Variation in Psychiatric Hospitalisations: A Multiple-Membership Multiple-Classification Analysis
Abstract
:1. Introduction
Andersen Behavioural Model of Utilisation
2. Methods
2.1. Setting
2.2. Study Population and Sampling
2.3. Data and Variables
2.3.1. Data
2.3.2. Outcome: Hospitalisations
2.3.3. Characteristics: Using the Andersen Behavioural Model of Utilisation
2.3.4. Individual-Level Characteristics
2.3.5. HCP-Level Characteristics
2.3.6. HCPM-Level Characteristics
2.4. Analytical Framework
2.4.1. Zero Inflation
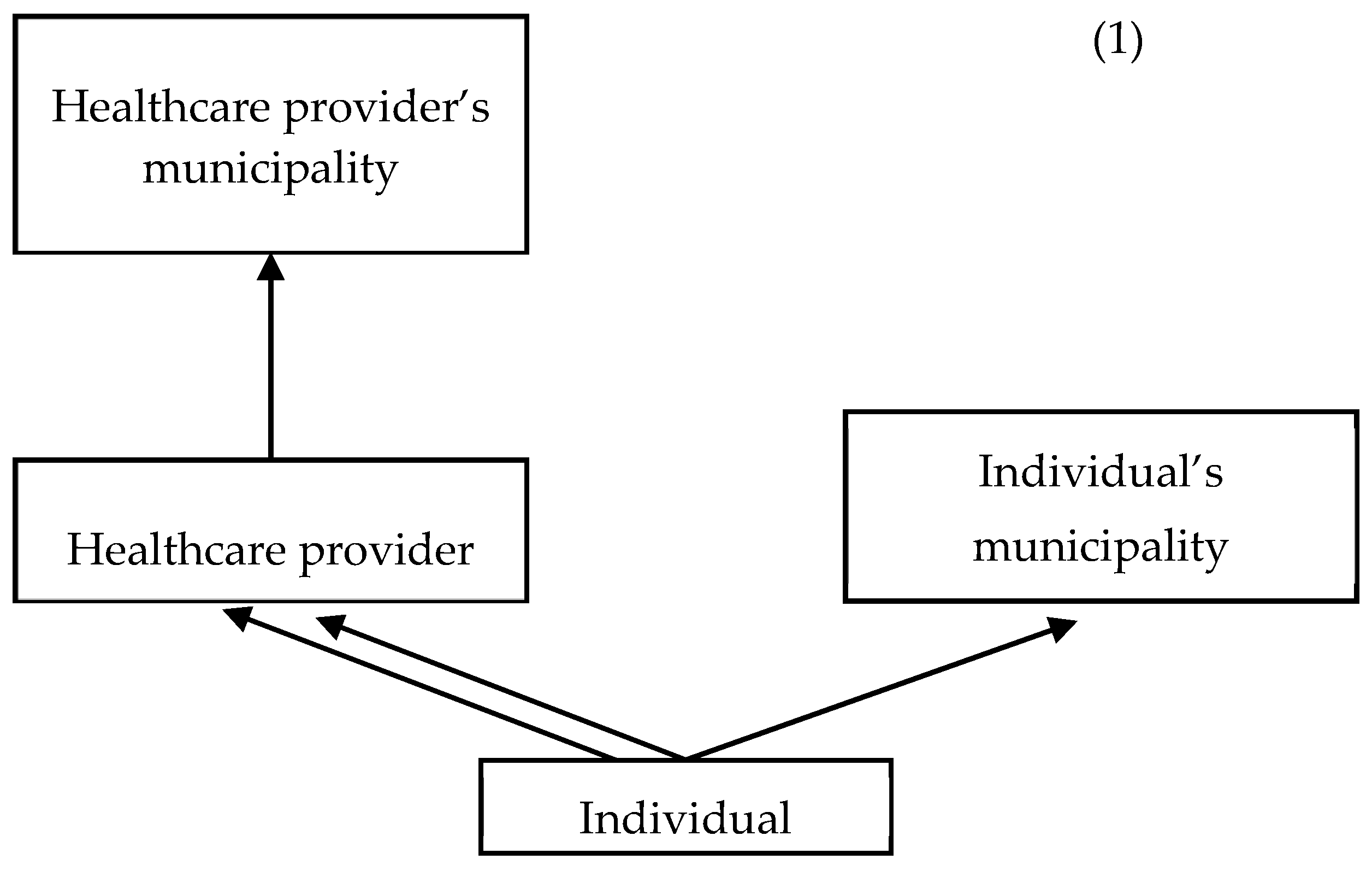
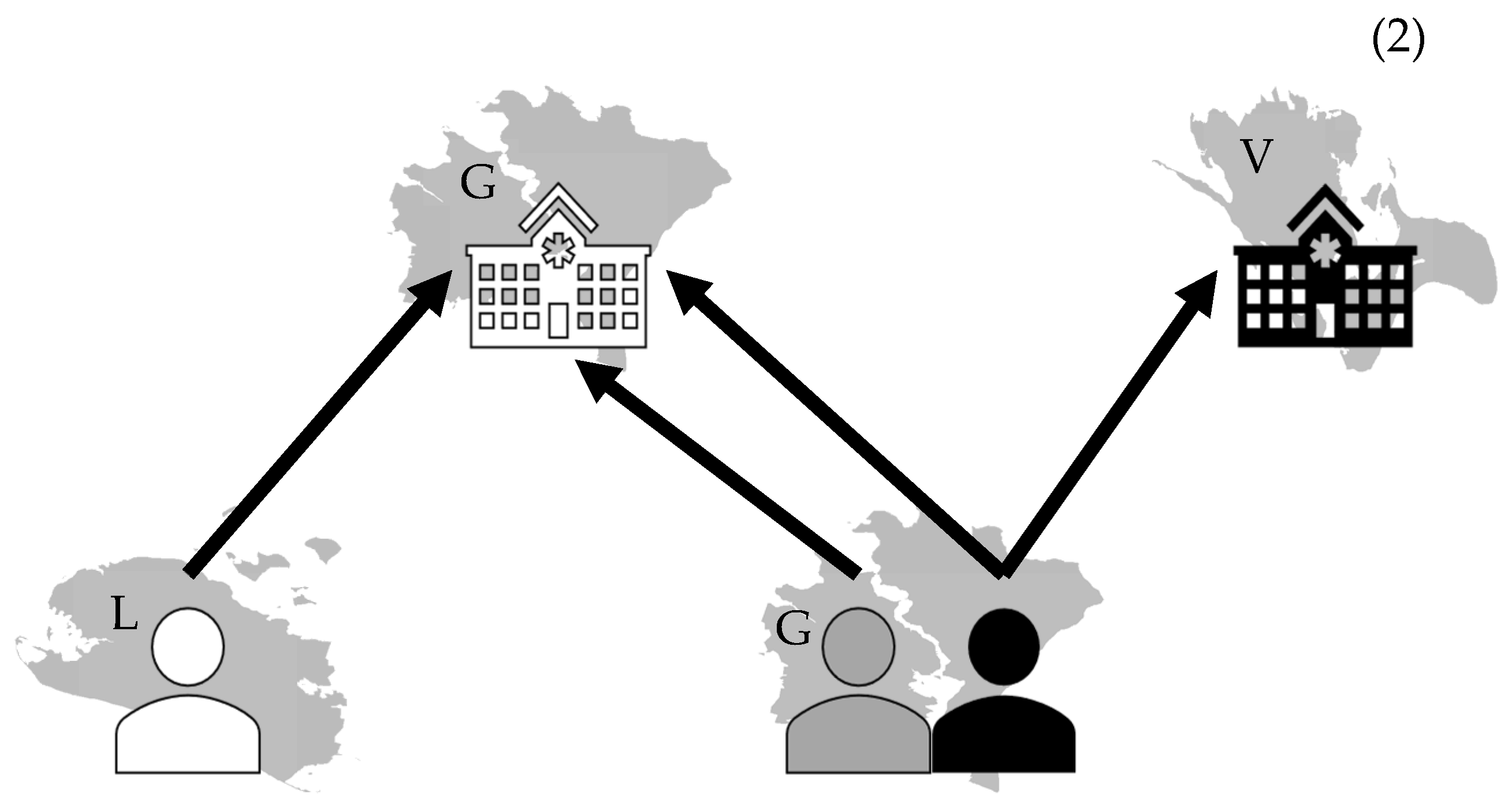
2.4.2. Clustered Structure
2.4.3. Model
2.5. Robustness Check and Subgroup Analyses
2.6. Ethics
3. Results
3.1. Participants
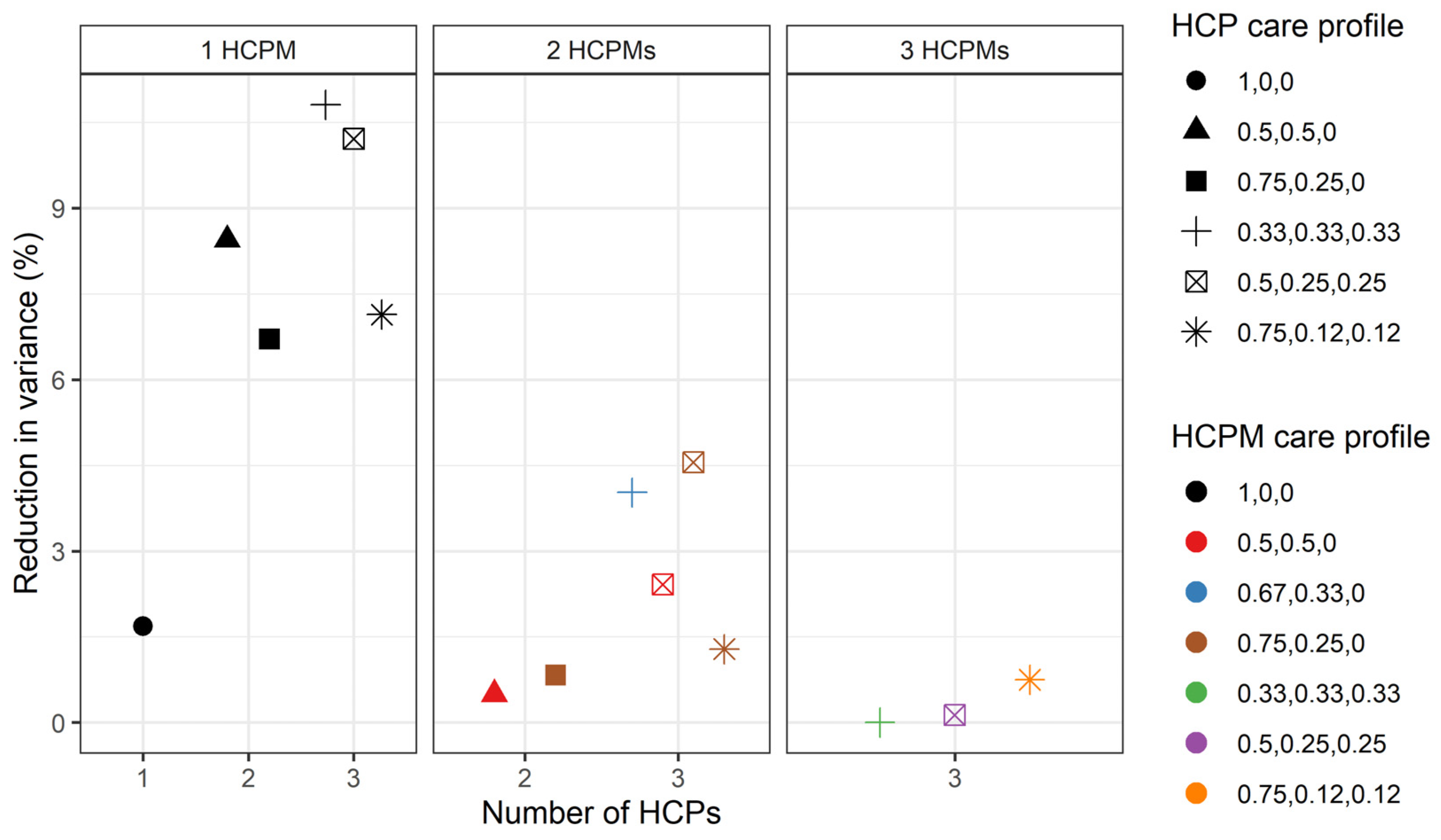
3.2. HCP Utilisation Pattern
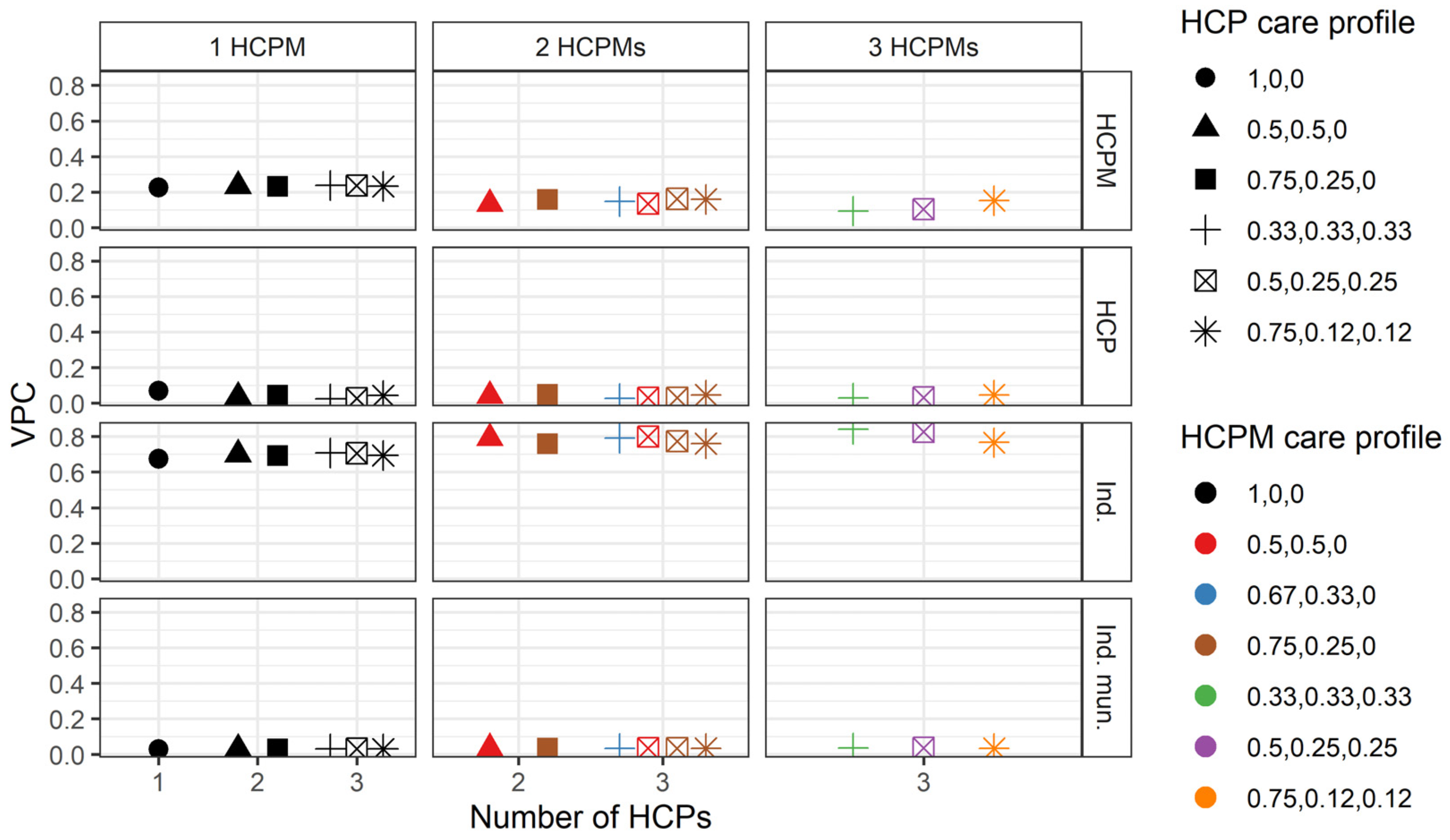
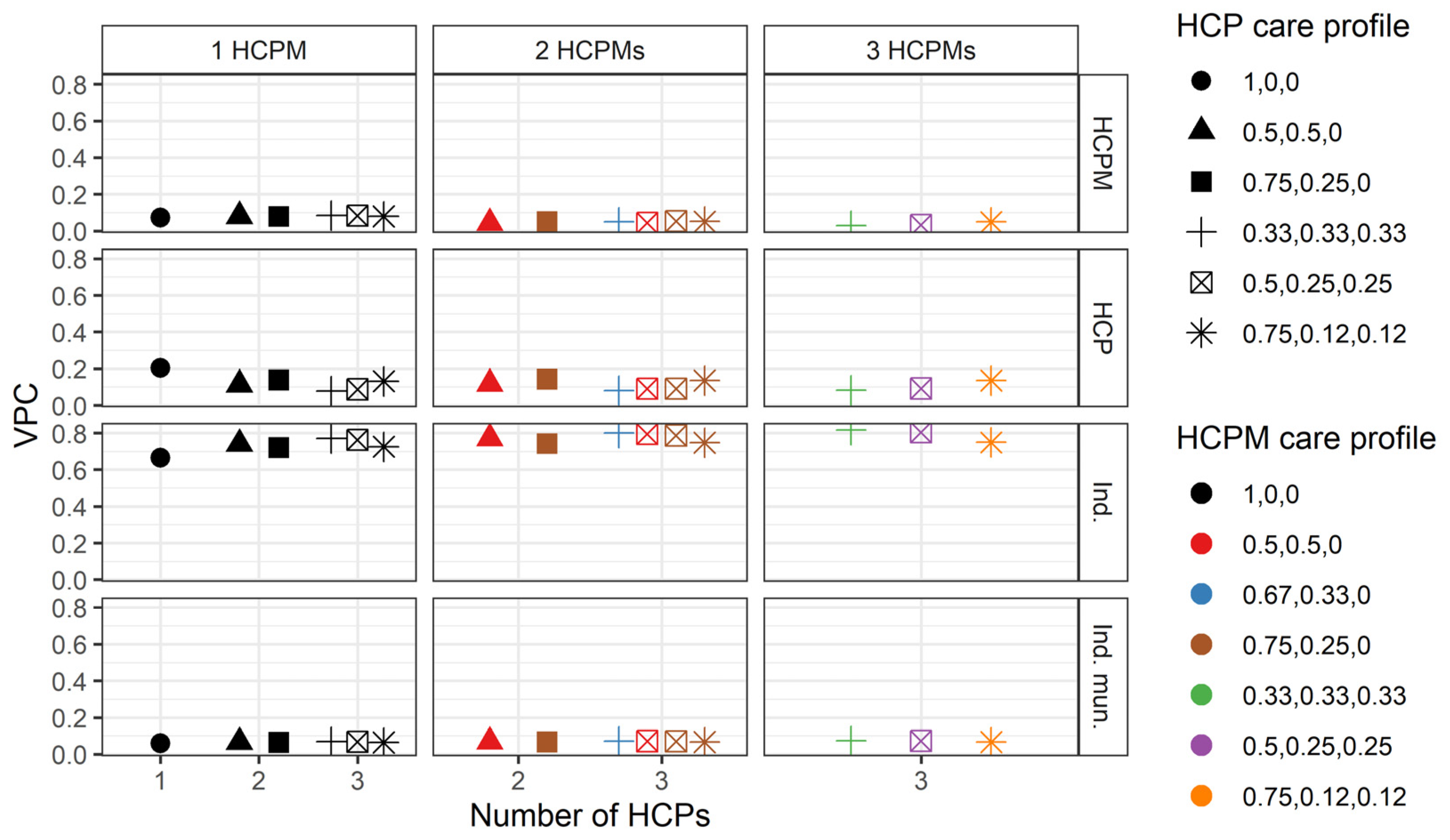
3.3. Variation in the Number of Hospitalisations
3.4. Robustness Checks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| HCP | healthcare provider |
| HCPM | healthcare provider municipality |
| MMMC | multiple-membership, multiple-classification |
| GP | general practitioner |
| OECD | Organisation for Economic Co-operation and Development |
| VCM | variance component model |
| Coef. | coefficient |
| Var. | variance |
| SE | standard error |
References
- Wennberg, J.; McPherson, K.; Goodman, D. Small Area Analysis and the Challenge of Practice Variation. In Medical Practice Variations; Johnson, A., Stukel, T., Eds.; Springer: Boston, MA, USA, 2016; pp. 1–25. [Google Scholar]
- OECD. Mental Health and Work: Denmark; OECD: Paris, France, 2013. [Google Scholar]
