CO2 Breathing Prior to Simulated Diving Increases Decompression Sickness Risk in a Mouse Model: The Microbiota Trail Is Not Forgotten
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Animal Batches
2.3. Evaluation of Superficial Temperature by Infrared Imaging
2.4. CO2 Exposition
2.5. Hyperbaric Exposure
2.6. Physical Examination
2.7. Anesthesia and Biopsy
2.8. Blood Biochemical Analysis
2.9. Stools Bacterial Content: 16s rDNA Extraction and Sequencing
2.10. Bioinformatic Processing
2.11. Statistical Analyses
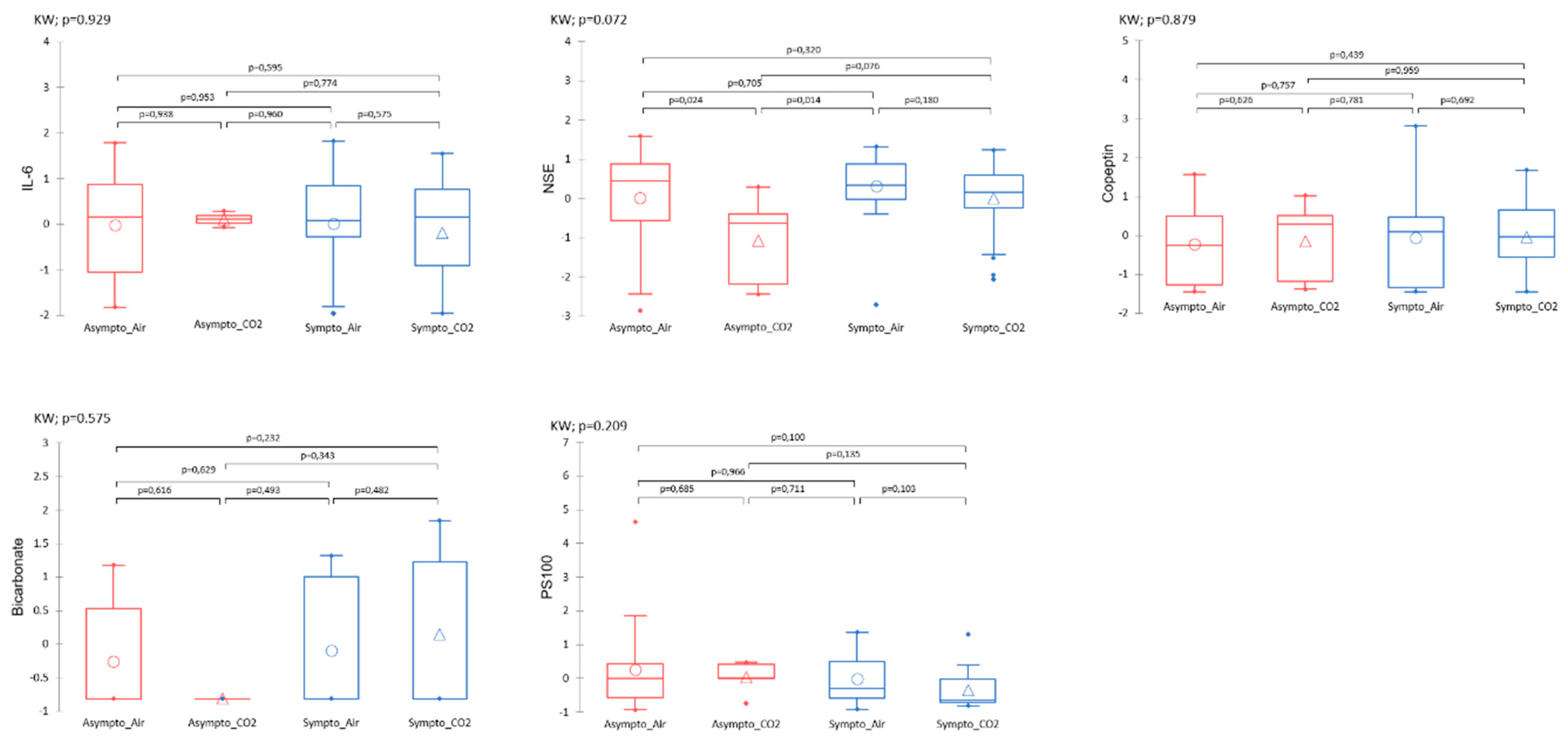
3. Results
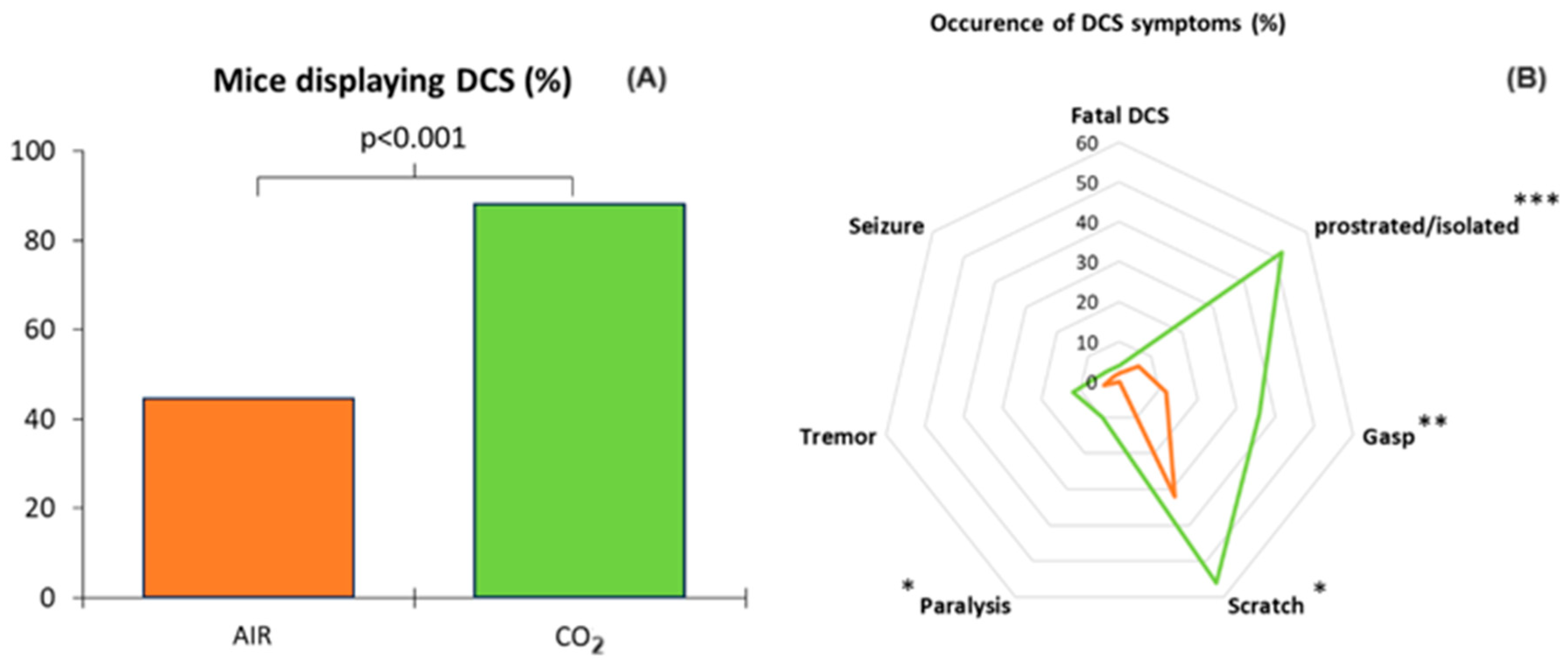
3.1. Clinical Status of the Animals Following the Dive
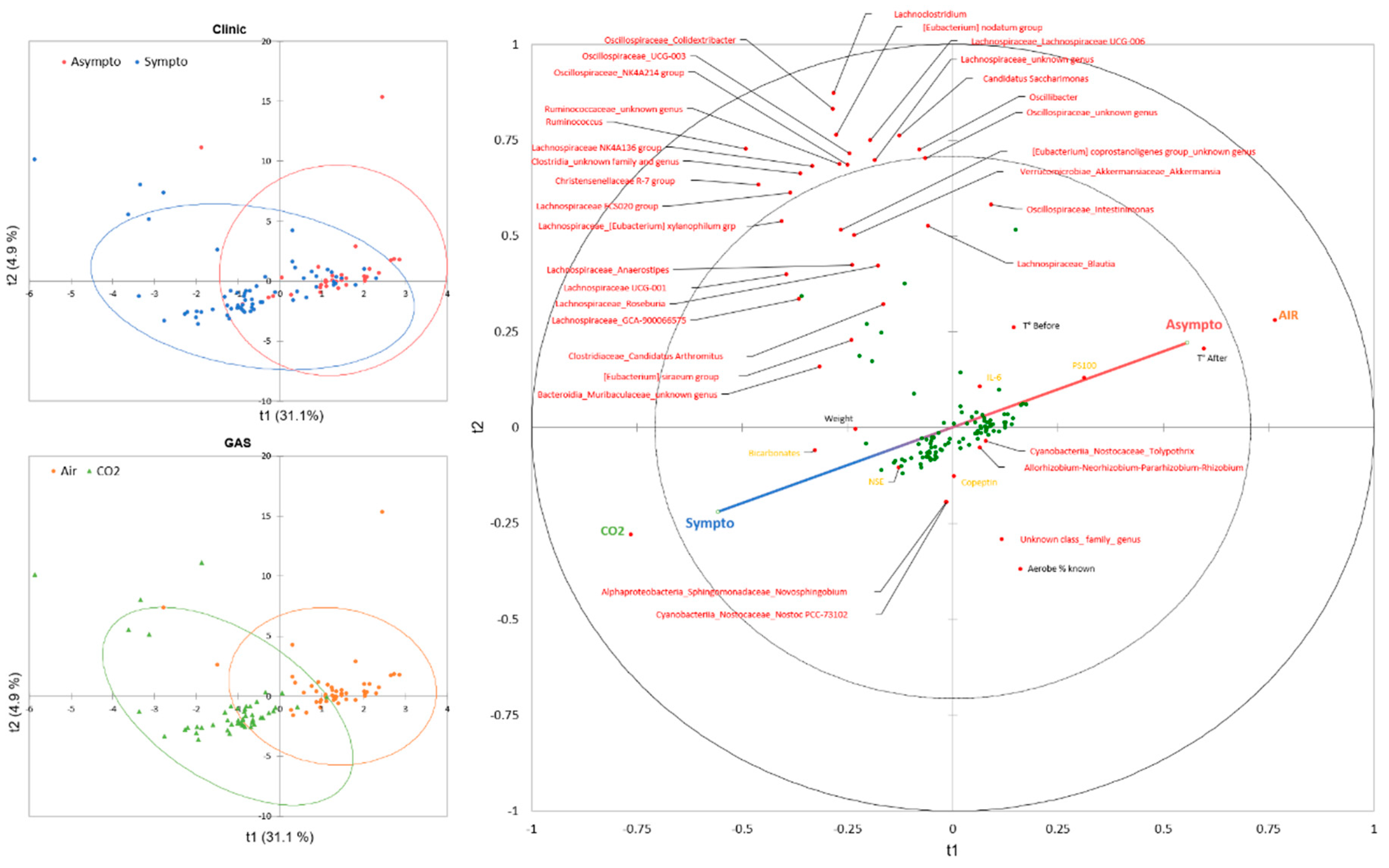
3.2. General Description of the Data
3.2.1. Two-Way Analysis
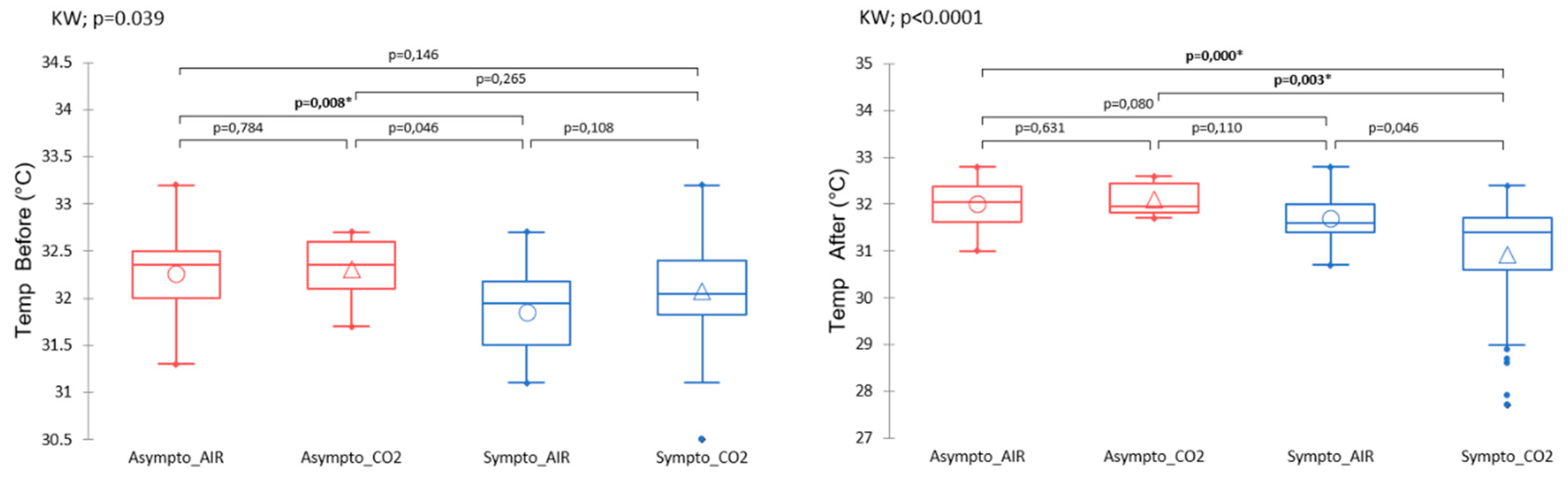
Body Temperature
Plasma Analysis
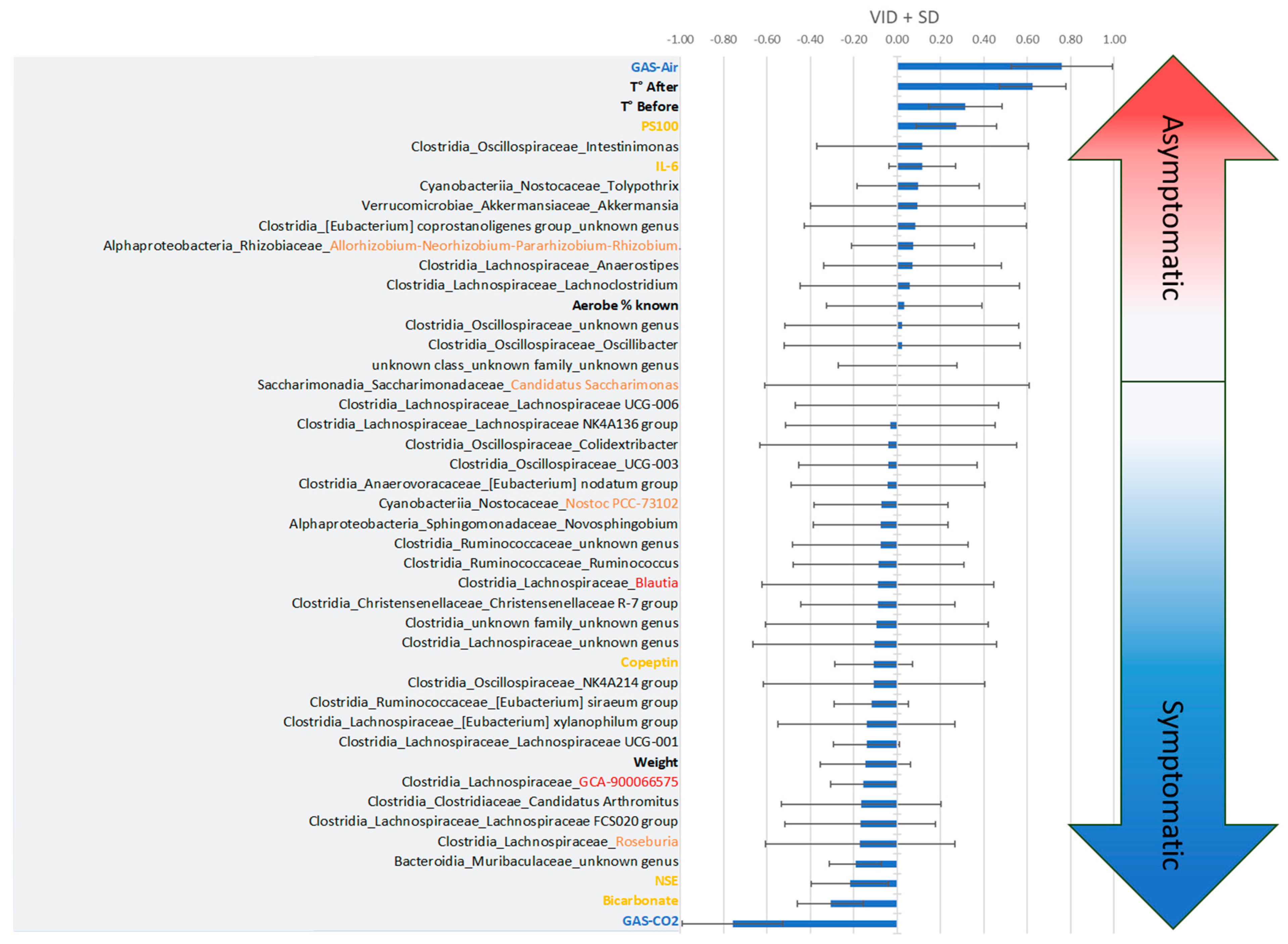
3.2.2. Initial Fecal Content Analysis
4. Discussion
4.1. Effects of CO2 and Diving on Circulating Markers of Neuronal Stress
4.2. Effects of CO2 and Diving on Plasma Markers, Thermoregulation, and Vasomotion
4.3. Temperature
4.4. Impact of the Microbiota in the Stress Response
4.5. Context
4.6. Moderation, Study Limits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andicochea, C.T.; Henriques, M.E.; Fulkerson, J.; Jay, S.; Chen, H.; Deaton, T. Elevated environmental carbon dioxide exposure confounding physiologic events in aviators? Mil. Med. 2019, 184, e863–e867. [Google Scholar] [CrossRef] [PubMed]
- Doolette, D.J.; Mitchell, S.J. Hyperbaric conditions. Compr. Physiol. 2010, 1, 163–201. [Google Scholar]
- Gennser, M.; Blogg, S.L. Oxygen or carbogen breathing before simulated submarine escape. J. Appl. Physiol. 2008, 104, 50–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daubresse, L.; Amara, J.J.; Grau, M.M.; De Maistre, S.; Guillaume, C.G.; Lehot, H.H.; Texier, G.G.; Blatteau, J.-E. Case series of unusual neurologic symptoms after scuba diving training sessions, in the context of SARS COV2 preventive measures and restrictions. In Proceedings of the EUBS, Prague, Czech Republic, 31 August–3 September 2022. [Google Scholar]
- Mano, Y.; D’Arrigo, J. Relationship between CO2 levels and decompression sickness: Implications for disease prevention. Aviat. Space Environ. Med. 1978, 49, 349–355. [Google Scholar] [PubMed]
- Daubresse, L.; Vallée, N.; Druelle, A.; Castagna, O.; Guieu, R.; Blatteau, J.-E. Effects of CO2 on the occurrence of decompression sickness: Review of the literature. Diving Hyperb. Med. 2024, 54, 110–119. [Google Scholar] [CrossRef]
