Time-Use Sequences: A Mixed-Methods Study Exploring How, When, and Where Spatiotemporal Patterns of Everyday Routines Can Strengthen Public Health Interventions
Abstract
1. Background
1.1. Advancements in Behavior Change Interventions
1.2. Limitations of Behavior Change Interventions
1.3. Methods to Assess Contexts Influencing Behaviors
- (1)
- Investigate spatiotemporal contexts about patients’ day-to-day health activities;
- (2)
- Explore how interventions can use spatiotemporal information about patients’ day-to-day health activities to overcome challenges to physical activity within clinical interventions.
2. Methods
2.1. Study Design
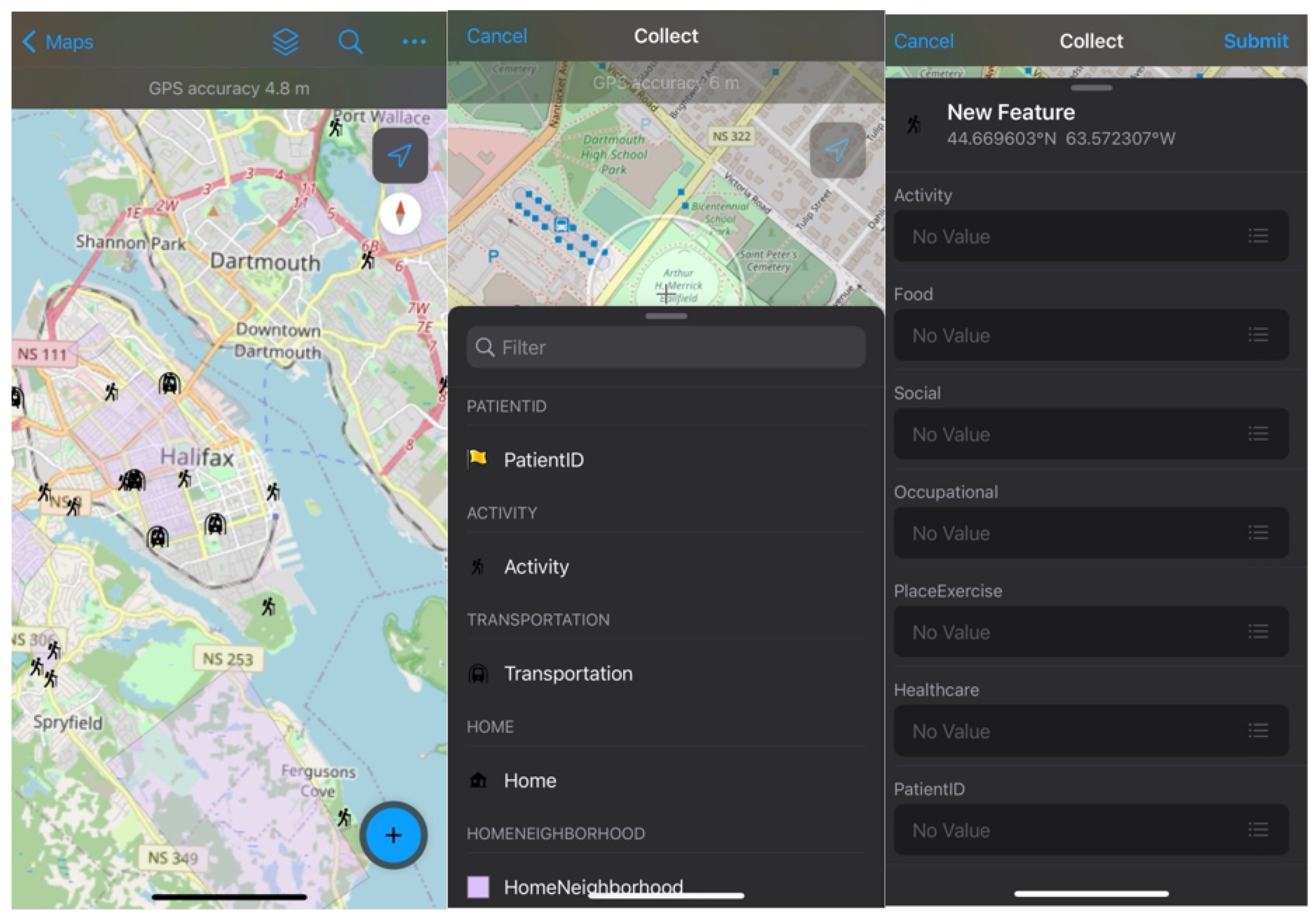
2.2. Geo-Ethnography Techniques
2.3. Research Setting
2.4. Patient Recruitment and Data Collection
2.5. Ethical Approval
2.6. Analysis
3. Results
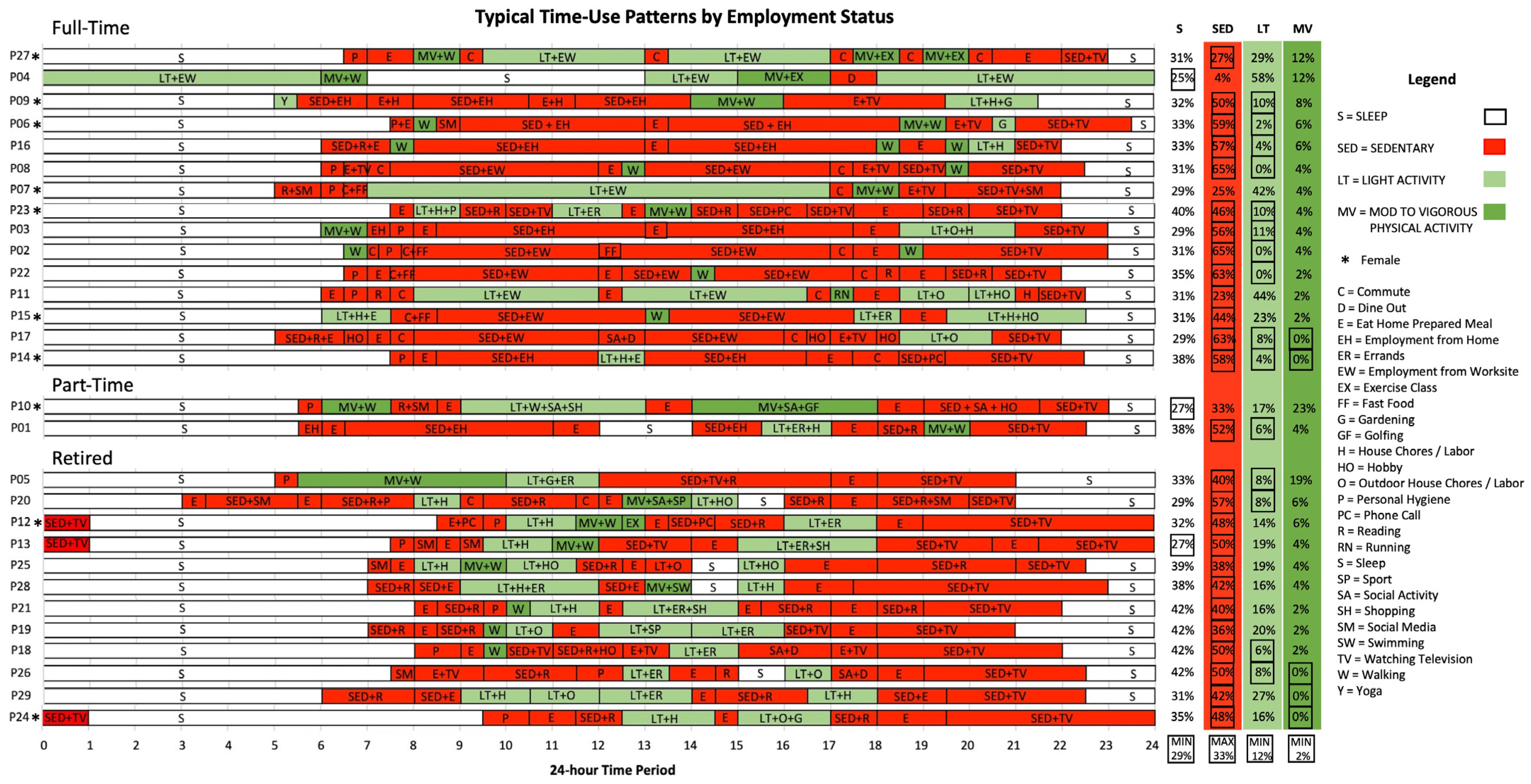
3.1. Sequencing and Visualization of Time-Use Patterns
3.2. What Are Spatiotemporal Contexts of Patients’ Day-to-Day Health Activities?
3.3. Spatiotemporal Characteristics of Patients’ Time-Use Patterns
3.4. How Can Interventions Use Spatiotemporal Information about Patients’ Day-to-Day Health Activities?
3.5. Characterizing Heterogeneity of Patients’ Time-Use Patterns
3.6. Characterizing the Space-Time Continuum of Where Activities Occur
4. Discussion
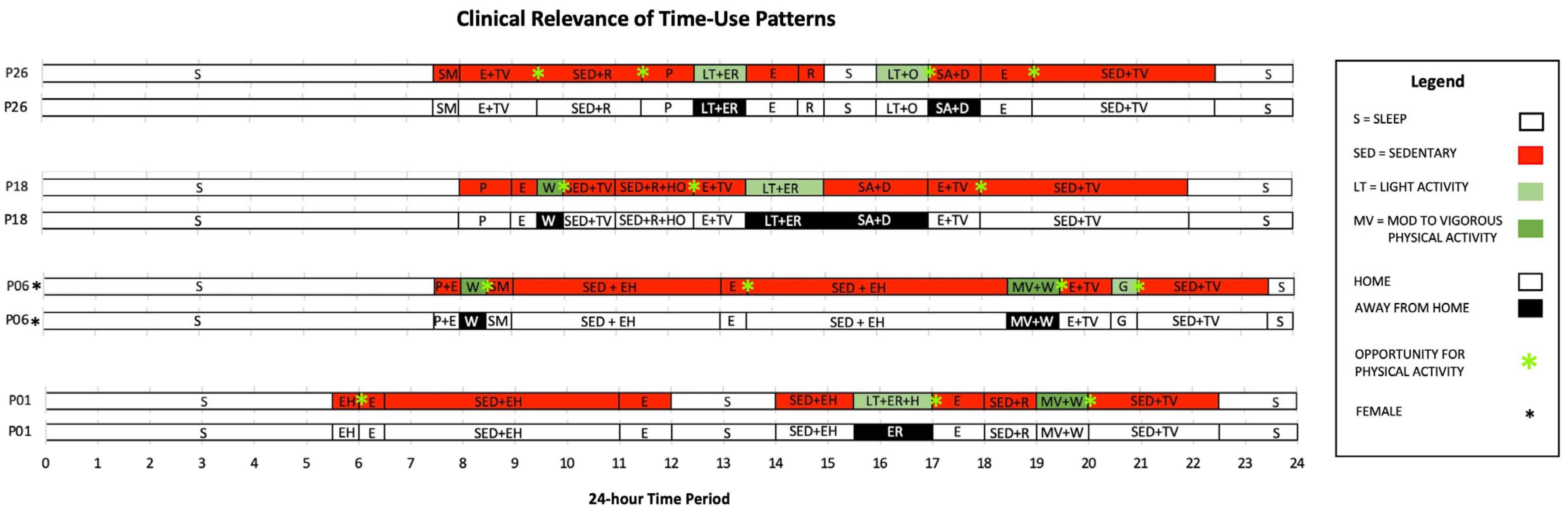
4.1. Clinical Relevance of Time-Use Patterns
4.2. Strengthening Behavior Change Interventions
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| EMA | ecological momentary assessment |
| GPS | global positioning system |
| METs | metabolic equivalents scores |
| PA | physical activity |
| MVPA | moderate-to-vigorous physical activity |
Appendix A
References
- Graham, H.; Prue-Owens, K.; Kirby, J.; Ramesh, M. Systematic Review of Interventions Designed to Maintain or Increase Physical Activity Post-Cardiac Rehabilitation Phase II. Rehabil. Process Outcome 2020, 9, 1179572720941833. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 3 September 2022).
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 29 July 2022).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases: Avoiding Heart Attacks and Strokes. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 18 July 2022).