- Grytten, J.; Sørensen, R. Practice variation and physician-specific effects. J. Health Econ. 2003, 22, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, J.E. Unwarranted variations in healthcare delivery: Implications for academic medical centres. BMJ 2002, 325, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.; Gafni, A. Medical practice variations: What the literature tells us (or does not) about what are warranted and unwarranted variations. J. Eval. Clin. Pract. 2011, 17, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Granados, N.; Georgiades, K.; Boyle, M.H. Regional and individual influences on use of mental health services in Canada. Can. J. Psychiatry 2010, 55, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gandre, C.; Gervaix, J.; Thillard, J.; Mace, J.-M.; Roelandt, J.-L.; Chevreul, K. Thirty-day readmission rates and associated factors: A multilevel analysis of practice variations in French public psychiatry. J. Ment. Health Policy Econ. 2018, 21, 17–28. [Google Scholar] [PubMed]
- Gifford, E.; Foster, E.M. Provider-level effects on psychiatric inpatient length of stay for youth with mental health and substance abuse disorders. Med. Care 2008, 46, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Sakata, N.; Tachimori, H.; Takeshima, T. Geographical Variation in Psychiatric Admissions Among Recipients of Public Assistance. J. Epidemiol. 2019, 29, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Weich, S.; McBride, O.; Twigg, L.; Duncan, C.; Keown, P.; Crepaz-Keay, D.; Bhui, K. Variation in compulsory psychiatric inpatient admission in England: A cross-classified, multilevel analysis. Lancet Psychiatry 2017, 4, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Crossley, N.; Sweeney, B. Patient and service-level factors affecting length of inpatient stay in an acute mental health service: A retrospective case cohort study. BMC Psychiatry 2020, 20, 438. [Google Scholar] [CrossRef]
