Abstract
Dimba Cave is a large array of natural galleries in limestone mountains of the Democratic Republic of the Congo that contains highly valued pre-historic archaeological artifacts. The cave attracts a high number of tourists every year and is used by local populations as a water supply source. The main objective of the research undertaken in Dimba Cave consisted of assessing the quality of water and sediments from Dimba Cave ponds through evaluating contamination by heavy metals (15 elements analyzed, including As, Cd, Pb, and Hg) and by microbial populations (including Escherichia coli and total coliforms) in order to estimate the ecotoxicological risk to humans and to non-human biota. All water samples collected in the cave ponds showed very high metal concentrations exceeding the internationally recommended limits for drinking water, particularly for Cr, Mn, As, Pb, and Hg. Most sediment samples from cave ponds also displayed high heavy metal concentrations. The calculated pollution parameters, such as the enrichment factor (EF), and ecological risk parameters, such as the ecological risk index (Eri), indicated that the sediment may be toxic to aquatic biota. Furthermore, the microbiological analysis of pond waters indicated a widespread contamination with bacteria such as Escherichia coli, Enterococcus spp., total coliforms, and Pseudomonas spp., probably from anthropogenic and/or animal sources. Therefore, the consumption of Dimba Cave water as a drinking water represents a threat to public health. Urgent management measures should be enforced to protect public health and the cave ecosystem.
1. Introduction
Dimba Cave was discovered in the beginning of the 20th century, around 1920, and it was found to contain vast archeological artifacts of considerable interest to the investigation of pre-historic times of the region [1,2]. This cave is located in the territory of Mbanza-Ngungu, in the central part of the Democratic Republic of the Congo (DRC). Currently, the cave has high touristic frequency and because of the archaeological vestiges found therein, it has potential for being inscribed in the UNESCO World Heritage List. Therefore, the investigation and preservation of this unique Dimba Cave should be a priority.
Caves are common in limestone formations and are karstic structures carved by water. In general, the cave environment is dark, humid, and with minor temperature fluctuations, and, although offering a scarce supply of nutrients to the cave biota, provides habitats with stable conditions for many unique and endemic species [3,4,5,6,7]. The preservation and research on the fauna and flora of this uncommon environment is nowadays a priority in many countries (e.g., USA, Turkey, Slovenia).
Over time, many natural caves have been widely used by humans for different purposes, such as temporary shelter, residence, religious sanctuaries, extraction of natural resources, and tourism as show caves. Caves have also been used for disposal of household waste, especially in karst areas close to dwellings and, in the absence of regulations and suitable management, this has led to cave pollution and menace to this special environment [8,9].
Several studies on cave pollution have focused on contamination by heavy metals and invasive bacteria and, in particular, on fecal bacteria that have been used as an indicator of water and sediment contamination caused by waste from anthropogenic and animal origins [5,10,11,12,13]. These studies have shown the need for evaluating the concentration and distribution of heavy metals and microorganisms in cave waters and sediments in order to enable the assessment of ecotoxicological risks in touristic caves and to help their conservation [14,15,16,17].
The current investigation on Dimba Cave focused on the determination of 15 metallic elements in the water and sediments from cave ponds and on the microbiological analysis of cave water. This study is the first physicochemical and microbiological evaluation of water and sediments from Dimba Cave and is aimed at providing an assessment of the status of the cave environment and at identifying ecological threats as well as establishing the need for cave-management procedures.
2. Material and Methods
2.1. Description of the Study Area
Dimba Cave is an underground array of tunnels of natural origin carved by groundwater in limestone. The cave entrance is located 5 km south of the village of Mbamba Ntoto, in the Mbanza-Ngungu territory, DRC (geographic coordinates 05°17′42″ S and 014°52′23″ E). This cave spreads across a large part of the Cataracts district of DRC (Figure 1). A few meters into the cave entrance, there is a gallery of 6 m × 4 m on the left, arranged during colonization for meetings between settlers and villagers. Nearby, there is the tomb of an old chief of the Mbamba Ntoto village that is a place of ritual ceremonies by villagers.
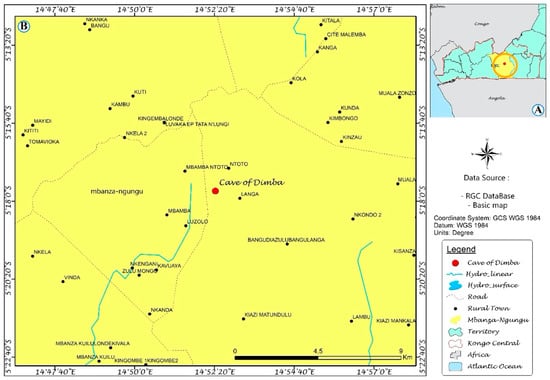
Figure 1.
(A) Mbanza-Ngungu territory located in Kongo central province of the DR Congo, (B) location of the Dimba Cave (red dot).
The local population developed special beliefs about Dimba Cave and practice spiritual rites therein. Furthermore, the population consumes cave water based on the belief that it cures several diseases, hunts bats for food, and carries out artisanal mining of lime and bat guano from the cave. Meanwhile, the cave has also become a major touristic attraction of the region.
Since 1997, this cave has been on the indicative list of properties that the Democratic Republic of the Congo government wants to propose for inscription into the UNESCO World Heritage List [18]. So far, the cave is not protected by specific regulations, and access is unrestricted.
Dimba Cave has abundant water and contains several ponds, with average diameters of 3 m and depths of about 1 m, which are located at approximately 400 m from the entrance of the cave (Figure 2).