- World Health Organization. Report of the First Meeting of the Strategic and Technical Advisory Group for Noncommunicable Diseases: Virtual Meeting, 27–28 October 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Room, J.; Hannink, E.; Dawes, H.; Barker, K. What Interventions Are Used to Improve Exercise Adherence in Older People and What Behavioural Techniques Are They Based on? A Systematic Review. BMJ Open 2017, 7, e019221. [Google Scholar] [CrossRef]
- Winter, S.J.; Sheats, J.L.; King, A.C. The Use of Behavior Change Techniques and Theory in Technologies for Cardiovascular Disease Prevention and Treatment in Adults: A Comprehensive Review. Prog. Cardiovasc. Dis. 2016, 58, 605–612. [Google Scholar] [CrossRef]
- Guiraud, T.; Granger, R.; Gremeaux, V.; Bousquet, M.; Richard, L.; Soukarié, L.; Babin, T.; Labrunée, M.; Sanguignol, F.; Bosquet, L.; et al. Telephone Support Oriented by Accelerometric Measurements Enhances Adherence to Physical Activity Recommendations in Noncompliant Patients After a Cardiac Rehabilitation Program. Arch. Phys. Med. Rehabil. 2012, 93, 2141–2147. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Schwalm, J.-D.; Khatib, R.; Yusuf, S. Why Are We Failing to Implement Effective Therapies in Cardiovascular Disease? Eur. Heart J. 2013, 34, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Berwanger, O.; Guimarães, H.P.; Laranjeira, L.N.; Cavalcanti, A.B.; Kodama, A.A.; Zazula, A.D.; Santucci, E.V.; Victor, E.; Tenuta, M.; Carvalho, V.; et al. Effect of a Multifaceted Intervention on Use of Evidence-Based Therapies in Patients with Acute Coronary Syndromes in Brazil: The BRIDGE-ACS Randomized Trial. JAMA 2012, 307, 2041–2049. [Google Scholar] [CrossRef]
- Emberson, M.A.; Lalande, A.; Wang, D.; McDonough, D.J.; Liu, W.; Gao, Z. Effectiveness of Smartphone-Based Physical Activity Interventions on Individuals’ Health Outcomes: A Systematic Review. BioMed Res. Int. 2021, 2021, 6296896. [Google Scholar] [CrossRef]
- Davis, A.J.; Parker, H.M.; Gallagher, R. Gamified Applications for Secondary Prevention in Patients with High Cardiovascular Disease Risk: A Systematic Review of Effectiveness and Acceptability. J. Clin. Nurs. 2021, 30, 3001–3010. [Google Scholar] [CrossRef]
- Nigg, C.R.; Long, C.R. A Systematic Review of Single Health Behavior Change Interventions vs. Multiple Health Behavior Change Interventions among Older Adults. Transl. Behav. Med. 2012, 2, 163–179. [Google Scholar] [CrossRef]