- Jacobs, R.; Gutacker, N.; Mason, A.; Goddard, M.; Gravelle, H.; Kendrick, T.; Gilbody, S. Determinants of hospital length of stay for people with serious mental illness in England and implications for payment systems: A regression analysis. BMC Health Serv. Res. 2015, 15, 439. [Google Scholar] [CrossRef]
- Lee, S.; Rothbard, A.B.; Noll, E.L. Length of inpatient stay of persons with serious mental illness: Effects of hospital and regional characteristics. Psychiatr. Serv. 2012, 63, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Karolaakso, T.; Autio, R.; Näppilä, T.; Leppänen, H.; Rissanen, P.; Tuomisto, M.T.; Karvonen, S.; Pirkola, S. Contextual and mental health service factors in mental disorder-based disability pensioning in Finland—A regional comparison. BMC Health Serv. Res. 2021, 21, 1081. [Google Scholar] [CrossRef] [PubMed]
- Hofstad, T.; Rugkåsa, J.; Ose, S.O.; Nyttingnes, O.; Husum, T.L. Measuring the level of compulsory hospitalisation in mental health care: The performance of different measures across areas and over time. Int. J. Methods Psychiatr. Res. 2021, 30, e1881. [Google Scholar] [CrossRef] [PubMed]
- Ahammer, A.; Schober, T. Exploring variations in health-care expenditures—What is the role of practice styles? Health Econ. 2020, 29, 683–699. [Google Scholar] [CrossRef]
- Chen, B.; Lawson, K.A.; Finelli, A.; Saarela, O. Causal mediation analysis decomposition of between-hospital variance. Health Serv. Outcomes Res. Methodol. 2022, 22, 118–144. [Google Scholar] [CrossRef]
- Ginja, R.; Riise, J.; Willage, B.; Willén, A. Does Your Doctor Matter? Doctor Quality and Patient Outcomes; CESifo: Munich, Germany, 2022. [Google Scholar]
- Andersen, R.; Newman, J.F. Societal and individual determinants of medical care utilization in the United States. Milbank Meml. Fund. Q. Health Soc. 1973, 51, 95–124. [Google Scholar] [CrossRef]