- Le Chatelier, H.L. Sur un énoncé général des lois des équilibres chimiques. Comptes Rendus Acad. Des. Sci. 1884, 99, 786–789. [Google Scholar]
- Caldwell, H.G.; Carr, J.M.; Minhas, J.S.; Swenson, E.R.; Ainslie, P.N. Acid–base balance and cerebrovascular regulation. J. Physiol. 2021, 599, 5337–5359. [Google Scholar] [CrossRef]
- Brubakk, A.O.; Ross, J.A.; Thom, S.R. Saturation diving; physiology and pathophysiology. Compr. Physiol. 2011, 4, 1229–1272. [Google Scholar]
- Pickles, D.; Ogston, D.; MacDonald, A. Effects of gas bubbling and other forms of convection on platelets in vitro. J. Appl. Physiol. 1989, 67, 1250–1255. [Google Scholar] [CrossRef]
- Giry, P.B.; Porlier, G.; Eastman, D.; Radomski, M.W. Dive-induced modifications in platelet kinetics in rats. Undersea Biomed. Res. 1977, 4, 147–157. [Google Scholar]
- Pontier, J.M.; Jimenez, C.; Blatteau, J.E. Blood platelet count and bubble formation after a dive to 30 msw for 30 min. Aviat. Space Environ. Med. 2008, 79, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, T.; Klausen, H.; Lie, R.T.; Holmsen, H. Bubble-induced aggregation of platelets: Effects of gas species, proteins, and decompression. Undersea Hyperb. Med. 1993, 20, 101–119. [Google Scholar] [PubMed]
- Brubakk, A.O.; Neuman, T.S.; Elliott, D.H. Bennett and Elliott’s Physiology and Medicine of Diving; Saunders: London, UK, 2003; pp. 578–650. [Google Scholar]
- Gempp, E.; Blatteau, J.E. Risk factors and treatment outcome in scuba divers with spinal cord decompression sickness. J. Crit. Care 2010, 25, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Maccallum, M.; Caldwell, J. Intravascular bubble composition in guinea pigs: A possible explanation for differences in decompression risk among different gases. Undersea Biomed. Res. 1992, 19, 375–386. [Google Scholar]
- Ishiyama, A. Analysis of gas composition of intravascular bubbles produced by decompression. Bull. Tokyo Med. Dent. Univ. 1983, 30, 25–35. [Google Scholar]
- Hyldegaard, O.; Kerem, D.; Melamed, Y. Effect of isobaric breathing gas shifts from air to heliox mixtures on resolution of air bubbles in lipid and aqueous tissues of recompressed rats. Eur. J. Appl. Physiol. 2011, 111, 2183–2193. [Google Scholar] [CrossRef][Green Version]
- Hayashi, K.; Honda, Y.; Miyakawa, N.; Fujii, N.; Ichinose, M.; Koga, S.; Kondo, N.; Nishiyasu, T. Effect of CO2 on the ventilatory sensitivity to rising body temperature during exercise. J. Appl. Physiol. 2011, 110, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Hodes, R.; Larrabee, M.G. The relation between alveolar carbon dioxide tension and susceptibility to decompression sickness. Am. J. Physiol. Leg. Content 1946, 147, 603–615. [Google Scholar] [CrossRef]
