A Geospatial Analysis of the Lung Cancer Burden in Philadelphia, Using Pennsylvania Cancer Registry Data from 2008–2017
Abstract
:1. Introduction
1.1. Lung Cancer Morbidity and Mortality: A Global and Local Public Health Issue
1.2. Emerging Emphasis on Social and Neighborhood Determinants of Cancer
1.3. Geographic Information Systems and the Assessment of Cancer Burden
2. Materials and Methods
2.1. Data Sources
2.2. Statistical Analysis
3. Results
3.1. Geographic Distribution of SIR and SMR
3.2. Multivariable Model of Census Tract-Level Predictors of SIR and SMR
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar]
- National Center for Health Statistics. Age-Adjusted Lung Cancer Death* Rates, by State—National Vital Statistics System, United States, 2018. Morb. Mortal. Wkly. Rep. 2020, 69, 1273. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevention. Lung Cancer Statistics. Available online: https://www.cdc.gov/lung-cancer/statistics/?CDC_AAref_Val=https://www.cdc.gov/cancer/lung/statistics/index.htm (accessed on 26 October 2024).
- Mariotto, A.B.; Enewold, L.; Zhao, J.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef]
- American Lung Association. State of Lung Cancer: Pennsylvania. State Data. Available online: https://www.lung.org/research/state-of-lung-cancer/states/pennsylvania (accessed on 26 October 2024).
- Pennsylvania Department of Health. Pennsylvania Healthy People, State Level. C-02: Lung Cancer Death Rate. 2023. Available online: https://www.pa.gov/content/dam/copapwp-pagov/en/health/documents/topics/healthstatistics/healthypeople/documents/current/state/c-02-lung-cancer-death-rate.html (accessed on 26 October 2024).
- Pennsylvania Department of Health. The Burden of Cancer in Pennsylvania: A Report of the Cancer Control, Prevention and Research Advisory Board; PA Department of Health: Harrisburg, PA, USA, 2019.
- Camiña, N.; McWilliams, T.L.; McKeon, T.P.; Penning, T.M.; Hwang, W.-T. Identification of spatio-temporal clusters of lung cancer cases in Pennsylvania, USA: 2010–2017. BMC Cancer 2022, 22, 555. [Google Scholar]
- Zhu, Y.; McKeon, T.P.; Tam, V.; Vachani, A.; Penning, T.M.; Hwang, W.-T. Geographic differences in lung cancer incidence: A study of a major metropolitan area within Southeastern Pennsylvania. Int. J. Environ. Res. Public Health 2020, 17, 9498. [Google Scholar] [CrossRef]
- Barta, J.A.; Erkmen, C.P.; Shusted, C.S.; Myers, R.E.; Saia, C.; Cohen, S.; Wainwright, J.; Zeigler-Johnson, C.; Dako, F.; Wender, R.; et al. The Philadelphia Lung Cancer Learning Community: A multi-health-system, citywide approach to lung cancer screening. JNCI Cancer Spectr. 2023, 7, pkad071. [Google Scholar] [CrossRef] [PubMed]
- McIntire, R.K.; Juon, H.-S.; Keith, S.W.; Simone, N.L.; Waters, D.; Lewis, E.; Zeigler-Johnson, C. A novel method for measuring the burden of breast cancer in neighborhoods. Prev. Med. Rep. 2023, 33, 102218. [Google Scholar]
- McIntire, R.K.; Keith, S.W.; Boamah, M.; Leader, A.E.; Glanz, K.; Klassen, A.C.; Zeigler-Johnson, C.M. A prostate cancer composite score to identify high burden neighborhoods. Prev. Med. 2018, 112, 47–53. [Google Scholar] [CrossRef]