- Walker, S.; Mackay, E.; Barnett, P.; Rains, L.S.; Leverton, M.; Dalton-Locke, C.; Johnson, S. Clinical and social factors associated with increased risk for involuntary psychiatric hospitalisation: A systematic review, meta-analysis, and narrative synthesis. Lancet Psychiatry 2019, 6, 1039–1053. [Google Scholar] [CrossRef]
- Landersø, R.A.-O.; Fallesen, P.A.-O. Psychiatric hospital admission and later crime, mental health, and labor market outcomes. Health Econ. 2021, 30, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Mackay, E.; Barnett, P.; Rains, L.S.; Leverton, M.; Dalton-Locke, C.; Johnson, S. Application and Use of Andersen’s Behavioral Model as Theoretical Framework: A Systematic Literature Review from 2012–2021. Iran. J. Public Health 2023, 52, 1346. [Google Scholar]
- Zhao, Y.; Hoenig, J.M.; Protacio, A.; Lim, S.; Norman, C.C. Identification of risk factors for early psychiatric rehospitalization. Psychiatry Res. 2020, 285, 112803. [Google Scholar] [CrossRef]
- Browne, W.J.; Goldstein, H.; Rasbash, J. Multiple membership multiple classification (MMMC) models. Stat. Model. 2001, 1, 103–124. [Google Scholar] [CrossRef]
- Phillips, K.A.; Morrison, K.R.; Andersen, R.; Aday, L.A. Understanding the context of healthcare utilization: Assessing environmental and provider-related variables in the behavioral model of utilization. Health Serv. Res. 1998, 33, 571–596. [Google Scholar]
- Vrangbæk, K. Structural Reform in Denmark, 2007–2009: Central Reform Processes in a Decentralised Environment. Local Gov. Stud. 2010, 36, 205–221. [Google Scholar] [CrossRef]
- Christiansen, T. Ten years of structural reforms in Danish healthcare. Health Policy 2012, 106, 114–119. [Google Scholar] [CrossRef]
- The Danish Parliament. Danish Health Care Act, WHO, Editor. 2010. Available online: https://extranet.who.int/mindbank/item/1194 (accessed on 22 June 2024).