- Camporesi, E.M.; Bosco, G. Ventilation, Gas Exchange and Exercise Under Pressure. In Bennett and Elliott’s Physiology and Medicine of Diving; Elsevier Science Limited: Amsterdam, The Netherlands, 2003; pp. 77–114. [Google Scholar]
- Lanphier, E.H. Carbon dioxide poisoning. Case Hist. Diving Hyperb. Accid. 1988, 199–213. [Google Scholar]
- Eynan, M.; Arieli, Y.; Taran, B.; Yanir, Y. Symptoms of central nervous system oxygen toxicity during 100% oxygen breathing at normobaric pressure with increasing inspired levels of carbon dioxide: A case report. Diving Hyperb. Med. 2020, 50, 70–74. [Google Scholar] [CrossRef]
- Eynan, M.; Arieli, R.; Adir, Y. Response to CO2 in novice closed-circuit apparatus divers and after 1 year of active oxygen diving at shallow depths. J. Appl. Physiol. 2005, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Vallée, N.; Dugrenot, E.; Desruelle, A.-V.; Richard, S.; Coupé, S.; Ramdani, C.; Guieu, R.; Risso, J.-J.; Gaillard, S.; Guerrero, F. Highlighting of the interactions of MYD88 and NFKB1 SNPs in rats resistant to decompression sickness: Toward an autoimmune response. Front. Physiol. 2023, 14, 1253856. [Google Scholar] [CrossRef]
- Blatteau, J.E.; Gaillard, S.; De Maistre, S.; Richard, S.; Louges, P.; Gempp, E.; Druelles, A.; Lehot, H.; Morin, J.; Castagna, O.; et al. Reduction in the Level of Plasma Mitochondrial DNA in Human Diving, Followed by an Increase in the Event of an Accident. Front. Physiol. 2018, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, K.; de Maistre, S.; Abraini, J.H.; Blatteau, J.-E.; Risso, J.-J.; Vallée, N. Tirofiban, a Glycoprotein IIb/IIIa Antagonist, Has a Protective Effect on Decompression Sickness in Rats: Is the Crosstalk Between Platelet and Leukocytes Essential? Front. Physiol. 2018, 9, 906. [Google Scholar] [CrossRef]
- Desruelle, A.V.; Louge, P.; Richard, S.; Blatteau, J.E.; Gaillard, S.; De Maistre, S.; David, H.; Risso, J.J.; Vallee, N. Demonstration by Infra-Red Imaging of a Temperature Control Defect in a Decompression Sickness Model Testing Minocycline. Front. Physiol. 2019, 10, 933. [Google Scholar] [CrossRef]
- Cosnard, C.; De Maistre, S.; Abraini, J.H.; Chazalviel, L.; Blatteau, J.E.; Risso, J.J.; Vallee, N. Thirty-five Day Fluoxetine Treatment Limits Sensory-Motor Deficit and Biochemical Disorders in a Rat Model of Decompression Sickness. Front. Physiol. 