- McQuoid, J.; Jowsey, T.; Talaulikar, G. Contextualising Renal Patient Routines: Everyday Space-Time Contexts, Health Service Access, and Wellbeing. Soc. Sci. Med. 2017, 183, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Alageel, S.; Gulliford, M.C.; McDermott, L.; Wright, A.J. Implementing Multiple Health Behaviour Change Interventions for Cardiovascular Risk Reduction in Primary Care: A Qualitative Study. BMC Fam. Pract. 2018, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Bostock, Y.; Backett-Milburn, K. Recovering from a Heart Attack: A Qualitative Study into Lay Experiences and the Struggle to Make Lifestyle Changes. Fam. Pract. 2006, 23, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.; Brawley, L.; Millen, J. Relationship of Proxy Efficacy and Reliance to Home-Based Physical Activity after Cardiac Rehabilitation. Rehabil. Psychol. 2006, 51, 224–231. [Google Scholar] [CrossRef]
- Coorey, G.; Peiris, D.; Neubeck, L.; Redfern, J. A Realist Evaluation Approach to Explaining the Role of Context in the Impact of a Complex eHealth Intervention for Improving Prevention of Cardiovascular Disease. BMC Health Serv. Res. 2020, 20, 764. [Google Scholar] [CrossRef]
- Leijon, M.E.; Bendtsen, P.; Ståhle, A.; Ekberg, K.; Festin, K.; Nilsen, P. Factors Associated with Patients Self-Reported Adherence to Prescribed Physical Activity in Routine Primary Health Care. BMC Fam. Pract. 2010, 11, 38. [Google Scholar] [CrossRef]
- Leijon, M.E.; Faskunger, J.; Bendtsen, P.; Festin, K.; Nilsen, P. Who Is Not Adhering to Physical Activity Referrals, and Why? Scand. J. Prim. Health Care 2011, 29, 234–240. [Google Scholar] [CrossRef]
- Macintyre, S.; Ellaway, A.; Cummins, S. Place Effects on Health: How Can We Conceptualise, Operationalise and Measure Them? Soc. Sci. Med. 2002, 55, 125–139. [Google Scholar] [CrossRef]
- Bauman, A.; Bittman, M.; Gershuny, J. A Short History of Time Use Research; Implications for Public Health. BMC Public Health 2019, 19 (Suppl. S2), 607. [Google Scholar] [CrossRef]
- Chau, J.Y.; Gomersall, S.R.; van der Ploeg, H.P.; Milton, K. The Evolution of Time Use Approaches for Understanding Activities of Daily Living in a Public Health Context. BMC Public Health 2019, 19 (Suppl. S2), 451. [Google Scholar] [CrossRef]
- Sullivan, O.; Gershuny, J.; Sevilla, A.; Foliano, F.; Vega-Rapun, M.; Lamote de Grignon, J.; Harms, T.; Walthéry, P. Using Time-Use Diaries to Track Changing Behavior across Successive Stages of COVID-19 Social Restrictions. Proc. Natl. Acad. Sci. USA 2021, 118, e2101724118. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Johnson, W.D.; Katzmarzyk, P.T. Frequently Reported Activities by Intensity for U.S. Adults: The American Time Use Survey. Am. J. Prev. Med. 2010, 39, e13–e20. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, T.; Zanda, A.; Fillekes, M.P.; Bereuter, P.; Portegijs, E.; Rantanen, T.; Schmidt-Trucksäss, A.; Zeller, A.W.; Weibel, R. Map-Based Assessment of Older Adults’ Life Space: Validity and Reliability. Eur. Rev. Aging Phys. Act. 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, H.; Kahila, M. The SoftGIS Approach to Local Knowledge. J. Environ. Manag. 2009, 90, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Fornace, K.M.; Surendra, H.; Abidin, T.R.; Reyes, R.; Macalinao, M.L.M.; Stresman, G.; Luchavez, J.; Ahmad, R.A.; Supargiyono, S.; Espino, F.; et al. Use of Mobile Technology-Based Participatory Mapping Approaches to Geolocate Health Facility Attendees for Disease Surveillance in Low Resource Settings. Int. J. Health Geogr. 2018, 17, 21. [Google Scholar] [CrossRef]
- Sasaki, J.E.; da Silva, K.S.; da Costa, B.G.G.; John, D. Measurement of Physical Activity Using Accelerometers. In Computer-Assisted and Web-Based Innovations in Psychology, Special Education, and Health; Elsevier Academic Press: San Diego, CA, USA, 2016; pp. 33–60. [Google Scholar]
- Lee, I.-M.; Shiroma, E.J. Using Accelerometers to Measure Physical Activity in Large-Scale Epidemiologic Studies: Issues and Challenges. Br. J. Sports Med. 2014, 48, 197–201. [Google Scholar] [CrossRef]
- Perchoux, C.; Chaix, B.; Kestens, Y. Activity Spaces in Place and Health Research_ Novel Exposure Measures, Data Collection Tools, and Designs. Health Place 2019, 58, 3. [Google Scholar] [CrossRef]
- Rainham, D.; Krewski, D.; McDowell, I.; Sawada, M.; Liekens, B. Development of a Wearable Global Positioning System for Place and Health Research. Int. J. Health Geogr. 2008, 7, 59. [Google Scholar] [CrossRef]
- Fuller, D.; Stanley, K. The Future of Activity Space and Health Research. Health Place 2019, 58, 3. [Google Scholar] [CrossRef]
- Nethery, E.; Mallach, G.; Rainham, D.; Goldberg, M.S.; Wheeler, A.J. Using Global Positioning Systems (GPS) and Temperature Data to Generate Time-Activity Classifications for Estimating Personal Exposure in Air Monitoring Studies: An Automated Method. Environ. Health 2014, 33, 11. Available online: http://www.ehjournal.net/content/13/1/33 (accessed on 9 August 2022). [CrossRef] [PubMed]