- Brewin, C.R.; Thornicroft, G.J.; Wing, J.K. Measuring Mental Health Needs, 2nd ed.; Gaskell: London, UK, 1993. [Google Scholar]
- Dickerson, F.B.; Lehman, A.F. Evidence-based psychotherapy for schizophrenia: 2011 update. J. Nerv. Ment. Dis. 2011, 199, 520–526. [Google Scholar] [CrossRef]
- Anckarsäter, H.; Radovic, S.; Svennerlind, C.; Höglund, P.; Radovic, F. Mental disorder is a cause of crime: The cornerstone of forensic psychiatry. Int. J. Law Psychiatry 2009, 32, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Blæhr, E.E.; Madsen, J.V.; Christiansen, N.L.S.; Ankersen, P.V. The influence of crisis resolution treatment on employment: A retrospective register-based comparative study. Nord. J. Psychiatry 2017, 71, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Statistics Denmark. Population Register. 2023. Available online: https://www.dst.dk/extranet/ForskningVariabellister/BEF%20-%20Befolkningen.html (accessed on 22 June 2024).
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Statistics Denmark. The Disability Register. 2023. Available online: https://www.dst.dk/extranet/ForskningVariabellister/HANDIC%20-%20Handicapydelser.html (accessed on 22 June 2024).
- Andersen, J.S.; Nde, F.O.; Krasnik, A. The Danish National Health Service Register. Scand. J. Public Health 2011, 39 (Suppl. 7), 34–37. [Google Scholar] [CrossRef]
- Baadsgaard, M.; Quitzau, J. Danish registers on personal income and transfer payments. Scand. J. Public Health 2011, 39 (Suppl. 7), 103–105. [Google Scholar] [CrossRef]
- Statistics Denmark. Reported Criminal Offences. 2023. Available online: https://www.dst.dk/en/Statistik/dokumentation/documentationofstatistics/reported-criminal-offences (accessed on 22 June 2024).
- Petersson, F.; Baadsgaard, M.; Thygesen, L.C. Danish registers on personal labour market affiliation. Scand. J. Public Health 2011, 39 (Suppl. 7), 95–98. [Google Scholar] [CrossRef]
- Jensen, V.M.; Rasmussen, A.W. Danish Education Registers. Scand. J. Public Health 2011, 39 (Suppl. 7), 91–94. [Google Scholar] [CrossRef] [PubMed]
- Deza, M.; Maclean, J.C.; Solomon, K. Local access to mental healthcare and crime. J. Urban Econ. 2022, 129, 103410. [Google Scholar] [CrossRef]
- Plana-Ripoll, O.; Pedersen, C.B.; Holtz, Y.; Benros, M.E.; Dalsgaard, S.; de Jonge, P.; Fan, C.C.; Degenhardt, L.; Ganna, A.; Greve, A.N.; et al. Exploring Comorbidity Within Mental Disorders Among a Danish National Population. JAMA Psychiatry 2019, 76, 259–270. [Google Scholar] [CrossRef]
- Gandré, C.; Gervaix, J.; Thillard, J.; Macé, J.M.; Roelandt, J.L.; Chevreul, K. Geographic variations in involuntary care and associations with the supply of health and social care: Results from a nationwide study. BMC Health Serv. Res. 2018, 18, 253. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.K.; Perez-Vicente, R.; Leckie, G.; Johnsen, S.P.; Merlo, J. Disentangling the contribution of hospitals and municipalities for understanding patient level differences in one-year mortality risk after hip-fracture: A cross-classified multilevel analysis in Sweden. PLoS ONE 2020, 15, e0234041. [Google Scholar] [CrossRef] [PubMed]
- Næss, Ø.; Leyland, A.H. Analysing the effect of area of residence over the life course in multilevel epidemiology. Scand. J. Public Health 2010, 38 (Suppl. 5), 119–126. [Google Scholar] [CrossRef]
- Stendorff Nielsen, K. plotDK: Plot Summary Statistics as Choropleth Maps of Danish Administrative Areas. 2021. Available online: https://CRAN.R-project.org/package=plotDK (accessed on 22 June 2024).
- Vrangbaek, K. The Danish health care system. Int. Profiles Health Care Syst. 2020, 47. Available online: https://www.commonwealthfund.org/international-health-policy-center/countries/denmark (accessed on 22 June 2024).
- Mullahy, J. Specification and testing of some modified count data models. J. Econ. 1986, 33, 341–365. [Google Scholar] [CrossRef]
- Farbmacher, H. Estimation of Hurdle Models for Overdispersed Count Data. Stata J. 2011, 11, 82–94. [Google Scholar] [CrossRef]
- Hall, D.B. Zero-Inflated Poisson and Binomial Regression with Random Effects: A Case Study. Biometrics 2004, 56, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D. Zero-Inflated Poisson Regression, with an Application to Defects in Manufacturing. Technometrics 1992, 34, 1–14. [Google Scholar] [CrossRef]
- Greene, W.H. Accounting for Excess Zeros and Sample Selection in Poisson and Negative Binomial Regression Models; NYU Working Paper, No. EC-94-10.1994. Available online: https://ssrn.com/abstract=1293115 (accessed on 22 June 2024).
- Feng, C.X. A comparison of zero-inflated and hurdle models for modeling zero-inflated count data. J. Stat. Distrib. Appl. 2021, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Skrondal, A.; Rabe-Hesketh, S. Multilevel modelling. In Sage Benchmarks in Social Research Methods; SAGE: London, UK, 2010. [Google Scholar]
- Cutler, D.; Skinner, J.S.; Stern, A.D.; Wennberg, D. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. American economic journal. Econ. Policy 2019, 11, 192–221. [Google Scholar]
- Aakvik, A.; Holmås, T.H.; Islam, M.K. Does variation in general practitioner (GP) practice matter for the length of sick leave? A multilevel analysis based on Norwegian GP-patient data. Soc. Sci. Med. 2010, 70, 1590–1598. [Google Scholar] [CrossRef]
- Peri, G. Young People, Skills and Cities. SSRN 292246. 2001. Available online: https://www.cesifo.org/en/publications/2001/working-paper/young-people-skills-and-cities (accessed on 22 June 2024).
