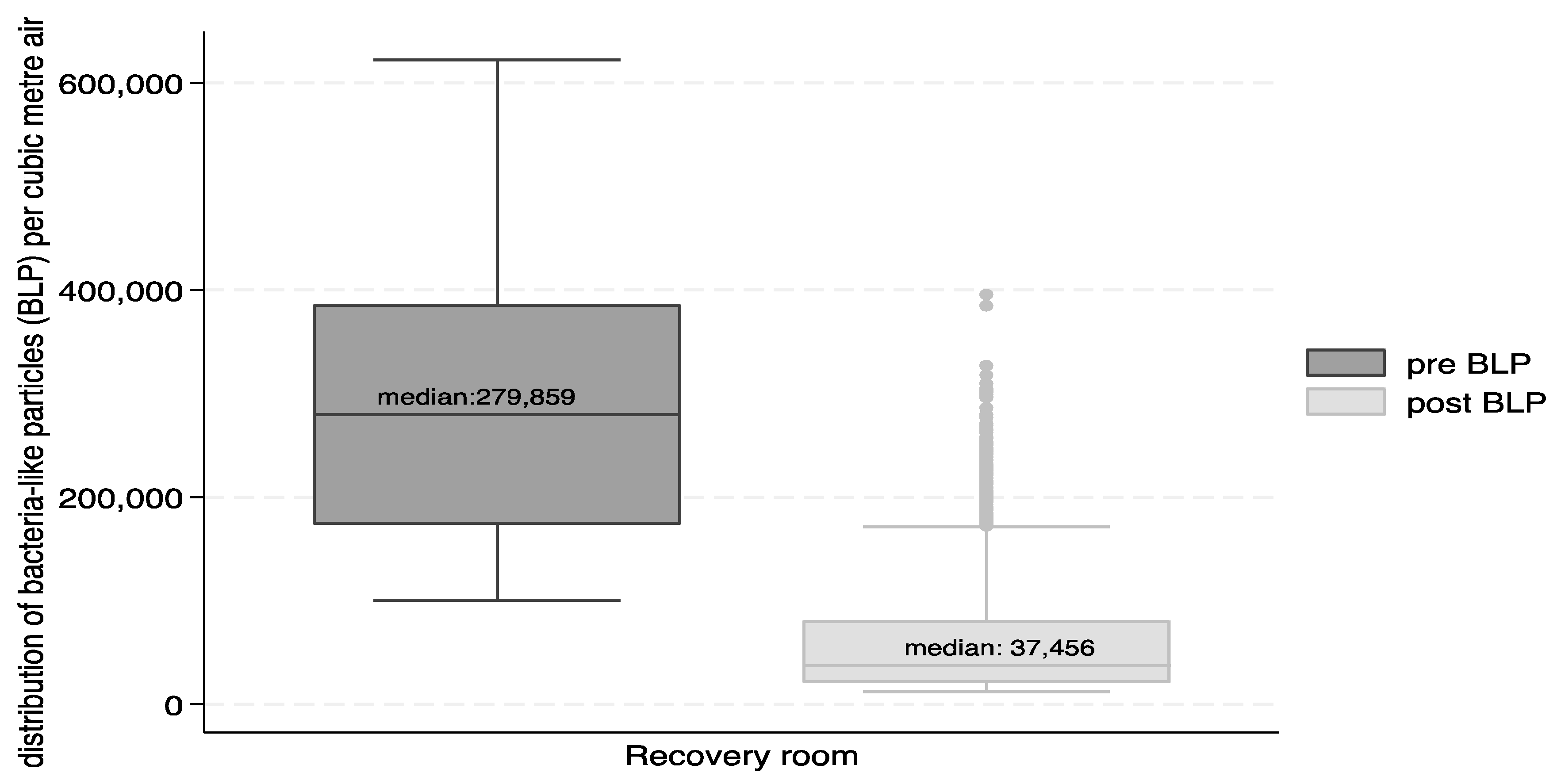
3.3. Culturable Microbial Counts
3.4. VOCs, Formaldehyde, Ozone, Nitrogen Dioxide, Sulphur Dioxide, Carbon Monoxide and Carbon Dioxide
3.4.1. Volatile Organic Compounds
3.4.2. Formaldehyde
3.4.3. Ozone
3.4.4. Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide
3.4.5. Carbon Dioxide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, J.G.; MacNaughton, P.; Satish, U.; Santanam, S.; Vallarino, J.; Spengler, J.D. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environ. Health Perspect. 2016, 124, 805–812. [Google Scholar] [CrossRef]
- Morawska, L.; Marks, G.B.; Monty, J. Healthy indoor air is our fundamental need: The time to act is now. Med. J. Aust. 2022, 217, 578–581. [Google Scholar] [CrossRef]
- Bolten, A.; Kringos, D.S.; Spijkerman, I.J.B.; Sperna Weiland, N.H. The carbon footprint of the operating room related to infection prevention measures: A scoping review. J. Hosp. Infect. 2022, 128, 64–73. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Nissen, K.; Krambrich, J.; Akaberi, D.; Hoffman, T.; Ling, J.; Lundkvist, A.; Svensson, L.; Salaneck, E. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci. Rep. 2020, 10, 19589. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Technical Consultation Report on Proposed Terminology for Pathogens That Transmit through the Air; World Health Organization: Geneva, Switzerland, 2024; Available online: https://cdn.who.int/media/docs/default-source/documents/emergencies/global-technical-consultation-report-on-proposed-terminology-for-pathogens-that-transmit-through-the-air.pdf?sfvrsn=de07eb5f_1&download=true (accessed on 17 May 2024).
- Tang, J.; Tellier, R.; Li, Y. Hypothesis: All respiratory viruses (including SARS-CoV-2) are aerosol-transmitted. Indoor Air 2022, 32, e12937. [Google Scholar] [CrossRef]
- Christie, B. COVID-19: Bring back mandatory mask wearing in health settings, say Scottish workers. BMJ 2023, 382, 1648. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Concerns over catching COVID-19 in hospital. New Sci. 2022, 255, 19. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Ventilation in Buildings. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html (accessed on 17 May 2024).