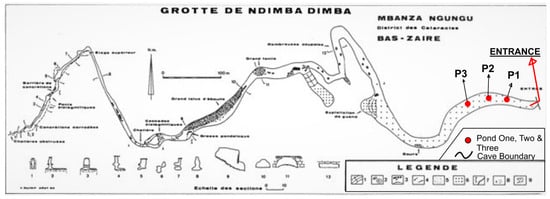
Figure 2.
Dimba Cave plan (based on Quinif et al. [3]).
The cave ponds are fed by the hydrological cycle of the karst system. This karst has morphological and hydrodynamic zonation that is mainly organized vertically and allows differentiating four layers in the karst aquifer: the epi-karst, the infiltration zone, the pinnate zone, and the drowned zone. The epi-karst is the superficial layer of the limestone formation that is relatively thick (with a few meters to a few tens of meters thickness) and collects surface waters into the karst. The infiltration zone temporarily stores the water and gradually transfers it to the deeper pinnate and drowned zones.
2.2. Sampling
Water and sediment sampling were conducted at three ponds in Dimba Cave on August 2021. Ponds were numbered 1, 2, and 3 in sequence from the nearest to the furthest to the cave entrance. Three water samples of 250 mL each were collected from ponds and labelled 1A, 1B, 1C for the first pond, 2A, 2B, 2C for the second pond, and 3A, 3B, 3C for the third pond. These samples were directly collected with clean and sterilized polypropylene bottles. The water samples for heavy metal analysis were acidified with 1% v/v HNO3. Replicate water samples for microbiological analysis were also collected with sterilized bottles and were not acidified [19].
In the same ponds, five samples of bottom sediments were collected in each pond. These samples, each with approximately 250 g from the sediment surface layer (0–3 cm), were manually collected at 1 m from the shore and at a depth of 0.5–1 m. These 15 sediment samples were labelled 1S1, 1S2, 1S3, 1S4, and 1S5 for the first pond, 2S1, 2S2, 2S3, 2S4, and 2S5 for the second pond, and 3S1, 3S2, 3S3, 3S4, and 3S5 for the third pond. After collection, all samples were packaged and preserved at 4 °C.
Sediment and water samples were sent to University of Geneva for heavy metals’ analysis. Microbial analyses were carried out in the microbiological laboratory of the University of Kinshasa, Chemistry Department.
2.3. Physicochemical Parameters
In situ measurements of pond water parameters, including temperature (T), electrical conductivity (EC), and hydrogen potential (pH), were performed using a multiparameter probe HQ40D from HACH (Auckland, New Zeland).
The grain size of sediment particles was determined on aliquots of about 1 g of fresh sediment from the ponds. Following 5 min of ultrasonic sediment dispersion in deionized water, particle grain size was determined with a Coulter LS-100 Laser diffractometer (Beckman Coulter, Fullerton, CA, USA) [14].
Sediment water content (WC) was determined based on the weight loss after sediment drying in an oven at 100 °C to constant weight. Sediment organic matter (OM) content was determined based on the weight loss of dry sediment aliquots after 1 h of combustion at 550 °C in a muffle furnace (Salvis AG, Lucerne, Switzerland) [20].
2.4. Sediment and Water Samples Treatment for Metal Analysis
After freeze-drying, the sediment samples were ground to a fine powder, homogenized, and sieved using a 63 μm sieve to remove occasional stones and debris. The <63 μm sediment fraction was retained for analysis in weighed aliquots, digested as described in Atibu et al. [21]. In short, a weighed aliquot of about 10–15 mg of sample (<63 µm fraction) was subject to complete digestion in pure mineral acids inside Teflon “bombs” heated on a vitro-ceramic hot plate. Sediment digestion was performed in three steps using (a) 1 mL of HNO3 (suprapure, 65%), then (b) 0.5 mL of a HClO4 mixture (suprapure, 70%) and 0.5 mL of HF (suprapure, 40%), (c) and, finally, 0.5 mL of HNO3 (suprapure, 65%). Between each step, the solvents were evaporated to dry residue, and the residue dissolved with the next treatment. The residue from the final step was dissolved in 10 mL of a 1% HNO3 solution, and the metal analysis was performed within 24 h.
For water samples, 10 mL of acidified samples was filtered using 0.45 µm membrane filters (Millex®-LCR, Millipore, Darmstadt, Germany) to remove particulate matter. A solution of 1% HNO3 was used for specific dilutions prior to metal analysis.
2.5. Analysis of Heavy Metals in Sediment and Water Samples
The concentrations of heavy metals in the samples were determined by inductively coupled plasma mass spectrometry using the Agilent 7700 × ICP-MS series developed for the analysis of complex matrices. To avoid spectral interferences, a helium mode and specific interference equations were used. Calibration was performed using multi-element standard solutions at different concentrations (0, 0.02, 1, 5, 20, 100, and 200 mg L−1) [22].
The limit of detection (LOD) was determined for each element as three times the standard deviation of the blank (Table 1). The concentration of each metal and associated analytical uncertainty were determined as the average and standard deviation of triplicate analysis of the same sample. The relative standard errors of metal concentrations were generally less than 10%, while the procedural chemical blanks were less than 2% of the sample signal. The results were expressed in mg L−1 and mg kg−1 (dry weight) for water and sediment samples, respectively.

Table 1.
Limits of detection (LOD) for the analyzed elements, and results of the determination of metals in lake water and sediment certified reference materials (CRM). Triplicate laboratory determinations of individual elements in CRMs displayed relative standard deviations generally lower than 5%. Certified values of CRMs are included for comparison. “–”: CRM value not available or not determined.