- Kristensen, T.; Olsen, K.R.; Schroll, H.; Thomsen, J.L.; Halling, A. Association between fee-for-service expenditures and morbidity burden in primary care. Eur. J. Health Econ. 2014, 15, 599–610. [Google Scholar] [CrossRef]
- Steele, F.; Vignoles, A.; Jenkins, A. The Effect of School Resources on Pupil Attainment: A Multilevel Simultaneous Equation Modelling Approach. J. R. Stat. Soc. Ser. A Stat. Soc. 2007, 170, 801–824. [Google Scholar] [CrossRef]

| Level | N | |
|---|---|---|
| 1 | Individuals with psychiatric diagnoses | 64,694 |
| 2 | Healthcare providers | 2287 |
| General practice | 2100 | |
| Community-based care | 145 | |
| Hospital department-based care | 42 | |
| 3 | Individuals’ municipalities (area of residence) | 98 |
| 4 | Healthcare providers’ municipalities (local areas) | 98 |
| n (%) | |||
|---|---|---|---|
| Individuals n = 64,694 | Male | 29,396 | (45.44) |
| Age, mean (SD) | 36.186 | (13.188) | |
| Danish citizen | 58,624 | (90.62) | |
| Married | 12,637 | (19.53) | |
| Living alone | 28,923 | (44.71) | |
| Labour market attachment | 43,293 | (66.92) | |
| Disability pension | 12,813 | (19.81) | |
| Income, mean (SD) | 149,271 | (435,197) | |
| Sentenced to prison | 1584 | (2.45) | |
| Sentenced to a fine | 2734 | (4.23) | |
| Diagnoses | |||
| Organic disorders | 1393 | (2.15) | |
| Substance abuse | 6679 | (10.32) | |
| Schizophrenia and psychoses | 12,698 | (19.63) | |
| Mood disorders | 14,971 | (23.14) | |
| Neurotic disorders | 23,827 | (36.83) | |
| Eating disorders | 1107 | (1.71) | |
| Personality disorders | 7848 | (12.13) | |
| Intellectual disabilities | 1351 | (2.09) | |
| Developmental disorders | 1404 | (2.17) | |
| Behavioural disorder | 5155 | (7.97) | |
| Suffered from disease in past 5 years | 10,056 | (15.54) | |
| Psychiatric comorbidity, mean (SD) | 0.519 | (0.841) | |
| Somatic comorbidity, mean (SD) | 1.493 | (2.396) | |
| Depressive disorders | 13,273 | (20.52) | |
| Suicide attempt | 1703 | (2.63) | |
| Days per hospitalisation, mean (SD) | 8.617 | (16.582) | |
| Forensic psychiatry | 1729 | (2.67) | |
| Psychotherapy | 14,965 | (23.13) | |
| Psychiatric hospitalisation | 19,592 | (30.23) | |
| Number of hospitalisations, mean (SD) | 5.764 | (9.405) | |
| Std. number of hospitalisations, mean (SD) | 0.000 | (1.000) | |
| Individuals treated for the first time in 2016 | 3507 | (5.42) | |
| Healthcare providers n = 2287 | Regions | ||
| Capital | 797 | (34.85) | |
| Zealandic | 300 | (13.12) | |
| Southern | 416 | (18.19) | |
| Central | 576 | (25.19) | |
| Northern | 198 | (8.66) | |
| Hospital departments | 42 | ||
| Inpatient | 5 | (11.90) | |
| Outpatient | 37 | (88.10) | |
| Hospital characteristics | |||
| Teaching hospital dep. | 11 | (26.19) | |
| Bed capacity, mean (SD) | 30.294 | (28.855) | |
| General practitioners | 2100 | ||
| GP characteristics | |||
| Patients per GP, mean (SD) | 40.322 | (94.691) | |
| Consultations, mean (SD) | 338.759 | (539.969) | |
| Talk therapy, mean (SD) | 358.970 | (592.511) | |
| Phone/mail consultations, mean (SD) | 20.753 | (43.918) | |
| Community-based care departments | |||
| Outreaching teams | <5 | (<4.00) | |
| Private psychiatrists | 139 | (95.86) | |
| Community | <5 | (<4.00) | |
| Community-based care characteristics | |||
| Episodes per year, mean (SD) | 2554.433 | (6988.498) | |
| Local area (municipalities) n = 98 | Assisted living | 88 | (89.80) |
| Personal and practical support | 78 | (79.59) | |
| Therapy by psychologist | 21 | (21.43) | |
| Population density, mean (SD) | 36,671 | (50,033) | |
| Nationality density, mean (SD) | |||
| Danish | 31,309 | (38,580) | |
| Total Number of Different Health Care Providers for Each Individual | Full Population N = 64,694 | % | Population Excl. Organic, Intelligence and Developmental Disorders N = 60,603 | % | Population only incl. Schizophrenia and Mood Disorders N = 27,104 | % |
|---|---|---|---|---|---|---|
| 1 | 6486 | (10.03) | 6141 | (10.13) | 2659 | (9.81) |
| 2 | 27,583 | (42.64) | 25,806 | (42.58) | 11,000 | (40.58) |
| 3 | 17,214 | (26.61) | 16,214 | (26.75) | 7420 | (27.38) |
| 4 | 7709 | (11.92) | 7194 | (11.87) | 3396 | (12.53) |
| 5 | 3172 | (4.90) | 2953 | (4.87) | 1443 | (5.32) |
| 6 | 1247 | (1.93) | 1143 | (1.89) | 610 | (2.25) |
| 7 | 623 | (0.96) | 562 | (0.93) | 288 | (1.06) |
| 8 | 276 | (0.43) | 252 | (0.42) | 130 | (0.48) |
| 9 | 162 | (0.25) | 139 | (0.23) | 70 | (0.26) |
| 10 | 98 | (0.15) | 93 | (0.15) | 43 | (0.16) |
| 11 | 55 | (0.09) | 44 | (0.07) | <20 | (<0.07) |
| 12 | 44 | (0.07) | 40 | (0.07) | <20 | (<0.07) |
| 13 | 25 | (0.04) | 22 | (0.04) | <20 | (<0.07) |
| Total Number of Different Health Care Provider Municipalities for each Individual | Full Population N = 64,694 | % | Population Excl. Organic, Intelligence and Developmental Disorders N = 60,603 | % | Population only incl. Schizophrenia and Mood Disorders N = 27,104 | % |
| 1 | 26,347 | (40.73) | 24,926 | (41.13) | 11,582 | (42.73) |
| 2 | 26,800 | (41.43) | 24,950 | (41.17) | 10,501 | (38.74) |
| 3 | 8664 | (13.39) | 8089 | (13.35) | 3687 | (13.60) |
| 4 | 2219 | (3.43) | 2036 | (3.36) | 1019 | (3.76) |