- Oreskovic, N.M.; Blossom, J.; Field, A.E.; Chiang, S.R.; Winickoff, J.P.; Kleinman, R.E. Combining Global Positioning System and Accelerometer Data to Determine the Locations of Physical Activity in Children. Geospat. Health 2012, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Dunton, G.F. Ecological Momentary Assessment in Physical Activity Research. Exerc. Sport Sci. Rev. 2017, 45, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.A.; Shiffman, S. Ecological Momentary Assessment (EMA) in Behavioral Medicine. Ann. Behav. Med. 1994, 16, 199–202. [Google Scholar] [CrossRef]
- Degroote, L.; DeSmet, A.; De Bourdeaudhuij, I.; Van Dyck, D.; Crombez, G. Content Validity and Methodological Considerations in Ecological Momentary Assessment Studies on Physical Activity and Sedentary Behaviour: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 35. [Google Scholar] [CrossRef]
- Park, S.H.; Petrunoff, N.A.; Wang, N.X.; van Dam, R.M.; Sia, A.; Tan, C.S.; Müller-Riemenschneider, F. Daily Park Use, Physical Activity, and Psychological Stress: A Study Using Smartphone-Based Ecological Momentary Assessment amongst a Multi-Ethnic Asian Cohort. Ment. Health Phys. Act. 2022, 22, 100440. [Google Scholar] [CrossRef]
- Matthews, S.A.; Detwiler, J.E.; Burton, L.M. Geo-Ethnography: Coupling Geographic Information Analysis Techniques with Ethnographic Methods in Urban Research. Cartogr. Int. J. Geogr. Inf. Geovis. 2005, 40, 75–90. [Google Scholar] [CrossRef]
- Milton, S.; Pliakas, T.; Hawkesworth, S.; Nanchahal, K.; Grundy, C.; Amuzu, A.; Casas, J.-P.; Lock, K. A Qualitative Geographical Information Systems Approach to Explore How Older People over 70 Years Interact with and Define Their Neighbourhood Environment. Health Place 2015, 36, 127–133. [Google Scholar] [CrossRef] [PubMed]
- MacNell, L. A Geo-Ethnographic Analysis of Low-Income Rural and Urban Women’s Food Shopping Behaviors. Appetite 2018, 128, 311–320. [Google Scholar] [CrossRef]
- Barber, B.V.; Kephart, G.; Martin-Misener, R.; Vallis, M.; Matthews, S.; Atkins, L.; Cassidy, C.; Curran, J.; Rainham, D. Integrating Health Geography and Behavioral Economic Principles to Strengthen Context-Specific Behavior Change Interventions. Transl. Behav. Med. 2024, 14, 257–272. [Google Scholar] [CrossRef]
- Hennink, M.; Kaiser, B.N. Sample Sizes for Saturation in Qualitative Research: A Systematic Review of Empirical Tests. Soc. Sci. Med. 2022, 292, 114523. [Google Scholar] [CrossRef]
- QSR International. Learn More about Data Analysis Software|NVivo. Available online: https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/about/nvivo (accessed on 29 August 2020).