2.6. Analysis of Total Mercury in Sediment Samples
An atomic absorption spectrometer Advanced Mercury Analyzer (AMA 254, Altec s.r.l., Czech Republic) was used for the quantitative determination of total mercury in the samples. The method of analysis consisted in the combustion of sediments, followed by the fusion of mercury in a gold trap and, finally, by the measurement of gaseous mercury with the AMA. The detection limit, determined as three times the standard deviation of the blank, was 0.005 mg kg−1 (Table 1) [23].
2.7. Analysis of Total Mercury in Water Samples
Cold vapor atomic fluorescence spectrometry (CVAFS) (Merx Model III, Brooks Rand, Seattle, WA, USA) was used for the analysis of total mercury (THg) in water samples. Sample preparation was carried out according to the procedure described by Gallorini and Loizeau [24]. This procedure is similar to that of EPA 1631 [25]. It consists of weighing 100 mL of a pre-acidified water sample with 0.5 mL of HNO3 and adding 0.5 mL of SnCl2 at 20% to reduce Hg(II) to volatile mercury Hg(0). Oxygen was removed by purging the sample with argon for 20 min at a flow rate of 350 mL min−1, while the gold trap was purged with argon for 5 min at a flow rate of 60 mL min−1. THg was then measured using CVAFS. The detection limit, calculated as three times the standard deviation of the blank, was 0.20 ng L−1.
2.8. Analytical Quality Control
The reliability of the analytical procedure, the sensitivity of the device and trueness of the results were tested for water and sediment matrices through the repeated analyses of the certified reference materials TMDA-70 and LKSD4 (Environment Canada), respectively (Table 1). Certified reference materials (CRM) were selected to match the matrix of the samples from Dimba Cave. The metal concentrations determined in the CRMs were in excellent agreement with the CRM certified reference values (Table 1).
2.9. Assessment of Sediment Pollution
Sediment pollution was evaluated by calculating two parameters, the enrichment factor (EF) and the geoaccumulation index (Igeo) [26]. In order to detect metal pollution from anthropogenic origin and discriminate it from the natural geochemical background, the EF was calculated as follows:
where “metal” is the heavy metal concentration in the sediment sample or in the geochemical background and Sc is the scandium concentration in the sediment sample or in the geochemical background. Sc was used to carry out a normalization of analytical results, and the Upper Continental Crust (UCC) metal concentrations were considered as the geochemical background concentrations for the investigated heavy metals [27].
EF = [metal/Sc]sample/[metal/Sc]background
The degree of pollution was assessed by calculating the Igeo as follows:
where Cm is the concentration of metal (m) in the sediment, Bm is the concentration of the same metal (m) in the geochemical background, and 1.5 is the correlation matrix safety factor to account for the variation of the natural geochemical background [20].
Igeo = Log2[Cm]/1.5[Bm]
2.10. Ecological Risk Parameters
To quantify the heavy metal concentration in sediment samples, the contamination factor (CF) was calculated as follows [28,29]:
where Cn is the concentration of heavy metals in the sediment sample and Bn is the concentration of heavy metals in the geochemical background.
CF = (Cn/Bn)
The assessment of the polymetallic contamination level for each sediment sample was carried out by calculating the contamination degree (CD) as follows [30]:
where i stands for a specific heavy metal and CFi is the contamination factor for the heavy metal i. The heavy metals Hg, Cd, As, Co, Cu, Pb, Cr, and Zn were considered in the CD calculation.
CD = ΣCFi
The ecological risk index (Eri) was calculated to assess the harmful impact of heavy metals on both the environment and human health. This parameter reflects the ecological sensitivity and the toxicity of pollutants [31]. Eri was determined as follows:
where Tri represents the response factor to a given toxic heavy metal or biological toxic factor of the heavy metal. Tri values used for each element (in parenthesis) were 40 (Hg), 30 (Cd), 10 (As), 5 (Co), 5 (Cu), 5 (Pb), 2 (Cr), and 1 (Zn) [32]. CFi is the contamination factor for the heavy metal i.
Eri = Tri × CFi
The potential ecological risk index (RI) for polymetallic contamination in each sample was calculated by summing the singular ecological risk indices. This RI factor takes into account the synergy between the toxic level, the concentration of heavy metals, and the ecological sensitivity of biological communities to heavy metals [33]. The RI was calculated as follows:
where Eri is the ecological risk index for the heavy metal i.
RI = ΣEri
The ecotoxicological risk posed by metal pollutants in sediments was also assessed through comparison of metal concentrations determined in Dimba Cave sediments against the limits recommended in Sediment Quality Guidelines (SQG) for the protection of aquatic life and the Probable Effect Levels (PEL) on aquatic biota, as adopted by Canada [34].
2.11. Microbial Analysis in Water Samples
The quantification of microbial populations such as those of Escherichia coli (E. coli), enterococci (ENT), total coliforms (TC), and Pseudomonas spp. (P. spp.) was performed using the membrane filtration method. In brief, after filtration of water samples through 47 mm diameter and 0.45 μm pore-size membrane sterile filters (Millipore, Bedford, MA, USA), the filters were placed on selective culture media (Biolife, Italy) supplemented with the antifungal compound Nystatin (100 μg mL−1 final concentration).
The following incubation conditions were used: E. coli: Tryptone Soy Agar (TSA) medium, incubated at 37 °C for 4 h and transferred to Tryptone Bile X-Gluc Agar (TBX) medium at 44 °C for 24 h; ENT: Slanetz Bartley Agar (SBA) medium, incubated at 44 °C for 48 h and transferred into Bile Aesculin Agar (BAA) medium at 44 °C for 4 h; TC: Endo Agar, incubated at 35 °C for 24 h; P. spp.: Pseudomonas selective Agar (PSA), incubated at 37 °C for 24 h.