| 5 | 527 | (0.81) | 477 | (0.79) | 253 | (0.93) |
| 6 | 113 | (0.17) | 105 | (0.17) | 49 | (0.18) |
| 7 | 24 | (0.04) | 20 | (0.03) | <20 | (<0.07) |
| Dependent Variable: Indicator of Hospitalisation or Not | ||||||||
|---|---|---|---|---|---|---|---|---|
| (1) VC model | (2) All characteristics | |||||||
| Coef. | SE | Coef. | SE | p-val. | ||||
| Male | 0.113 ** | 0.012 | 0.000 | |||||
| Age | 0.010 ** | 0.000 | 0.000 | |||||
| Danish citizen | 0.110 ** | 0.020 | 0.000 | |||||
| Diagnosis group | Organic disorders | 0.411 ** | 0.038 | 0.000 | ||||
| Substance abuse | 0.937 ** | 0.020 | 0.000 | |||||
| Mood disorders | 0.273 ** | 0.015 | 0.000 | |||||
| Neurotic disorders | 0.074 ** | 0.014 | 0.000 | |||||
| Eating disorders | 0.293 ** | 0.053 | 0.000 | |||||
| Personality disorders | 0.008 | 0.019 | 0.667 | |||||
| Intellectual disabilities | −0.059 | 0.042 | 0.156 | |||||
| Behavioural disorder | −0.161 ** | 0.024 | 0.000 | |||||
| Developmental disorders | 0.030 | 0.041 | 0.467 | |||||
| Suffered from disease in past 5 years | 0.541 ** | 0.016 | 0.000 | |||||
| Regions | Suicide attempt | 1.070 ** | 0.036 | 0.000 | ||||
| Capital | 5.005 | 163.997 | 0.976 | |||||
| Zealandic | 5.828 | 163.997 | 0.972 | |||||
| Southern | 5.273 | 163.997 | 0.974 | |||||
| Central | 5.382 | 163.997 | 0.974 | |||||
| Northern | 5.256 | 163.997 | 0.974 | |||||
| HCP Characteristics | Teaching hospital | 0.593 ** | 0.038 | 0.000 | ||||
| Outpatient | −1.488 ** | 0.022 | 0.000 | |||||
| GP | −0.569 ** | 0.028 | 0.000 | |||||
| Private psychiatrist | −0.689 ** | 0.081 | 0.000 | |||||
| Outreaching teams | −0.385 ** | 0.041 | 0.000 | |||||
| Bed capacity | 0.021 ** | 0.000 | 0.000 | |||||
| Patients per GP | 0.000 | 0.000 | 0.559 | |||||
| Var. | SE | Var. | SE | |||||
| Levels | Individual’s municipality | 0.022 | 0.004 | 0.031 | 0.006 | |||
| Observations | 64,694 | 64,694 | ||||||
| Dependent Variable: Log-Transformed number of psychiatric hospitalisations in 2016 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) VC Model | (2) Individual Characteristics | (3) HCP Characteristics | (4) Municipality Characteristics | (5) All Characteristics | |||||||||||
| Coef. | SE | Coef. | SE | p-val. | Coef. | SE | p-val. | Coef. | SE | p-val. | Coef. | SE | p-val. | ||
| Individual | Male | −0.021 ** | 0.005 | 0.000 | −0.079 ** | 0.016 | 0.000 | ||||||||
| Age | −0.001 ** | 0.000 | 0.008 | 0.001 ** | 0.000 | 0.001 | |||||||||
| Danish citizen | 0.005 | 0.015 | 0.767 | 0.032 | 0.023 | 0.164 | |||||||||
| Married | −0.020 | 0.013 | 0.116 | −0.018 | 0.010 | 0.080 | |||||||||
| Living alone | 0.039 ** | 0.012 | 0.001 | 0.033 * | 0.014 | 0.016 | |||||||||
| Labour market attachment | −0.021 | 0.022 | 0.354 | 0.011 | 0.006 | 0.079 | |||||||||
| Disability pension | 0.219 ** | 0.032 | 0.000 | 0.226 ** | 0.006 | 0.000 | |||||||||
| Income | 0.000 | 0.000 | 0.873 | 0.000 ** | 0.000 | 0.000 | |||||||||
| Sentenced to prison | −0.007 | 0.044 | 0.880 | 0.007 | 0.022 | 0.761 | |||||||||
| Sentenced to a fine | −0.100 ** | 0.006 | 0.000 | −0.075 ** | 0.014 | 0.000 | |||||||||
| Diagnoses | |||||||||||||||
| Organic disorders | −0.176 ** | 0.008 | 0.000 | −0.121 ** | 0.027 | 0.000 | |||||||||
| Substance abuse | −0.220 ** | 0.011 | 0.000 | −0.111 ** | 0.026 | 0.000 | |||||||||
| Schizophrenia and psychoses | Ref. | Ref. | |||||||||||||
| Mood disorders | 0.108 ** | 0.010 | 0.000 | 0.016 | 0.016 | 0.313 | |||||||||
| Neurotic disorders | −0.321 ** | 0.008 | 0.000 | −0.263 ** | 0.008 | 0.000 | |||||||||
| Eating disorders | −0.084 | 0.071 | 0.230 | −0.081 | 0.092 | 0.379 | |||||||||
| Personality disorders | −0.138 ** | 0.027 | 0.000 | −0.126 ** | 0.021 | 0.000 | |||||||||
| Intellectual disabilities | −0.157 ** | 0.030 | 0.000 | −0.131 ** | 0.010 | 0.000 | |||||||||
| Developmental disorders | −0.171 ** | 0.017 | 0.000 | −0.146 ** | 0.042 | 0.000 | |||||||||
| Behavioural disorder | −0.089 ** | 0.019 | 0.000 | −0.099 ** | 0.038 | 0.009 | |||||||||
| Suffered from disease in past 5 years | 0.202 ** | 0.020 | 0.000 | 0.179 ** | 0.006 | 0.000 | |||||||||
| Psychiatric comorbidity | 0.134 ** | 0.006 | 0.000 | 0.099 ** | 0.004 | 0.000 | |||||||||
| Somatic comorbidity | 0.022 ** | 0.001 | 0.000 | 0.035 ** | 0.002 | 0.000 | |||||||||
| Depressive disorders | 0.162 ** | 0.007 | 0.000 | 0.165 ** | 0.017 | 0.000 | |||||||||
| Suicide attempt | 0.253 ** | 0.004 | 0.000 | 0.204 ** | 0.011 | 0.000 | |||||||||
| Bed days per hospitalisation | −0.004 ** | 0.000 | 0.000 | −0.007 ** | 0.000 | 0.000 | |||||||||
| Forensic psychiatry | 0.476 ** | 0.026 | 0.000 | 0.384 ** | 0.020 | 0.000 | |||||||||
| Psychotherapy | −0.003 | 0.072 | 0.410 | −0.169 ** | 0.014 | 0.000 | |||||||||
| HCP | Regions | ||||||||||||||
| Capital | - | −0.132 ** | 0.035 | 0.000 | −0.080 ** | 0.007 | 0.000 | ||||||||
| Zealandic | - | −0.214 ** | 0.039 | 0.000 | −0.214 ** | 0.018 | 0.000 | ||||||||
| Southern | - | −0.164 ** | 0.035 | 0.000 | −0.139 ** | 0.015 | 0.000 | ||||||||
| Central | - | 0.922 ** | 0.050 | 0.000 | 0.907 ** | 0.067 | 0.000 | ||||||||
| Northern | - | 0 (omitted) | 0 (omitted) | ||||||||||||