2017, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Vallee, N.; Lambrechts, K.; De Maistre, S.; Royal, P.; Mazella, J.; Borsotto, M.; Heurteaux, C.; Abraini, J.; Risso, J.J.; Blatteau, J.E. Fluoxetine Protection in Decompression Sickness in Mice is Enhanced by Blocking TREK-1 Potassium Channel with the “spadin” Antidepressant. Front. Physiol. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Lautridou, J.; Buzzacott, P.; Belhomme, M.; Dugrenot, E.; Lafere, P.; Balestra, C.; Guerrero, F. Evidence of Heritable Determinants of Decompression Sickness in Rats. Med. Sci. Sports Exerc. 2017, 49, 2433–2438. [Google Scholar] [CrossRef]
- Orsat, J.; Guernec, A.; Le Maréchal, C.; Pichereau, V.; Guerrero, F. Association between rat decompression sickness resistance, transthyretin single nucleotide polymorphism, and expression: A pilot study. Physiol. Rep. 2024, 12, e16160. [Google Scholar] [CrossRef]
- Oya, M.; Tokunaga, T.; Tadano, Y.; Ogawa, H.; Fujii, S.; Murakami, W.; Tamai, K.; Ikomi, F.; Morimoto, Y. The composition of the human fecal microbiota might be significantly associated with fecal SCFA levels under hyperbaric conditions. Biosci. Microbiota Food Health 2021, 40, 168–175. [Google Scholar] [CrossRef]
- Hagberg, M.; Ornhagen, H. Incidence and risk factors for symptoms of decompression sickness among male and female dive masters and instructors--a retrospective cohort study. Undersea Hyperb. Med. 2003, 30, 93–102. [Google Scholar] [PubMed]
- Oya, M.; Tadano, Y.; Takihata, Y.; Murakami, W.; Fujii, S.; Tamai, K.; Morimoto, Y.; Ikomi, F.; Tokunaga, T. Effects of hyperbaric conditions on fecal microbiota. Biosci. Microbiota Food Health 2019, 38, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Tang, L.; Xu, X.; Liu, M.; Wen, J.; Lv, J.; Wang, D. Role of gut microbiota in the postnatal thermoregulation of Brandt’s voles. Cell Rep. 2023, 42, 113021. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, K.; Li, Y.; Nagaoka, K.; Li, C. Gut microbiota intervention attenuates thermogenesis in broilers exposed to high temperature through modulation of the hypothalamic 5-HT pathway. J. Anim. Sci. Biotechnol. 2023, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J. The cause of multiple sclerosis is autoimmune attack of adenosyltransferase thereby limiting adenosylcobalamin production. Med. Hypotheses 2017, 109, 29–37. [Google Scholar] [CrossRef]
- Phan, H.T.; Ho, T.T.; Chu, H.H.; Vu, T.H.; Gresch, U.; Conrad, U. Neutralizing immune responses induced by oligomeric H5N1-hemagglutinins from plants. Vet. Res. 2017, 48, 53. [Google Scholar] [CrossRef][Green Version]