The number of colony-forming units per 100 mL of water (CFU 100 mL−1) was used to express the results. The reproducibility of the whole experimental procedure was tested by means of triplicate analyses of selected samples. The results of triplicate analysis displayed a mean coefficient of variation of 8% for E. coli and 9% for both ENT and TC [35,36].
2.12. Data Treatment
Triplicate determinations of heavy metal concentrations were carried out on each sample and averaged. Statistical data treatment (Spearman correlation) was performed using SigmaStat 11.0 software (Systat Software, Inc., San Jose, CA, USA) and XLSTAT software version 2021.1 from Addinsoft [37] (Statistical and data analysis solution, New York, United States; https://www.xlstat.com, accessed on 3 June 2024).
3. Results and Discussion
3.1. Physicochemical Characteristics of Water and Sediment Samples
Table 2 shows the results of determination of water (Table 2a) and sediment (Table 2b) physicochemical parameters, including the temperature (T), hydrogen potential (pH), electrical conductivity (EC), water content (WC), organic matter (OM), and mean size of sediment particles.

Table 2.
Physicochemical parameters determined in Dimba Cave samples.
For the three ponds investigated in Dimba Cave, the water temperature ranged from 23 to 24 °C. These values were within the 12–25 °C range set by the WHO for drinking water of acceptable quality. Dimba Cave waters were acidic, with pH values in the range of 3.25–4.44, and were well below the WHO range of pH recommended values (6.5–9.5) for drinking water of acceptable quality [38]. Acidic groundwater in limestone lithology is unusual and may indicate intrusion of acidic and contaminated water from waste releases into the environment and/or contamination of the ponds by miners and bat guano collectors. The EC values in Dimba Cave water samples varied from 312 to 444 µS cm−1 and were within the range recommended by the WHO (200–800 µS cm−1) for drinking water [38].
The sediment water content (WC) ranged from 48 to 60% for all samples, while organic matter (OM) ranged from 6.34 to 7.91% of sediment dry weight (Table 2b). The mean size of sediment particles ranged from 4.72 to 10.95 μm for all samples. According to the descriptive terminology adopted in the GRADISTAT program, the sediment samples consisted mostly of medium and fine silts [39].
3.2. Heavy Metal Concentrations in the Water Samples
The concentration values of heavy metals in the water samples are reported in Table 3. Pond 3 showed the highest metal concentrations, but in the three ponds, almost all samples showed very high concentrations of Cr, Mn, Ni, Cu, As, Cd, Pb, and Hg that practically exceeded the limit values set for drinking water [40]. The concentration levels of other metals, namely Sc, Ti, V, Fe, Co, Zn, and Ba, were often elevated as well but not exceeding the permissible limits for drinking water. Limits recommended by the WHO for heavy metals in drinking water were included for the elements available in Table 3, as were the limits adopted by the European Union for drinking water quality [41]. From the comparison of concentrations, several heavy metals in these pond waters do not allow its use as drinking water for human consumption.

Table 3.
Heavy metal content (mg L−1) in filtered water samples from Dimba Cave ponds. The heavy metal concentration values that exceeded the recommended limit values set by the WHO for drinking water are shown in bold [40].
3.3. Concentrations of Heavy Metals in the Sediment Samples
Table 4 shows the results of heavy metal analysis in sediment samples. The highest concentration values in the samples were 15.2 mg kg−1 (Sc), 270 mg kg−1 (Ti), 72.3 mg kg−1 (V), 62.0 mg kg−1 (Cr), 10,430 mg kg−1 (Mn), 46,698 mg kg−1 (Fe), 15.3 mg kg−1 (Co), 57.4 mg kg−1 (Ni), 339 mg kg−1 (Cu), 1065 mg kg−1 (Zn), 6.1 mg kg−1 (As), 1.2 mg kg−1 (Cd), 1023 mg kg−1 (Ba), 24.6 mg kg−1 (Pb), and 1.2 mg kg−1 (Hg).

Table 4.
Heavy metal concentrations in sediment samples (mg kg−1 dry weight) from Dimba Cave ponds. The heavy metal concentration values exceeding the Sediment Quality Guidelines for the Protection of Aquatic Life are shown in bold [34]. SQG—sediment quality guidelines, PEL—probable effect levels.
Most sediments’ concentrations of Cr, Cu, Zn, As, Cd, and Hg exceeded the sediment quality guideline values (SQG) for the protection of aquatic life [34]. Concentrations even higher than SQG values and exceeding the Probable Effect Levels (PEL) for aquatic biota were determined in almost all samples for Cr, Cu, Zn, Cd, and Hg, with Hg and Zn displaying concentrations 2.5 to 3.5 times higher than their respective PEL values. Therefore, the sediments from cave ponds were contaminated with heavy metals in concentrations largely exceeding the sediment quality guidelines. The current contamination levels of these sediments may put in danger the aquatic biota of the cave’s ecosystem [42].
3.4. Estimation of the Pollution Level
In order to discriminate heavy metals of anthropogenic origin from metals of geogenic origin, the EF and Igeo indices were calculated and used to build a criterion (a scale) to classify the level of sediment pollution by heavy metals [43]. The results are presented in Table 5 and Table 6, respectively.

Table 5.
Enrichment factor (EF) values of heavy metals in sediment samples from Dimba Cave ponds.

Table 6.