- ParticipACTION. ParticipACTION 2021 Report Card on Physical Activity for Adults; ParticipACTION: Toronto, ON, Canada, 2021; p. 80. [Google Scholar]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project Process and Outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- The Canadian Society for Exercise Physiology. The Canadian 24-Hour Movement Guidelines for Adults. Available online: https://csepguidelines.ca/guidelines/adults-18-64/ (accessed on 14 June 2022).
- Moore, C.J.; Williams, T.N.; Berg, A.C.; Durward, C.M. An Evaluation of Inter-Coder and Intra-Coder Reliability for 24-Hour Dietary Recall Data Entered in WebNEERS. J. Nutr. Educ. Behav. 2019, 51, 432–439. [Google Scholar] [CrossRef]
- Abbott, A. Sequence Analysis: New Methods for Old Ideas. Annu. Rev. Sociol. 1995, 21, 93–113. [Google Scholar] [CrossRef]
- Shoval, N.; Isaacson, M. Sequence Alignment as a Method for Human Activity Analysis in Space and Time. Ann. Assoc. Am. Geogr. 2007, 97, 282–297. [Google Scholar] [CrossRef]
- Müller-Riemenschneider, F.; Petrunoff, N.; Yao, J.; Ng, A.; Sia, A.; Ramiah, A.; Wong, M.; Han, J.; Tai, B.C.; Uijtdewilligen, L. Effectiveness of Prescribing Physical Activity in Parks to Improve Health and Wellbeing—The Park Prescription Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Orrow, G.; Kinmonth, A.-L.; Sanderson, S.; Sutton, S. Effectiveness of Physical Activity Promotion Based in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ 2012, 344, e1389. [Google Scholar] [CrossRef] [PubMed]
- Riera-Sampol, A.; Bennasar-Veny, M.; Tauler, P.; Aguilo, A. Effectiveness of Physical Activity Prescription by Primary Care Nurses Using Health Assets: A Randomized Controlled Trial. J. Adv. Nurs. 2021, 77, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, L.M.; Johnson, S.S.; Prochaska, J.M. Meeting Patients Where They Are at: Using a Stage Approach to Facilitate Engagement. In Practical Strategies and Tools to Promote Treatment Engagement; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–44. [Google Scholar] [CrossRef]
- Asiamah, N.; Agyemang, S.M.; Yarfi, C.; Jnr, R.A.-M.; Muhonja, F.; Khan, H.T.A.; Kouveliotis, K.; Sghaier, S. Associations of Social Networks with Physical Activity Enjoyment among Older Adults: Walkability as a Modifier through a STROBE-Compliant Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3341. [Google Scholar] [CrossRef]
- Latkin, C.A.; Knowlton, A.R. Social Network Assessments and Interventions for Health Behavior Change: A Critical Review. Behav. Med. 2015, 41, 90–97. [Google Scholar] [CrossRef]
- Abrahams, N.; Khodabakhsh, S.; Toumpakari, Z.; Marais, F.; Lambert, E.V.; Foster, C. Using Social Networks to Scale up and Sustain Community-Based Programmes to Improve Physical Activity and Diet in Low-Income and Middle-Income Countries: A Scoping Review. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.; Moller, A.C.; Coons, M.J. Multiple Health Behaviours: Overview and Implications. J. Public Health Oxf. Engl. 2012, 34 (Suppl. S1), i3–i10. [Google Scholar] [CrossRef]
- Tzelepis, F.; Mitchell, A.; Wilson, L.; Byrnes, E.; Haschek, A.; Leigh, L.; Oldmeadow, C. The Long-Term Effectiveness of Internet-Based Interventions on Multiple Health Risk Behaviors: Systematic Review and Robust Variance Estimation Meta-Analysis. J. Med. Internet Res. 2021, 23, e23513. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Health Benefits of Physical Activity: A Systematic Review of Current Systematic Reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum Amount of Physical Activity for Reduced Mortality and Extended Life Expectancy: A Prospective Cohort Study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.M.; De Craemer, M.; De Cocker, K.; Powell, L.; Van Cauwenberg, J.; Dall, P.; Hamer, M.; Stamatakis, E. How Does Light-Intensity Physical Activity Associate with Adult Cardiometabolic Health and Mortality? Systematic Review with Meta-Analysis of Experimental and Observational Studies. Br. J. Sports Med. 2019, 53, 370–376. [Google Scholar] [CrossRef]