- World Health Organization. Roadmap to Improve and Ensure Good Indoor Ventilation in the Context of COVID-19; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240021280 (accessed on 17 May 2024).
- Victorian Department of Health. Ventilation: Guidelines for Optimising Ventilation to Reduce the Risk of Transmission of COVID-19 in Healthcare Settings. 2024. Available online: https://www.health.vic.gov.au/covid-19-infection-prevention-control-guidelines/ventilation (accessed on 17 May 2024).
- National Health and Medical Research Council (NHMRC). The Role of Airflow and Ventilation in Relation to SARS-CoV-2 Transmission in Quarantine Assessment. In National COVID-19 Health and Research Advisory Committee; Australian Government: Canberra, Australia, 2021. Available online: https://www.nhmrc.gov.au/sites/default/files/2023-02/NCHRAC-Advice-18-Airflow-and-ventilation-in-quarantine-arrangements.pdf (accessed on 17 May 2024).
- AS 4260-1997; High Efficiency Particulate Air (HEPA) Filters—Classification, Construction and Performance. Standards Australia: Sydney, Australia. Available online: https://www.standards.org.au/standards-catalogue/standard-details?designation=as-4260-1997 (accessed on 17 May 2024).
- International Agency for Research and Cancer (IARC). Outdoor Air Pollution. IARC Scientific Publication No. 16. 2016. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Outdoor-Air-Pollution-2015 (accessed on 17 May 2024).
- Fernández de Mera, I.G.; Granda, C.; Villanueva, F.; Sánchez-Sánchez, M.; Moraga-Fernández, A.; Gortázar, C.; de la Fuente, J. HEPA filters of portable air cleaners as a tool for the surveillance of SARS-CoV-2. Indoor Air. 2022, 32, e13109. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Optimising Ventilation for Infection Prevention and Control in Healthcare Settings. 2022. Available online: https://www.safetyandquality.gov.au/our-work/infection-prevention-and-control/optimising-ventilation-infection-prevention-and-control-healthcare-settings (accessed on 17 May 2024).