Igeo values of heavy metals in sediment samples from Dimba Cave ponds.
The highest EF values of Hg (2 samples) and Cu (1 sample) were between 25 and 50, indicating “very severe enrichment” of this metal in sediment. Other samples showed “severe enrichment” for Hg and “severe enrichment” to “moderately severe enrichment” for Cu. The EF values of Cr, Mn, Co, Ni, Zn, As, and Cd varied across sampling sites, ranging from “minor enrichment” to “severe enrichment”, while “no enrichment” was observed for Pb in the samples from Ponds 2 and 3, and a “minor enrichment” for the samples from Pond 1. On the one hand, according to the calculated Igeo values, all the samples analyzed were “heavily polluted” by Hg, while only some samples collected in Pond 2 were “heavily polluted” by Mn, Cu, Zn, and Cd (Table 5). On the other hand, all the samples were “practically unpolluted” by Co and Pb and ranked as “unpolluted to moderately polluted” by Cr and Ni. Regarding Mn, Cu, Zn, As, and Cd, the samples were classified from “unpolluted to moderately polluted” and from “moderately to heavily polluted”.
Therefore, although there was a wide range of contamination levels for individual metals, globally, the pond sediments were highly contaminated by several toxic metals.
3.5. Statistical Correlations
The Spearman rank correlation was applied to parameters measured in water and sediment samples. The results are shown in Table 7 and Table 8, respectively.

Table 7.
Spearman rank order correlation for physicochemical parameters analyzed in water samples from Dimba Cave. Significant coefficients, with p < 0.05, are indicated in bold.

Table 8.
Spearman rank order correlation for physicochemical parameters analyzed in sediment samples from Dimba Cave ponds. Significant coefficients, with p < 0.05, are shown in bold.
A correlation analysis of concentrations of several heavy metals with temperature (T), pH, and electrical conductivity (EC) was carried out, and the results are reported in Table 7. The results indicated strong and positive correlations (p < 0.05) between T and V, Mn, Fe, Co, As, and Hg, while negative correlations were observed between pH and V, Cr, Fe, Ni, Zn, As, Pb, and Hg. EC also had negative correlations with V, Cr, Mn, Fe, Co, Ni, Zn, As, Pb, and Hg. In general, strong and positive correlations were observed between all heavy metals, except between Cd and V, Cr, Mn, Fe, Co, and Pb ; between Hg and Cd; between Pb and V, Fe, and Co ; between Zn and V and Fe; and between Cu and V, Mn, Fe, and Co, for which no correlations were observed. The strong and positive correlations suggested that the heavy metals came from a common source and were transported into the ponds’ water by the same route. Additionally, the positive correlation would indicate that T had a positive impact on this transport. The negative correlation between heavy metals, pH, and EC indicated that the migration of heavy metals was negatively influenced by these parameters [44,45].
Regarding the sediment matrix, a correlation analysis between several heavy metals, WC, OM, and grain size was performed, and Table 8 shows the obtained results. The positive correlations between heavy metals and WC, OM, and grain size indicated that the accumulation of heavy metals in the sediments would be influenced by OM, WC, and grain size. Positive correlations were also observed between metals such as Cr and Pb (r = 0.587); Mn and Ni (r = 0.811), Cu (r = 0.800), Zn (r = 0.979), As (r = 0.782), and Hg (r = 0.926); Co and Ni (r = 0.500) and Cu (r = 0.581); Ni and Cu (r = 0.791), Zn (r = 0.808), As (r = 0.850), and Hg (r = 0.847); Cu and Zn (r = 0.814), As (r = 0.665), Cd (r = 0.612), and Hg (r = 0.786); Zn and As (r = 0.814) and Hg (r = 0.926); As and Hg (r = 0.815). These correlations suggest that these metals may have originated from the same sources and were transported together [45].
3.6. Ecological Risk Parameters
Table 9 shows the results of the ecological risk parameters, including the contamination factor (CF), the potential ecological risk factor (Eri), the contamination degree (CD), and the ecological risk index (RI). The CF showed that, in general, the sediments were “considerably contaminated” (3 < CF < 6) or “very strongly contaminated” (6 < CF) by Cu, Zn, As, Cd, and Hg. Some samples displayed “moderate contamination” (1 < CF < 3) or “low contamination” (CF < 1) with Cr, Co, As, and Pb. Concerning the CD values, all the sediment samples showed “very high contamination” (32 ≤ CD).

Table 9.
Values of ecological risk parameters in sediment samples from Dimba Cave for contamination factor (CF), contamination degree (CD), ecological risk index (Eri), and potential ecological index (RI).
It was noticed that the sediment samples had low ecological risks (Eri < 40) or moderate ecological risks (40 < Eri < 80) for Cr, Co, Cu, Zn, As, and Pb, whereas high ecological risks (160 < Eri < 320) or very high ecological risks (Eri > 320) were detected for Cd and Hg. Regarding the RI parameter, all the samples showed very high ecological risks or a serious ecological pollution level (IR > 600).
In general, these parameters indicated that anthropic activities, including activities of local populations, resulted in serious contamination of the cave environment. This contamination may have occurred at the surface and reached the cave through water infiltration in the karst, but might have occurred also in the cave from activities such as guano and lime exploitation.
3.7. Microbiological Parameters of Water Samples
Table 10 shows the population densities of Escherichia coli (E. coli), Enterococcus spp. (ENT), total coliforms (TC), and Pseudomonas spp. (P. spp.) determined in the water samples from Dimba Cave ponds. To assess the sanitary quality of water, the European Union (EU) and the United States Environmental Protection Agency (USEPA) recommend the use of E. coli (a fecal coliform) and ENT (the members of Enterococcus genus) as indicators [46,47].