- Khakisahneh, S.; Zhang, X.-Y.; Nouri, Z.; Wang, D.-H. Gut microbiota and host thermoregulation in response to ambient temperature fluctuations. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Yang, F.; Wang, G.; Zeng, M.; Zhang, Z.; Yang, M.; Wang, Z.; Li, Z. Gut microbiota of two invasive fishes respond differently to temperature. Front. Microbiol. 2023, 14, 1087777. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.-D.; Wang, Y.-Y.; Liao, Z.-Z.; Xiao, X.-H. Skeletal muscles and gut microbiota-derived metabolites: Novel modulators of adipocyte thermogenesis. Front. Endocrinol. 2023, 14, 1265175. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S. Gut microbiota orchestrates energy homeostasis during cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Fu, M.; Su, J.; Yan, M.; Yu, J.; Wang, C.; Niu, Z.; Du, Y.; Hu, X.; Zheng, J. Gut microbiota dysbiosis and intestinal barrier impairment in diarrhea caused by cold drink and high-fat diet. Toxicology 2024, 502, 153728. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Campaña-Duel, E.; Ceccato, A.; Morales-Quinteros, L.; Camprubí-Rimblas, M.; Artigas, A. Hypercapnia and its relationship with respiratory infections. Expert Rev. Respir. Med. 2024, 18, 41–47. [Google Scholar] [CrossRef]
- Ruiz, A. Carbon dioxide response curves during hypothermia. Pflügers Arch. 1975, 358, 125–133. [Google Scholar] [CrossRef]
- Santiago-Marrero, I.; Liu, F.; Wang, H.; Arzola, E.P.; Xiong, W.-C.; Mei, L. Energy expenditure homeostasis requires ErbB4, an obesity risk gene, in the paraventricular nucleus. Eneuro 2023, 10. [Google Scholar] [CrossRef]
- Branco, L.; Wood, S.C. Role of central chemoreceptors in behavioral thermoregulation of the toad, Bufo marinus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 266, R1483–R1487. [Google Scholar] [CrossRef]
- Tsuji, B.; Hayashi, K.; Kondo, N.; Nishiyasu, T. Characteristics of hyperthermia-induced hyperventilation in humans. Temperature 2016, 3, 146–160. [Google Scholar] [CrossRef]
- White, M.D. Components and mechanisms of thermal hyperpnea. J. Appl. Physiol. 2006, 101, 655–663. [Google Scholar] [CrossRef]
- Sunanaga, J.; Deng, B.-S.; Zhang, W.; Kanmura, Y.; Kuwaki, T. CO2 activates orexin-containing neurons in mice. Respir. Physiol. Neurobiol. 2009, 166, 184–186. [Google Scholar] [CrossRef]
- Hacquemand, R.; Buron, G.; Pourié, G.; Karrer, M.; Jacquot, L.; Brand, G. Effects of CO2 inhalation exposure on mice vomeronasal epithelium. Cell Biol. Toxicol. 2010, 26, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.T.C.; da Silva, E.N.; José de Anchieta, C.; Gargaglioni, L.H.; Dias, M.B. Glutamate metabotropic receptors in the lateral hypothalamus/perifornical area reduce the CO2 chemoreflex. Respir. Physiol. Neurobiol. 2019, 260, 122–130. [Google Scholar] [CrossRef]