- Schwartz, J.; Rhodes, R.; Bredin, S.S.D.; Oh, P.; Warburton, D.E.R. Effectiveness of Approaches to Increase Physical Activity Behavior to Prevent Chronic Disease in Adults: A Brief Commentary. J. Clin. Med. 2019, 8, 295. [Google Scholar] [CrossRef]
- Strazdins, L.; Welsh, J.; Korda, R.; Broom, D.; Paolucci, F. Not All Hours Are Equal: Could Time Be a Social Determinant of Health? Sociol. Health Illn. 2016, 38, 21–42. [Google Scholar] [CrossRef]
- Stuart-Shor, E.M.; Berra, K.; Kamau, M.W.; Kumanyika, S. Behavioral Strategies for Cardiovascular Risk Reduction in Diverse and Underserved Racial/Ethnic Groups. Circulation 2012, 125, 171–184. [Google Scholar] [CrossRef]
- Gardner, B. A Review and Analysis of the Use of ‘Habit’ in Understanding, Predicting and Influencing Health-Related Behaviour. Health Psychol. Rev. 2015, 9, 277–295. [Google Scholar] [CrossRef]
- Kaushal, N.; Rhodes, R.E.; Meldrum, J.T.; Spence, J.C. The Role of Habit in Different Phases of Exercise. Br. J. Health Psychol. 2017, 22, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.; Lally, P.; Wardle, J. Making Health Habitual: The Psychology of ‘Habit-Formation’ and General Practice. Br. J. Gen. Pract. 2012, 62, 664–666. [Google Scholar] [CrossRef] [PubMed]


| Layer Name | Feature Class | Field ID | Value ID | |
|---|---|---|---|---|
| Patient ID | Point | ID | P01–P29 | |
| Neighborhood | Polygon | Neighborhood | P01–P29 | |
| Activity | Point | Activity | Walking | Running/Jogging |
| Bicycling | Yoga/Stretching | |||
| Strength Training | Playing Sport | |||
| Swimming | Gardening | |||
| Housework | Watching TV | |||
| Cooking/Baking | Shopping | |||
| Point | Food | Grocery Store | Restaurant | |
| Fast Food | Local Market | |||
| Convenience Store | Specialty Store | |||
| Bar/Brewery | Meal at Home | |||
| Café | ||||
| Point | Social | Family | Friends | |
| Point | Occupational | Employment | Volunteer | |
| Caregiver | ||||
| Point | Place of Exercise | Gym Membership | Park | |
| Community Rec Centre | Home | |||
| Mall/Shopping Centre | Senior Centre | |||
| Outdoor place | Home Condo Facilities | |||
| Point | Healthcare | Healthcare Appointment | Hospital | |
| Transportation | Point | Mode of Transportation | Car | Public Bus |
| Walk | Bike | |||
| Home | Point | Dwelling Type | House | Condo |
| Apartment | ||||
| Patient Characteristics | n | % |
|---|---|---|
| Age | ||
| 45–54 | 7 | 24% |
| 55–64 | 7 | 24% |
| 65–74 | 9 | 31% |
| 75–84 | 6 | 21% |
| Sex | ||
| Male | 19 | 66% |
| Female | 10 | 34% |
| Education Level | ||
| High school | 5 | 18% |
| College/trades | 12 | 41% |
| University/graduate degree | 12 | 41% |
| Marital Status | ||
| Married/common law | 22 | 76% |
| Single/widow | 7 | 24% |
| Household Composition | ||
| Alone | 7 | 24% |
| With partner | 15 | 52% |
| Partner and children | 7 | 24% |
| Employment Status | ||
| Part time | 2 | 7% |
| Full time | 15 | 52% |
| Retired | 12 | 41% |
| Smoking Status | ||
| No | 14 | 48.5% |
| Current smoker | 1 | 3% |
| Previous smoker | 14 | 48.5% |
| Reason for Referral | ||
| Prevention—no comorbidity | 2 | 7% |
| Heart attack/stroke—no comorbidity | 14 | 48.5% |
| Heart attack—comorbidities | 13 | 44.5% |
| Term | Definition |
|---|---|
| Sleep (S) | Sleep routine is an important component of health that affects attention, behavior, memory, and overall mental and physical health. Not getting enough quality sleep is linked to a wide variety of health problems, including obesity, type 2 diabetes, cardiovascular disease, and depression [48]. Canadian guidelines recommend getting 7 to 9 h of quality sleep on a regular basis, with consistent bed and wake-up times [48,50]. |
| Sedentary Behavior (SED) | Sedentary behavior is any waking behavior characterized by very low energy expenditure below 1.5 METs (i.e., less than 1.5 times the intensity of rest) while standing, sitting, reclining, or lying down [48,49,50]. Canadian guidelines recommend limiting sedentary time to 8 h or less, including no more than 3 h of recreational screen time and breaking up long periods of sitting as often as possible [48,50]. |