Table 10.
The population densities (CFU 100 mL−1) of E. coli, ENT, TC, and P. spp. in water samples from Dimba Cave. Limit values recommended by the WHO and adopted by the EU for drinking water were included for comparison.
The microbial population densities in Dimba Cave pond waters varied with the sampling sites between 123 and 243 CFU 100 mL−1 for E. coli, 8 and 16 CFU 100 mL−1 for ENT, 20 and 28 CFU 100 mL−1 for TC, and 10 and 16 CFU 100 mL−1 for P. spp. These values clearly showed microbiological contamination of the waters of Dimba Cave, and, according to the European Directive (EU) 2020/2184 [41] for drinking water quality, the cave water is not suitable for human consumption [47].
Spearman rank correlation coefficients applied to microbiological parameters measured in water samples are shown in Table 11.

Table 11.
Spearman rank order correlation for microbiological parameters analyzed in water samples. Positive and significant coefficients, with p < 0.05, are indicated in bold.
A positive and significant correlation (p < 0.05) was observed between E. coli/TC (r = 1). This is understandable because E. coli is a subset of the fecal coliform group. Significant correlations were observed between P. spp. (a non-fecal indicator bacteria) and E. coli (r = 0.5) and TC (r = 0.5), suggesting an origin other than fecal matter. Negative and significant correlations between E. coli/ENT (r = −0.5), ENT/TC (r = −0.5), and ENT/P. spp. (r = −1) suggested that they originated from different sources that could be either a natural source or human or animal fecal matter and that they were transported by different pathways [48].
To better define the natural or non-natural origin of E. coli and ENT, the E. coli and ENT genomic profiles of general origin should be examined by PCR tests using primers and specific operating conditions [48,49,50].
4. Conclusions
This work allowed obtaining the first information on heavy metal concentrations and related ecotoxicological risks, and on the microbial contamination of pond waters and sediments from Dimba Cave. The results underpin several conclusions, as follows.
Most water samples contained very high concentrations of V, Cr, Mn, Fe, Ni, Cu, Zn, As, Cd, Pb, and Hg, all exceeding the maximum permissible values set in the WHO recommendations for waters used for human consumption. Therefore, the use of Dimba Cave water by local populations as a drinking water is a threat to public health and must be discouraged. Because of the water–food–human health nexus, the use of this water for cooking and irrigation may also add metal contaminants to the diet and, therefore, should be discouraged.
In general, all sediment samples showed higher concentrations of Cr, Cu, Zn, Cd, and Hg when compared with the sediment quality guidelines for the protection of aquatic life.
In the majority of sediment samples, the enrichment factor and the geoaccumulation index parameters indicated serious pollution by heavy metals that possibly originated from anthropogenic activities. Nevertheless, further research shall be carried out to identify the pollution sources and to clarify whether the limestone could also be a source of these metals.
The calculated ecological risk parameters, including contamination factor, ecological risk factor, contamination degree, and ecotoxicological risk index, indicated that sediments may pose a very high ecotoxicological risk to the aquatic cave biota that, in general, is very fragile.
The microbiological analyses of pond waters revealed the presence of Escherichia coli, Enterococcus, total coliforms, and Pseudomonas spp. The presence of fecal coliform bacteria indicated strong contamination by human and/or animal manure. The Spearman correlation (p < 0.05) of these microbial parameters suggested different origins and pathways of microbial contamination, which could be anthropogenic sources at the surface and exploitation of bat guano in the cave.
Globally, this investigation showed that the Dimba Cave environment that was sealed from human intrusion until the 1920s is now highly polluted by toxic heavy metals and pathogenic bacteria and, thus, the groundwater from the cave is not suitable for human consumption.
The results of this work provide a sound basis for action by decision makers to enact Dimba Cave management to improve water quality and to protect the cave environment and public health, contributing also to the preservation of this candidate to UNESCO World Heritage. The improved management of Dimba cave and groundwater protection measures could also contribute to attaining the UN Sustainable Development Goals and meeting the objectives of the 2030 Agenda for Sustainable Development [51].