- Blatteau, J.E.; Barre, S.; Pascual, A.; Castagna, O.; Abraini, J.H.; Risso, J.J.; Vallee, N. Protective effects of fluoxetine on decompression sickness in mice. PLoS ONE 2012, 7, e49069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallee, N.; Meckler, C.; Risso, J.J.; Blatteau, J.E. Neuroprotective role of the TREK-1 channel in decompression sickness. J. Appl. Physiol. (1985) 2012, 112, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High throughput Sequence Data. 2010; Volume 17. p. 2018. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 21 August 2024).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, rapidly, OTUs with galaxy solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar] [CrossRef]
- Ostertagova, E.; Ostertag, O.; Kováč, J. Methodology and application of the Kruskal-Wallis test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Pinard, E.; Rigaud, A.; Riche, D.; Naquet, R.; Seylaz, J. Continuous determination of the cerebrovascular changes induced by bicuculline and kainic acid in unanaesthetized spontaneously breathing rats. Neuroscience 1987, 23, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Evans, K.A.; McEneny, J.; Young, I.S.; Hullin, D.A.; James, P.E.; Ogoh, S.; Ainslie, P.N.; Lucchesi, C.; Rockenbauer, A. Exercise-induced oxidative–nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood–brain barrier leakage. Exp. Physiol. 2011, 96, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R1689–R1694. [Google Scholar] [CrossRef]
- Stacey, M.J.; Hill, N.E.; Parsons, I.T.; Wallace, J.; Taylor, N.; Grimaldi, R.; Shah, N.; Marshall, A.; House, C.; O’Hara, J.P. Relative changes in brain and kidney biomarkers with Exertional Heat Illness during a cool weather marathon. PLoS ONE 2022, 17, e0263873. [Google Scholar] [CrossRef]
- Gempp, E.; Louge, P.; De Maistre, S.; Emile, L.; Blatteau, J.E. Neuron-specific enolase and S100B protein levels in recreational scuba divers with neurological decompression sickness. Diving Hyperb. Med. 2014, 44, 26–29. [Google Scholar]
- Havnes, M.B.; Hjelde, A.; Brubakk, A.O.; Møllerløkken, A. S100B and its relation to intravascular bubbles following decompression. Diving Hyperb. Med. 2010, 40, 210–212. [Google Scholar]
- Havnes, M.B.; Kerlefsen, Y.; Møllerløkken, A. S100B and NSE serum concentrations after simulated diving in rats. Physiol. Rep. 2015, 3, e12546. [Google Scholar] [CrossRef]
- Linér, M.H.; Andersson, J. Hypoxic syncope in a competitive breath-hold diver with elevation of the brain damage marker S100B. Aviat. Space Environ. Med. 2009, 80, 1066–1068. [Google Scholar] [CrossRef]