- Torres, A.Z.; Phelan-Emrick, D.; Castillo-Salgado, C. Evaluating neighborhood correlates and geospatial distribution of breast, cervical, and colorectal cancer incidence. Front. Oncol. 2018, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- McGee-Avila, J.K.; Richmond, J.; Henry, K.A.; Stroup, A.M.; Tsui, J. Disparities in geospatial patterns of cancer care within urban counties and structural inequities in access to oncology care. Health Serv. Res. 2023, 58, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, J.; Chen, Y.; Liu, Z.; Xia, H.; Xu, H. Gender disparities in lung cancer incidence in the United States during 2001–2019. Sci. Rep. 2023, 13, 12581. [Google Scholar] [CrossRef]
- Patel, M.I.; McKinley, M.; Cheng, I.; Haile, R.; Wakelee, H.; Gomez, S.L. Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: An analysis spanning 28 years. Lung Cancer 2017, 108, 140–149. [Google Scholar] [CrossRef]
- Sung, H.; Nisotel, L.; Sedeta, E.; Islami, F.; Jemal, A. Racial and ethnic disparities in survival among people with second primary cancer in the US. JAMA Netw. Open 2023, 6, e2327429. [Google Scholar] [CrossRef]
- Adie, Y.; Kats, D.J.; Tlimat, A.; Perzynski, A.; Dalton, J.; Gunzler, D.; Tarabichi, Y. Neighborhood disadvantage and lung cancer incidence in ever-smokers at a safety net health-care system: A retrospective study. Chest 2020, 157, 1021–1029. [Google Scholar] [CrossRef]
- Korycinski, R.W.; Tennant, B.L.; Cawley, M.A.; Bloodgood, B.; Oh, A.Y.; Berrigan, D. Geospatial approaches to cancer control and population sciences at the United States cancer centers. Cancer Causes Control 2018, 29, 371–377. [Google Scholar] [CrossRef]
- Fadiel, A.; Eichenbaum, K.D.; Abbasi, M.; Lee, N.K.; Odunsi, K. Utilizing geospatial artificial intelligence to map cancer disparities across health regions. Sci. Rep. 2024, 14, 7693. [Google Scholar] [CrossRef]
- Fu, Z.; Li, Y.; Chu, J.; Sun, J.; Lu, Z.; Zhang, J.; Chen, X.; Zhang, G.; Xue, F.; Guo, X. Lung cancer mortality clusters in urban and rural areas of Shandong Province, China: A spatial scan statistical analysis. Precis. Radiat. Oncol. 2019, 3, 15–22. [Google Scholar] [CrossRef]
- Jiang, F.; Fu, Z.; Lu, Z.; Chu, J.; Xu, A.; Guo, X.; Ma, J. Cancer survival analysis and spatial distribution during 2014–2016 in Shandong Province, China. Sci. Rep. 2023, 13, 10324. [Google Scholar]
- United States Census Bureau. TIGER/Line Shapefiles. Available online: https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html (accessed on 26 October 2024).
- Policy Map. Policy Map: About Us. Available online: https://policymap.wpengine.com/about (accessed on 26 October 2024).
- U.S. Centers for Disease Control and Prevention. Welcome to the 500 Cities & PLACES Data Portal. Available online: https://chronicdata.cdc.gov/browse?category=500+Cities+%26+Places&sortBy=newest&utf8= (accessed on 2 February 2023).
- Surveillance Research Program. SEER*Explorer: An interactive website for SEER cancer statistics. Available online: https://seer.cancer.gov/explorer/ (accessed on 26 October 2024).
- U.S. Census Bureau. Decennial Census by Decade. Available online: https://www.census.gov/programs-surveys/decennial-census/decade.2010.html (accessed on 26 October 2024).