Author Contributions
Conceptualization: D.M.M., E.K.A., F.P.C. and J.P.; sampling: D.M.M.; sample preparation, formal analysis, and data validation: D.M.M., E.K.A., F.P.C. and J.P.; methodology and data interpretation: D.M.M., E.K.A., F.P.C. and J.P.; writing—original draft: E.K.A.; writing, review and editing: D.M.M., A.M.N., J.M.K., S.N.L., E.K.A., F.P.C. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Maret, P.; Van Noten, F.; Cahen, D. Radiocarbon dates from West Central Africa: A synthesis. J. Afr. Hist. 1977, 4, 481–505. [Google Scholar] [CrossRef]
- Cranshof, E.; Nikis, N.; De Maret, P. Ceramics Decorated with Woven Motifs: An Archaeological Kongo Kingdom Identifier? Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Quinif, Y. 2e Semestre. Une Morphologie Karstique Typique en Zone Intertropicale: Les Karsts du Bas Zaïre. Karstologia Rev. De Karstol. Et De Spéléologie Phys. 1985, 6, 43–52. [Google Scholar] [CrossRef]
- Fourvel, J.-B.; Beaudet, A.; Zanolli, C.; Dandurand, G.; Moiana, M.; Stratford, D.; Chadelle, B.; Bruxelles, L. The HOMME project—Human Origins in Mozambique and Malawi Environments: Looking for our origin in the Mozambican karst. Karstologia Mémoires. In Proceedings of the 18th International Congress of Speleology—Savoie Mont Blanc 2022—SYMPOSIUM 04—Geomorphology and Speleogenesis, hal-03916737, Bourget-du-Lac, France, 24–31 July 2022; Volume 5, pp. 219–222. [Google Scholar]
- Çil, E.A.; Uncumusaoglu, A.A.; Ergen, S.F.; Gürbüzer, P. Evaluation of water and sediment quality of Inalti cave (Northern Turkey) by using multivariate statistical methods. Environ. Monit. Assess. 2023, 195, 667. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2009; 256p. [Google Scholar] [CrossRef]
- Rachmawati, S.; Matin, H.H.A.; Suhardono, S.; Setyono, P.; Kusumaningrum, L.; Rinawati, S.; Sholiqin, M. Biodiversity of Rambut Cave Sale Central Java. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012006. [Google Scholar] [CrossRef]
- Ribeiro, D.; Tičar, J. The problematics of cave pollution in Bela Krajina. Nat. Slov. 2017, 19, 43–45. [Google Scholar] [CrossRef]
- Prelovšek, M. Pollution and cleanup of karst caves in Slovenia. In Pressures and Protection of the Underground Karst—Cases from Slovenia and Croatia; Prelovšek, M., Zupan Hajna, N., Eds.; Karst Research Institute ZRC SAZU: Postojna, Slovenia, 2011; pp. 101–111. [Google Scholar]
- Balestra, V.; Bellopede, R. Microplastic pollution in show cave sediments: First evidence and detection technique. Environ. Pollut. 2022, 292, 118261. [Google Scholar] [CrossRef] [PubMed]
- Printarakul, N.; Meeinkuirt, W. The bryophyte community as bioindicator of heavy metals in a waterfall outflow. Sci. Rep. 2022, 12, 6942. [Google Scholar] [CrossRef]
- Ferrante, M.; Spena, M.T.; Hernout, B.V.; Grasso, A.; Messina, A.; Grasso, R.; Copat, C. Trace elements bioaccumulation in liver and fur of Myotis myotis from two caves of the eastern side of Sicily (Italy): A comparison between a control and a polluted area. Environ. Pollut. 2018, 240, 273–285. [Google Scholar] [CrossRef]
- Cuculić, V.; Cukrov, N.; Kwokal, Ž.; Mlakar, M. Distribution of trace metals in anchialine caves of Adriatic Sea, Croatia. Estuar. Coast. Shelf Sci. 2011, 95, 253–263. [Google Scholar] [CrossRef]
- Loizeau, J.-L.; Arbouille, D.; Santiago, S.; Vernet, J.-P. Evaluation of a wide range laser diffraction grain size analyser for use with sediments. Sedimentology 1994, 41, 353–361. [Google Scholar] [CrossRef]
- Rachid, N.A.; Güngör, N.D. Human activities’ impacts on cave microbial diversity: Perspectives for cave microbial diversity conservation. Int. J. Life Sci. Biotechnol. 2021, 4, 311–323. [Google Scholar] [CrossRef]
- Yong, N.; Jiang, X.; Wang, K. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci Total Environ. 2020, 700, 134509. [Google Scholar]
- Birch, G.F. A review of chemical-based sediment quality assessment methodologies for the marine environment. Mar. Pollut. Bull. 2018, 133, 218–232. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Grottes de Dimba et Ngovo. 2023. Available online: https://whc.unesco.org/en/tentativelists/961/ (accessed on 18 July 2023).
- Poté, J.; Mavingui, P.; Navarro, E.; Rosselli, W.; Wildi, W.; Simonet, P.; Vogel, T. Extracellular plant DNA in Geneva groundwater and traditional artesian drinking water fountains. Chemosphere 2009, 75, 498–504. [Google Scholar] [CrossRef]
- Atibu, E.K.; Devarajan, N.; Laffite, A.; Giuliani, G.; Salumu, J.A.; Muteb, R.C.; Mulaji, C.K.; Otamonga, J.-P.; Elongo, V.; Mpiana, P.T.; et al. Assessment of metal and rare earth elements contamination in rivers around abandoned mine areas. The case of Lubumbashi River and Tshiamilemba Canal, Katanga, Democratic Republic of the Congo. Chem. Der Erde 2016, 76, 353–362. [Google Scholar] [CrossRef]
- Atibu, E.K.; Oliveira, J.M.; Malta, M.; Santos, M.; Mulaji, C.K.; Mpiana, P.T.; Carvalho, F.P. Assessment of natural radioactivity in the copper belt region, Kolwezi Province, of the Democratic Republic of the Congo. J. Geosc. Environ. Protect. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Thevenon, F.; Adatte, T.; Wildi, W.; Poté, J. A high-resolution historical sediment record of nutrients, trace elements and organochlorines (DDT and PCB) deposition in a drinking water reservoir (Lake Brêt, Switzerland) points at local and regional pollutant sources. Chemosphere 2013, 90, 2444–2452. [Google Scholar] [CrossRef]
- Bravo, A.G.; Bouchet, S.; Amouroux, D.; Pote, J.; Dominik, J. Distribution of Mercury and Organic Matter in Particle-Size Classes in Sediments Contaminated by a Waste Water Treatment Plant: Vidy Bay, Lake Geneva, Switzerland. J. Environ. Monitor. 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Gallorini, A.; Loizeau, J.-L. Lake snow as a mercury methylation micro-environment in the oxic water column of a deep peri-alpine lake. Chemosphere 2022, 299, 134306. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry; EPA-821-R-02-019; U.S.E.P. Agency: Washington, DC, USA, 2002; p. 45.