- Li, J.; Kong, X.; Shu, W.; Zhang, W. The association of serum neuron-specific enolase with other disease markers in chronic obstructive pulmonary disease: A case-control study. Pak. J. Med. Sci. 2018, 34, 1172. [Google Scholar]
- Blatteau, J.E.; Brubakk, A.O.; Gempp, E.; Castagna, O.; Risso, J.J.; Vallee, N. Sidenafil pre-treatment promotes decompression sickness in rats. PLoS ONE 2013, 8, e60639. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063. e1058. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Torres, M.; Montserrat, J.M.; Sanchez-Alcoholado, L.; Cardona, F.; Tinahones, F.J.; Gozal, D.; Poroyko, V.A.; Navajas, D.; Queipo-Ortuño, M.I. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 2015, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Desruelle, A.V.; de Maistre, S.; Gaillard, S.; Richard, S.; Tardivel, C.; Martin, J.C.; Blatteau, J.E.; Boussuges, A.; Rives, S.; Risso, J.J.; et al. Cecal Metabolomic Fingerprint of Unscathed Rats: Does It Reflect the Good Response to a Provocative Decompression? Front. Physiol. 2022, 13, 882944. [Google Scholar] [CrossRef]
- de Maistre, S.; Gaillard, S.; Martin, J.C.; Richard, S.; Boussuges, A.; Rives, S.; Desruelle, A.V.; Blatteau, J.E.; Tardivel, C.; Risso, J.J.; et al. Cecal metabolome fingerprint in a rat model of decompression sickness with neurological disorders. Sci. Rep. 2020, 10, 15996. [Google Scholar] [CrossRef] [PubMed]
- Vallee, N.; Dugrenot, E.; Desruelle, A.V.; Tardivel, C.; Martin, J.C.; Guernec, A.; Boussuges, A.; Rives, S.; Risso, J.J.; Guerrero, F. Evidence of a hormonal reshuffle in the cecal metabolome fingerprint of a strain of rats resistant to decompression sickness. Sci. Rep. 2021, 11, 8317. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- de Maistre, S.; Vallee, N.; Gaillard, S.; Duchamp, C.; Blatteau, J.E. Stimulating fermentation by the prolonged acceleration of gut transit protects against decompression sickness. Sci. Rep. 2018, 8, 10128. [Google Scholar] [CrossRef]
- de Maistre, S.; Vallee, N.; Gempp, E.; Lambrechts, K.; Louge, P.; Duchamp, C.; Blatteau, J.E. Colonic Fermentation Promotes Decompression sickness in Rats. Sci. Rep. 2016, 6, 20379. [Google Scholar] [CrossRef]