- Li, P.; Koziel, J.; Macedo, N.; Zimmerman, J.J.; Wrzesinski, D.; Sobotka, E.; Balderas, M.; Walz, W.B.; Paris, R.V.; Lee, M.; et al. Evaluation of an air cleaning device equipped with filtration and UV: Comparison of removal efficiency on particulate matter and viable airborne bacteria in the inlet and treated air. Int. J. Environ. Res. Public Health 2022, 19, 16135. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Wolfe, S.; Brisendine, C.; Eid, S.; Zangari, M.; Ford, F.; Snyder, B.; Moyer, W.; Levicoff, L.; Burfeind, W.R. The impact of comprehensive air purification on patient duration of stay, discharge outcomes, and health care economics: A retrospective cohort study. Surgery 2020, 168, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, A.R.; Schlener, S.D.; Eid, S.; Bock, K.A.; Worrilow, K.C. The effects of an advanced air purification technology on environmental and clinical outcomes in a long-term care facility. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Arikan, I.; Genc, O.; Uyar, C.; Tokur, M.E.; Balci, C.; Percin Renders, D. Effectiveness of air purifiers in intensive care units: An intervention study. J. Hosp. Infect. 2022, 120, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Brainard, J.; Jones, N.R.; Swindells, I.C.; Archer, E.J.; Kolyva, A.; Letley, C.; Pond, K.; Lake, I.R.; Hunter, P.R. Effectiveness of filtering or decontaminating air to reduce or prevent respiratory infections: A systematic review. Prev. Med. 2023, 177, 107774. [Google Scholar] [CrossRef] [PubMed]
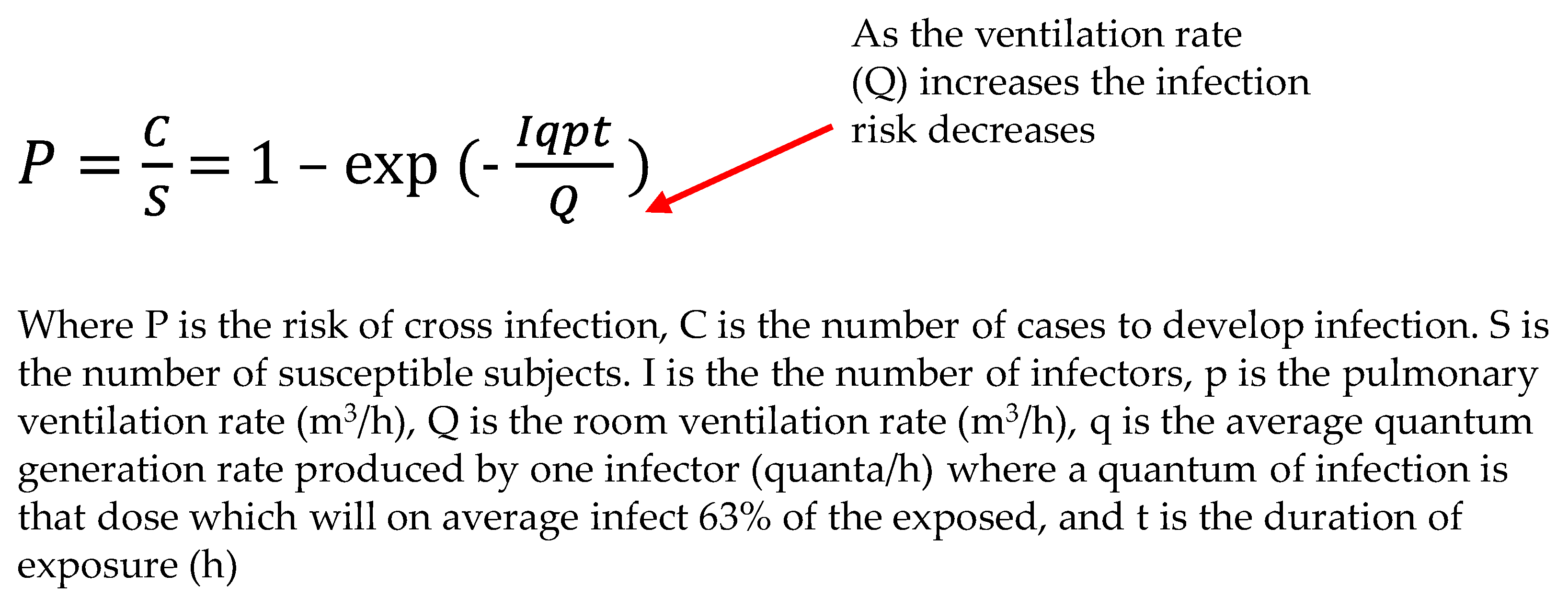
- Noakes, C.J.; Sleigh, P.A. Applying the Wells-Riley Equation to the Risk of Airborne Infection in Hospital Environments: The Importance of Stochastic and Proximity Effects. In Proceedings of the 11th International Conference on Indoor Air Quality. Indoor Air 2008, Copenhagen, Denmark, 17–22 August 2008; Available online: https://eprints.whiterose.ac.uk/7702/ (accessed on 17 May 2024).
- Lau, T.; Belusko, M. Airborne particulate matter filtration using non-thermal plasma air purification. ASHRAE J. 2023, 65, 38–48. [Google Scholar]