- Maanan, M.; Zourarah, B.; Carruesco, C.; Aajjane, A.; Naud, J. The distribution of heavy metals in the Sidi Moussa lagoon sediments (Atlantic Moroccan Coast). J. Afr. Earth Sci. 2004, 39, 473–483. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 2000GC000109. [Google Scholar] [CrossRef]
- Rubio, B.; Nombela, M.A.; Vilas, F. Geochemistry of Major and Trace Elements in Sediments of the Ría de Vigo (NW Spain): An Assessment of Metal Pollution. Mar. Pollut. Bull. 2000, 40, 968–980. [Google Scholar] [CrossRef]
- Förstner, U.; Ahlf, W.; Calmano, W. Studies on the Transfer of Heavy Metals between Sedimentary Phases with a Multi-Chamber Device: Combined Effects of Salinity and Redox Variation. Mar. Chem. 1989, 28, 145–158. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control: A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Suresh, G.; Sutharsan, P.; Ramasamy, V.; Venkatachalapath, R. Assessment of Spatial Distribution and Potential Ecological Risk of the Heavy Metals in Relation to Granulometric Contents of Veeranamlake Sediments India. Ecotoxicol. Environ. Saf. 2012, 84, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Han, S.S.; Ahmed, M.K.; Masunaga, S. Assessment of Trace Metal Contamination in Water and Sediment of Some Rivers in Bangladesh. J. Water Environ. Technol. 2014, 12, 109–121. [Google Scholar] [CrossRef]
- Singh, M.; Müller, G.; Singh, I.B. Heavy Metals in freshly deposited stream sediments of rivers associated with urbanization of the Ganga Plain, India. Water Air Soil Pollut. 2002, 141, 35–54. [Google Scholar] [CrossRef]
- CCME Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. EPC 98-E, Canadian Environmental Quality Guidelines. Canadian Council of Ministers of the Environment. 1999. Available online: http://www.ccme.ca/ (accessed on 3 June 2024).
- APHA (American Public Health Association); American Water Works Association & Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005; p. 1368. [Google Scholar]
- Devarajan, N.; Köhler, T.; Sivalingam, P.; van Delden, C.; Mulaji, C.K.; Mpiana, P.T.; Ibelings, B.W.; Poté, J. Antibiotic resistant Pseudomonas spp. in the aquatic environment: A prevalence study under tropical and temperate climate conditions. Water Res. 2017, 115, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft 2020, XLSTAT Statistical and Data Analysis Solution. New York, NY, USA. 2022. Available online: https://www.xlstat.com (accessed on 1 June 2024).
- WHO (World Health Organisation). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017; p. 2017. [Google Scholar]
- Blott, S.J.; Pye, K. Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- WHO (World Health Organisation). A Global Overview of National Regulations and Standards for Drinking-Water Quality; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- EU Directive 2020. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020: L 435/1. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 3 June 2024).
- Thevenon, F.; Guédron, S.; Chiaradia, M.; Loizeau, J.-L.; Pote, J. (Pre-) historic changes in natural and anthropogenic heavy metals deposition inferred from two contrasting Swiss Alpine lakes. Quat. Sci. Rev. 2011, 30, 224–233. [Google Scholar] [CrossRef]
- Adamo, P.; Arienzo, M.; Imperato, M.; Naimo, D.; Nardi, G.; Stanzione, D. Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere 2005, 61, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Wildi, W.; Dominik, J.; Loizeau, J.-L.; Thomas, R.L.; Favarger, P.-Y.; Haller, L.; Perroud, A.; Peytremann, C. River, reservoir and lake sediment contamination by heavy metals downstream from urban areas of Switzerland. Lakes Reservoirs: Res. Manag. 2004, 9, 75–87. [Google Scholar] [CrossRef]
- Haller, L.; Poté, J.; Loizeau, J.-L.; Wildi, W. Distribution and survival of faecal indicator bacteria in the sediments of the Bay of Vidy, Lake Geneva, Switzerland. Ecol. Ind. 2009, 9, 540–547. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). Improved Enumeration Methods for the Recreational Water Quality Indicators: Enterococci and Escherichia coli EPA-821/R-97/004; U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- EU 2016. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, L 064, 37–51. [Google Scholar]
- Tshibanda, J.B.; Devarajan, N.; Birane, N.; Mwanamoki, P.M.; Atibu, E.K.; Mpiana, P.T.; Prabakar, K.; Mubedi, J.J.; Wildi, W.; Poté, J. Microbiological and physicochemical characterization of water and sediment of an urban river: N’Djili River, Kinshasa, Democratic Republic of Congo. Sustain. Water Qual. Ecol. 2014, 3–4, 47–54. [Google Scholar] [CrossRef]
- Thevenon, F.; Regier, N.; Benagli, C.; Tonolla, M.; Adatte, T.; Wildi, W.; Poté, J. Characterization of faecal indicator bacteria in sediments cores from the largest freshwater lake of Western Europe (Lake Geneva, Switzerland). Ecotox. Environ. Safe. 2012, 78, 50–56. [Google Scholar] [CrossRef]
- Morrison, C.; Bachoon, D.; Gates, K. Quantification of enterococci and bifidobacteria in Georgia estuaries using conventional and molecular methods. Water Res. 2008, 42, 4001–4009. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development. Resolution Adopted by the United Nations General Assembly on 25 September 2015. Available online: https://documents.un.org/doc/undoc/gen/n15/291/89/pdf/n1529189.pdf?token=iynaYynoqfCaFd4jTr&fe=true (accessed on 18 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).