- de Maistre, S.; Vallee, N.; Gempp, E.; Louge, P.; Duchamp, C.; Blatteau, J.E. Gut fermentation seems to promote decompression sickness in humans. J. Appl. Physiol. (1985) 2016, 121, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.P.; Layman, J.; Willis, J. Physiologic effects of surgical masking in children versus adults. PeerJ 2023, 11, e15474. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Hirsch, O.; Wagner, S.; Wojtasik, B.; Funken, S.; Klosterhalfen, B.; Kanti Manna, S.; Prescher, A.; Sukul, P.; Sönnichsen, A. Physio-metabolic and clinical consequences of wearing face masks—Systematic review with meta-analysis and comprehensive evaluation. Front. Public Health 2023, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Giboni, P.; Prescher, A.; Klosterhalfen, B.; Graessel, D.; Funken, S.; Kempski, O.; Hirsch, O. Is a mask that covers the mouth and nose free from undesirable side effects in everyday use and free of potential hazards? Int. J. Environ. Res. Public Health 2021, 18, 4344. [Google Scholar] [CrossRef]
- Gireesan, S.; Pandit, A.B. Modeling the effect of carbon-dioxide gas on cavitation. Ultrason. Sonochem. 2017, 34, 721–728. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daubresse, L.; Portas, A.; Bertaud, A.; Marlinge, M.; Gaillard, S.; Risso, J.-J.; Ramdani, C.; Rostain, J.-C.; Adjiriou, N.; Desruelle, A.-V.; et al. CO2 Breathing Prior to Simulated Diving Increases Decompression Sickness Risk in a Mouse Model: The Microbiota Trail Is Not Forgotten. Int. J. Environ. Res. Public Health 2024, 21, 1141. https://doi.org/10.3390/ijerph21091141
Daubresse L, Portas A, Bertaud A, Marlinge M, Gaillard S, Risso J-J, Ramdani C, Rostain J-C, Adjiriou N, Desruelle A-V, et al. CO2 Breathing Prior to Simulated Diving Increases Decompression Sickness Risk in a Mouse Model: The Microbiota Trail Is Not Forgotten. International Journal of Environmental Research and Public Health. 2024; 21(9):1141. https://doi.org/10.3390/ijerph21091141
Chicago/Turabian StyleDaubresse, Lucille, Aurélie Portas, Alexandrine Bertaud, Marion Marlinge, Sandrine Gaillard, Jean-Jacques Risso, Céline Ramdani, Jean-Claude Rostain, Nabil Adjiriou, Anne-Virginie Desruelle, and et al. 2024. "CO2 Breathing Prior to Simulated Diving Increases Decompression Sickness Risk in a Mouse Model: The Microbiota Trail Is Not Forgotten" International Journal of Environmental Research and Public Health 21, no. 9: 1141. https://doi.org/10.3390/ijerph21091141
APA StyleDaubresse, L., Portas, A., Bertaud, A., Marlinge, M., Gaillard, S., Risso, J.-J., Ramdani, C., Rostain, J.-C., Adjiriou, N., Desruelle, A.-V., Blatteau, J.-E., Guieu, R., & Vallée, N. (2024). CO2 Breathing Prior to Simulated Diving Increases Decompression Sickness Risk in a Mouse Model: The Microbiota Trail Is Not Forgotten. International Journal of Environmental Research and Public Health, 21(9), 1141. https://doi.org/10.3390/ijerph21091141