- Whiley, H.; Keerthirathne, T.; Kuhn, E.; Nisar, M.; Sibley, A.; Speck, P.; Ross, K.E. Efficacy of the Plasmashield®, a non-thermal, plasma-basedair purification device, in removing airborne microorganisms. Electrochem 2022, 3, 276–284. [Google Scholar] [CrossRef]
- ANSI/ASHRAE. Standard 52.2-2017: Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size; 2017. Available online: https://www.ashrae.org/File%20Library/Technical%20Resources/COVID-19/52_2_2017_COVID-19_20200401.pdf (accessed on 17 May 2024).
- Prussin, A.J., 2nd; Garcia, E.B.; Marr, L.C. Total virus and bacteria concentrations in indoor and outdoor air. Environ. Sci. Technol. Lett. 2015, 2, 84–88. [Google Scholar] [CrossRef]
- Archer, J.; McCarthy, L.P.; Symons, H.E.; Watson, N.A.; Orton, C.M.; Browne, W.J.; Harrison, J.; Moseley, B.; Philip, K.E.J.; Calder, J.D.; et al. Comparing aerosol number and mass exhalation rates from children and adults during breathing, speaking and singing. Interface Focus 2022, 12, 20210078. [Google Scholar] [CrossRef]
- Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023.
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Geneva, Switzerland, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- Alsved, M.; Nygren, D.; Thuresson, S.; Fraenkel, C.J.; Medstrand, P.; Londahl, J. Size distribution of exhaled aerosol particles containing SARS-CoV-2 RNA. Infect. Dis. 2023, 55, 158–163. [Google Scholar] [CrossRef]
- Shah, Y.; Kurelek, J.W.; Peterson, S.D.; Yarusevych, S. Experimental investigations of indoor aerosol dispersion and accumulation in the context of COVID-19: Effects of masks and ventilation. Phys. Fluids 2021, 33, 073315. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE). ASHRAE Standard 241 Factsheet, Control of Infectious Aerosols; ASHRAE: Washington, DC, USA, 2023; Available online: https://www.ashrae.org/file%20library/about/government%20affairs/advocacy%20toolkit/virtual%20packet/standard-241-fact-sheet.pdf (accessed on 17 May 2024).