- Moore, K.D. Healthy Places: Towards a Transdisciplinary Mapping of the Theoretical Landscape. Enq. ARCC J. Archit. Res. 2020, 17, 1–20. [Google Scholar]
- Moore, J.X.; Royston, K.J.; Langston, M.E.; Griffin, R.; Hidalgo, B.; Wang, H.E.; Colditz, G.; Akinyemiju, T. Mapping hot spots of breast cancer mortality in the United States: Place matters for Blacks and Hispanics. Cancer Causes Control 2018, 29, 737–750. [Google Scholar]
- Nassel, A.F.; Root, E.D.; Haukoos, J.S.; McVaney, K.; Colwell, C.; Robinson, J.; Eigel, B.; Magid, D.J.; Sasson, C. Multiple cluster analysis for the identification of high-risk census tracts for out-of-hospital cardiac arrest (OHCA) in Denver, Colorado. Resuscitation 2014, 85, 1667–1673. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Richards, T.B.; Doria-Rose, V.P.; Soman, A.; Klabunde, C.N.; Caraballo, R.S.; Gray, S.C.; Houston, K.A.; White, M.C. Lung cancer screening inconsistent with US Preventive Services Task Force recommendations. Am. J. Prev. Med. 2019, 56, 66–73. [Google Scholar]
- Kane, G.C.; Barta, J.A.; Shusted, C.S.; Evans, N.R. Now is the time to make screening for lung cancer reportable. Ann. Intern. Med. 2022, 175, 888–889. [Google Scholar] [PubMed]
- U.S. Preventive Services Task Force. Lung Cancer: Screening; American Medical Association: Chicago, IL, USA, 2021; p. 962. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening (accessed on 19 December 2024).
- Pu, C.Y.; Lusk, C.M.; Neslund-Dudas, C.; Gadgeel, S.; Soubani, A.O.; Schwartz, A.G. Comparison between the 2021 USPSTF lung cancer screening criteria and other lung cancer screening criteria for racial disparity in eligibility. JAMA Oncol. 2022, 8, 374–382. [Google Scholar] [CrossRef] [PubMed]