| Light Physical Activity (LT) | Light intensity activities require low levels of energy and effort performed between 1.5 and 3 METs (i.e., greater than 1.5 but less than 3 times the intensity of rest). Light physical activity includes walking at a slower pace, standing work, or light housework [48]. Canadian guidelines recommend getting at least 3 h of light physical activity per day [48]. |
| Moderate-to-Vigorous Physical Activity (MV) | Moderate-to-vigorous physical activity is movement that requires substantial energy expenditure above resting levels performed above 3 METs (i.e., greater than 3 times the intensity of rest) [48]. Moderate-to-vigorous physical activity includes a variety of activities and intensities, like swimming, brisk walking, jogging, rowing, weightlifting, or bicycling. Canadian guidelines recommend participating in at least 30 min per day or 150 min per week of moderate-to-vigorous physical activity [48,50]. |
| n | Av. Time | ||
|---|---|---|---|
| (m, f) | (Range) | Av.% | |
| Sleep | |||
| ≥7 h Minimum Guideline | 26 | 8.25 | 34% |
| (17, 9) | (7–10) | ||
| <7 h Minimum Guideline | 3 | 6.25 | 26% |
| (2, 1) | (6–6.5) | ||
| Total Sleep Activity | 29 | 8 | 33% |
| (19, 10) | (6–10) | ||
| Sedentary Activity | |||
| ≥8 h Maximum Guideline | 24 | 12.25 | 51% |
| (17, 7) | (8.5–15.5) | ||
| <8 h Maximum Guideline | 5 | 5.4 | 22% |
| (2, 3) | (1–8) | ||
| Total Sedentary Activity | 29 | 11 | 46% |
| (19, 10) | (1–15.5) | ||
| Employment from Home | 6 | 7 | 29% |
| (3, 3) | (5–9) | ||
| Employment from Worksite | 5 | 8 | 33% |
| (4, 1) | (7–8.5) | ||
| Watching Television | 27 | 3.5 | 14% |
| (18, 9) | (1–8) | ||
| Reading | 19 | 2 | 8% |
| (14, 5) | (0.5–3.5) | ||
| Social Activities | 4 | 1.6 | 7% |
| (3, 1) | (1–2.5) | ||
| Light Physical Activity | |||
| ≥3 h Minimum Guideline | 14 | 6.25 | 26% |
| (8, 6) | (4–14) | ||
| <3 h Minimum Guideline | 15 | 1.4 | 6% |
| (11, 4) | (0–2.5) | ||
| Total Light Physical Activity | 29 | 3.7 | 15% |
| (19, 10) | (0–14) | ||
| Employment from Worksite | 4 | 9.75 | 40% |
| (2, 2) | (7–14) | ||
| House Chores/Labor | 17 | 2 | 8% |
| (11, 6) | (1–2.5) | ||
| Moderate-to-Vigorous Physical Activity (MVPA) | |||
| ≥30 min Minimum Guideline | 24 | 1.5 | 6% |
| (16, 8) | (0.5–5.5) | ||
| <30 min Minimum Guideline | 5 | 0 | 0% |
| (3, 2) | |||
| Total MVPA | 29 | 1.2 | 5% |
| (19, 10) | (0.5–4.5) | ||
| Walking | 22 | 1.2 | 5% |
| (15, 7) | (0.5–4.5) | ||
| Exercise Class | 3 | 1.5 | 6% |
| (1, 2) | (0.5–2) | ||
| Golf | 1 | 4 | 16% |
| (0, 1) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, B.V.; Kephart, G.; Vallis, M.; Matthews, S.A.; Martin-Misener, R.; Rainham, D.G. Time-Use Sequences: A Mixed-Methods Study Exploring How, When, and Where Spatiotemporal Patterns of Everyday Routines Can Strengthen Public Health Interventions. Int. J. Environ. Res. Public Health 2024, 21, 1128. https://doi.org/10.3390/ijerph21091128
Barber BV, Kephart G, Vallis M, Matthews SA, Martin-Misener R, Rainham DG. Time-Use Sequences: A Mixed-Methods Study Exploring How, When, and Where Spatiotemporal Patterns of Everyday Routines Can Strengthen Public Health Interventions. International Journal of Environmental Research and Public Health. 2024; 21(9):1128. https://doi.org/10.3390/ijerph21091128
Chicago/Turabian StyleBarber, Brittany V., George Kephart, Michael Vallis, Stephen A. Matthews, Ruth Martin-Misener, and Daniel G. Rainham. 2024. "Time-Use Sequences: A Mixed-Methods Study Exploring How, When, and Where Spatiotemporal Patterns of Everyday Routines Can Strengthen Public Health Interventions" International Journal of Environmental Research and Public Health 21, no. 9: 1128. https://doi.org/10.3390/ijerph21091128
APA StyleBarber, B. V., Kephart, G., Vallis, M., Matthews, S. A., Martin-Misener, R., & Rainham, D. G. (2024). Time-Use Sequences: A Mixed-Methods Study Exploring How, When, and Where Spatiotemporal Patterns of Everyday Routines Can Strengthen Public Health Interventions. International Journal of Environmental Research and Public Health, 21(9), 1128. https://doi.org/10.3390/ijerph21091128