- Azimi, P.; Stephens, B. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs. Build. Environ. 2013, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Graeffe, F.; Luo, Y.; Guo, Y.; Ehn, M. Unwanted indoor air quality effects from using ultraviolet C lamps for disinfection. Environ Sci. Technol. Lett. 2023, 10, 172–178. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Shaban, R.Z.; MacBeth, D.; Wood, C.J.; Russo, P.L. The burden of healthcare-associated infection in Australian hospitals: A systematic review of the literature. Infect. Dis. Health 2017, 22, 117–128. [Google Scholar] [CrossRef] [PubMed]
| Pre-Intervention (MERV-13) | Post-Intervention (PlasmaShield) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VLP | BLP | VLP | BLP | ||||||||||
| No. of Samples | Mean | SD | Median | Mean | SD | Median | No. of Samples | Mean | SD | Median | Mean | SD | Median |
| Outdoor air | |||||||||||||
| Total 2757 | 11,802 | 11,079 | 5682 | 389 | 591 | 358 | 2961 | 3562 | 1549 | 3662 | 4074 | 2429 | 4253 |
| Work 1328 | 7194 | 5733 | 4279 | 284 | 1588 | 253 | 1526 | 3251 | 1093 | 3571 | 3633 | 1533 | 4139 |
| Night 1429 | 15,907 | 12,956 | 12,101 | 473 | 2155 | 451 | 1435 | 3891 | 1861 | 3740 | 4540 | 3038 | 4674 |
| Supply Air | |||||||||||||
| Total 2757 | 1554 | 1205 | 964 | 389 | 179 | 358 | 2961 | 2 | 3 | 1 | 1 | 1 | 0.7 |
| Work 1328 | 1162 | 702 | 826 | 284 | 135 | 253 | 1526 | 3 | 4 | 2 | 1 | 1 | 1 |
| Night 1429 | 1867 | 1504 | 1544 | 473 | 165 | 451 | 1435 | 1 | 0.7 | 0.7 | 0.8 | 0.5 | 0.7 |
| Room Air | |||||||||||||
| Total 2757 | 1060 | 713 | 773 | 282 | 116 | 279 | 2961 | 74 | 112 | 43 | 61 | 59 | 37 |
| Work 1328 | 886 | 469 | 724 | 256 | 112 | 240 | 1526 | 108 | 149 | 67 | 94 | 67 | 79 |
| Night 1429 | 1216 | 899 | 1013 | 306 | 115 | 296 | 1435 | 38 | 14 | 36 | 27 | 13 | 21 |
| Pre-Intervention (CFU/m3) | Post-Intervention (CFU/m3) | |
|---|---|---|
| Bacteria | ||
| Outdoor air | 202 | 166 |
| Supply air | 120 | 4 |
| Reduction (%) | 41% | 98% |
| Fungi | ||
| Outdoor air | 314 | 320 |
| Supply air | 30 | 14 |
| Reduction (%) | 90% | 96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisaniello, D.; Nitschke, M. Relative Health Risk Reduction from an Advanced Multi-Modal Air Purification System: Evaluation in a Post-Surgical Healthcare Setting. Int. J. Environ. Res. Public Health 2024, 21, 1089. https://doi.org/10.3390/ijerph21081089
Pisaniello D, Nitschke M. Relative Health Risk Reduction from an Advanced Multi-Modal Air Purification System: Evaluation in a Post-Surgical Healthcare Setting. International Journal of Environmental Research and Public Health. 2024; 21(8):1089. https://doi.org/10.3390/ijerph21081089
Chicago/Turabian StylePisaniello, Dino, and Monika Nitschke. 2024. "Relative Health Risk Reduction from an Advanced Multi-Modal Air Purification System: Evaluation in a Post-Surgical Healthcare Setting" International Journal of Environmental Research and Public Health 21, no. 8: 1089. https://doi.org/10.3390/ijerph21081089
APA StylePisaniello, D., & Nitschke, M. (2024). Relative Health Risk Reduction from an Advanced Multi-Modal Air Purification System: Evaluation in a Post-Surgical Healthcare Setting. International Journal of Environmental Research and Public Health, 21(8), 1089. https://doi.org/10.3390/ijerph21081089