| Hospital departments | - | ||||||||||||||
| Inpatient | - | Ref. | |||||||||||||
| Outpatient | - | −0.026 | 0.017 | 0.116 | −0.019 ** | 0.004 | 0.000 | ||||||||
| Hospital characteristics | - | ||||||||||||||
| Teaching hospital | - | 0.296 ** | 0.042 | 0.000 | 0.273 ** | 0.042 | 0.000 | ||||||||
| Bed capacity | - | 0.000 | 0.000 | 0.420 | −0.001 ** | 0.000 | 0.007 | ||||||||
| General practitioners | - | −1.17 ** | 0.0 | 0.000 | −1.265 ** | 0.038 | 0.000 | ||||||||
| GP characteristics | - | ||||||||||||||
| Patients per GP | - | −0.000 ** | 0.000 | 0.000 | −0.000 ** | 0.000 | 0.000 | ||||||||
| Consultations | - | Ref. | |||||||||||||
| Talk therapy | - | 0.000 | 0.000 | 0.174 | 0.000 ** | 0.000 | 0.005 | ||||||||
| Phone/mail consultations | - | −0.000 | 0.000 | 0.945 | 0.000 * | 0.000 | 0.014 | ||||||||
| Community−based care departments | |||||||||||||||
| Outreaching teams | - | 0.017 | 0.119 | 0.885 | 0.111 ** | 0.015 | 0.000 | ||||||||
| Private psychiatrists | - | −1.242 ** | 0.047 | 0.000 | −1.121 ** | 0.042 | 0.000 | ||||||||
| Community | - | Ref. | |||||||||||||
| Community-based care characteristics | |||||||||||||||
| Episodes per year | - | −0.000 | 0.000 | 0.434 | −0.000 ** | 0.000 | 0.002 | ||||||||
| Municipality | Assisted living | - | - | 0.067 | 0.043 | 0.256 | 0.013 | 0.036 | 0.711 | ||||||
| Personal and practical support | - | - | 0.134 ** | 0.037 | 0.000 | −0.004 | 0.004 | 0.218 | |||||||
| Therapy by psychologist | - | - | −0.175 ** | 0.021 | 0.000 | −0.017 | 0.012 | 0.138 | |||||||
| Population density | - | - | −0.000 ** | 0.000 | 0.000 | −0.000 ** | 0.000 | 0.002 | |||||||
| Nationality density, Danish | - | - | 0.000 ** | 0.000 | 0.000 | 0.000 ** | 0.000 | 0.002 | |||||||
| Var. | SE | Var. | SE | Var. | SE | Var. | SE | Var. | SE | ||||||
| Levels | Individual | 0.720 | 0.007 | 0.773 | 0.002 | 0.849 | 0.005 | 0.849 | 0.005 | 0.699 | 0.004 | ||||
| HCP | 0.032 | 0.018 | 0.161 | 0.034 | 0.178 | 0.023 | 0.062 | 0.017 | 0.062 | 0.017 | |||||
| HCP’s municipality | 0.075 | 0.009 | 0.068 | 0.028 | 0.088 | 0.039 | 0.213 | 0.020 | 0.213 | 0.020 | |||||
| Individual’s municipality | 0.241 | 0.036 | 0.474 | 0.005 | 0.450 | 0.010 | 0.076 | 0.007 | 0.076 | 0.007 | |||||
| Observations | 19,592 | 19,592 | 19,592 | 19,592 | 19,592 | ||||||||||
| Dependent Variable: Number of Psychiatric Hospitalisations in 2016 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controlling for extreme cases | Not adjusting for diagnoses | Excl. intellectual disabilities, organic and developmental disorders | Incl. only schizophrenia and mood disorders | |||||||||||||||||
| VCM | Model with all controls | VCM | Model with all controls | VCM | Model with all controls | VCM | Model with all controls | |||||||||||||
| Variance | SE | Variance | SE | Variance | SE | Variance | SE | Variance | SE | Variance | SE | Variance | SE | Variance | SE | |||||
| Individual | 0.832 | 0.004 | 0.689 | 0.001 | 0.720 | 0.007 | 0.711 | 0.005 | 0.851 | 0.004 | 0.675 | 0.003 | 0.847 | 0.003 | 0.714 | 0.006 | ||||
| HCP | 0.178 | 0.010 | 0.073 | 0.003 | 0.032 | 0.018 | 0.044 | 0.012 | 0.488 | 0.007 | 0.037 | 0.324 | 0.099 | 0.012 | 0.000 | 0.000 | ||||
| HCPM | 0.081 | 0.042 | 0.218 | 0.158 | 0.075 | 0.009 | 0.220 | 0.021 | 0.087 | 0.033 | 0.287 | 0.072 | 0.157 | 0.022 | 0.328 | 0.038 | ||||
| Individual’s municipality | 0.475 | 0.011 | 0.005 | 0.075 | 0.241 | 0.036 | 0.077 | 0.005 | 0.489 | 0.007 | 0.080 | 0.005 | 0.494 | 0.010 | 0.099 | 0.010 | ||||
| Observations | 19,592 | 19,592 | 18,230 | 11,831 | ||||||||||||||||
| Excl. individuals treated for the first time in 2016 | Number of inpatient days as the dependent variable | |||||||||||||||||||
| VCM | Model with all controls | VCM | Model with all controls | |||||||||||||||||
| Variance | SE | Variance | SE | Variance | SE | Variance | SE | |||||||||||||
| Individual | 0.848 | 0.005 | 0.710 | 0.003 | 0.973 | 0.009 | 0.770 | 0.001 | ||||||||||||
| HCP | 0.186 | 0.041 | 0.072 | 0.016 | 0.157 | 0.004 | 0.064 | 0.014 | ||||||||||||
| HCPM | 0.081 | 0.018 | 0.170 | 0.023 | 0.213 | 0.034 | 0.304 | 0.013 | ||||||||||||
| Individual’s municipality | 0.488 | 0.010 | 0.078 | 0.018 | 0.148 | 0.007 | 0.066 | 0.005 | ||||||||||||
| Observations | 19,190 | 19,947 | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blæhr, E.E.; Gallo Cordoba, B.; Skipper, N.; Søgaard, R. Variation in Psychiatric Hospitalisations: A Multiple-Membership Multiple-Classification Analysis. Int. J. Environ. Res. Public Health 2024, 21, 973. https://doi.org/10.3390/ijerph21080973
Blæhr EE, Gallo Cordoba B, Skipper N, Søgaard R. Variation in Psychiatric Hospitalisations: A Multiple-Membership Multiple-Classification Analysis. International Journal of Environmental Research and Public Health. 2024; 21(8):973. https://doi.org/10.3390/ijerph21080973
Chicago/Turabian StyleBlæhr, Emely Ek, Beatriz Gallo Cordoba, Niels Skipper, and Rikke Søgaard. 2024. "Variation in Psychiatric Hospitalisations: A Multiple-Membership Multiple-Classification Analysis" International Journal of Environmental Research and Public Health 21, no. 8: 973. https://doi.org/10.3390/ijerph21080973