- McIntire, R.K.; Lewis, E.; Zeigler-Johnson, C.; Shusted, C.; Barta, J.; Juon, H.-S.; Keith, S.W.; Klein, G. Estimating eligibility for lung cancer screening by neighborhood in Philadelphia using previous and current USPSTF guidelines. Popul. Health Manag. 2022, 25, 254–263. [Google Scholar] [CrossRef]
- American Cancer Society. Lung Cancer Survival Rates. Available online: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 26 October 2024).
- Vachani, A.; Carroll, N.M.; Simoff, M.J.; Neslund-Dudas, C.; Honda, S.; Greenlee, R.T.; Rendle, K.A.; Burnett-Hartman, A.; Ritzwoller, D.P. Stage migration and lung cancer incidence after initiation of low-dose computed tomography screening. J. Thorac. Oncol. 2022, 17, 1355–1364. [Google Scholar]
- Wang, K.; Immergluck, D. The geography of vacant housing and neighborhood health disparities after the US foreclosure crisis. Cityscape 2018, 20, 145–170. [Google Scholar]
- Snider, N.G.; Hastert, T.A.; Nair, M.; Kc, M.; Ruterbusch, J.J.; Schwartz, A.G.; Peters, E.S.; Stoffel, E.M.; Rozek, L.S.; Purrington, K.S. Area-level socioeconomic disadvantage and cancer survival in metropolitan Detroit. Cancer Epidemiol. Biomark. Prev. 2023, 32, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Bottomley, A.; Smit, E.; Lianes, P.; Legrand, C.; Debruyne, C.; Schramel, F.; Smit, H.; Gaafar, R.; Biesma, B. Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann. Oncol. 2006, 17, 1698–1704. [Google Scholar] [PubMed]
- Sofianidi, A.; Karadimou, A.; Charpidou, A.; Syrigos, K.N. The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality. Cancers 2024, 16, 906. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Han, S.; Lim, J.U.; Kang, H.S.; Kim, S.K.; Kim, J.W.; Lee, S.H.; Kim, S.J.; Yeo, C.D. Association between quality of life questionnaire at diagnosis and survival in patients with lung cancer. Clin. Lung Cancer 2023, 24, 459–466. [Google Scholar]

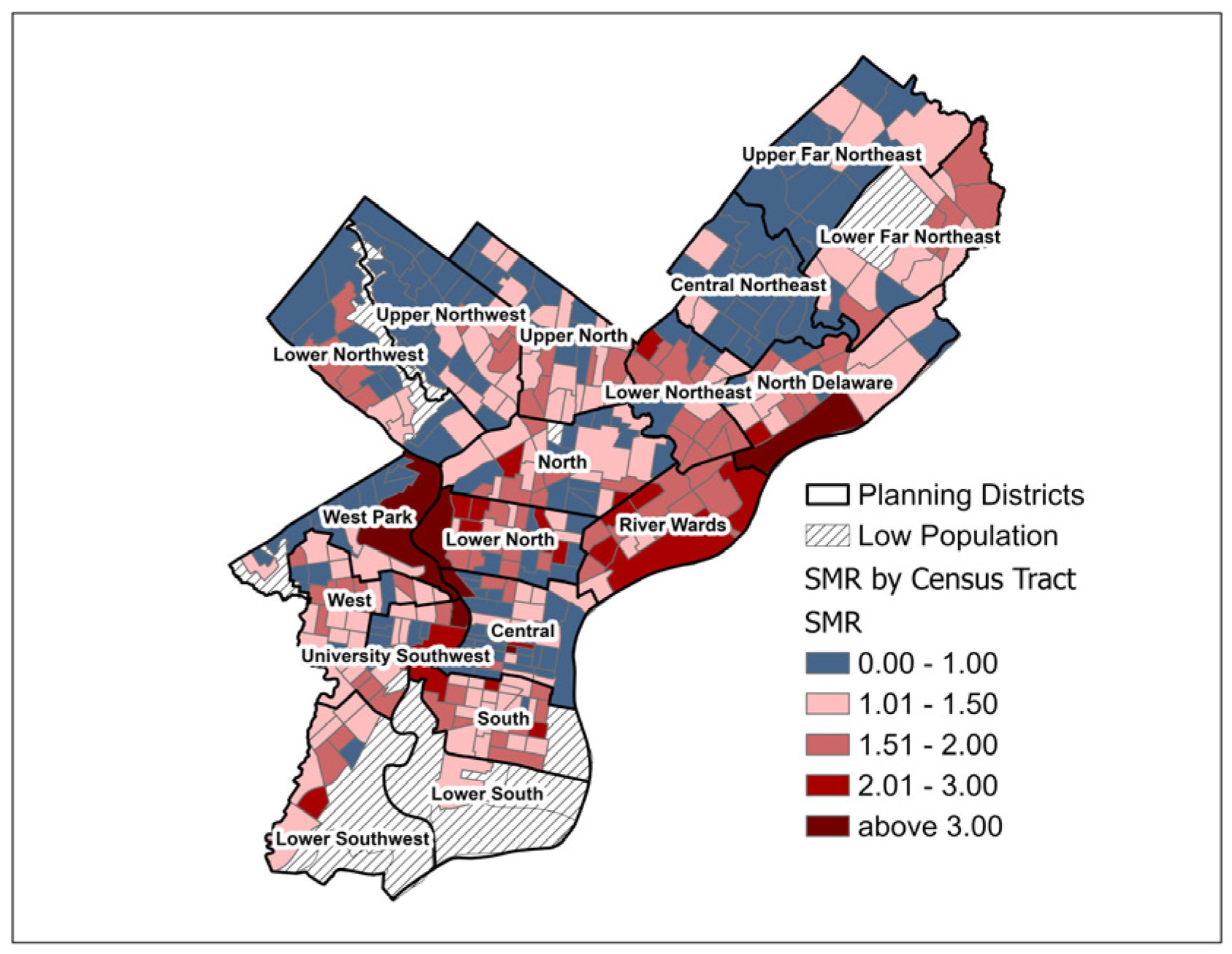

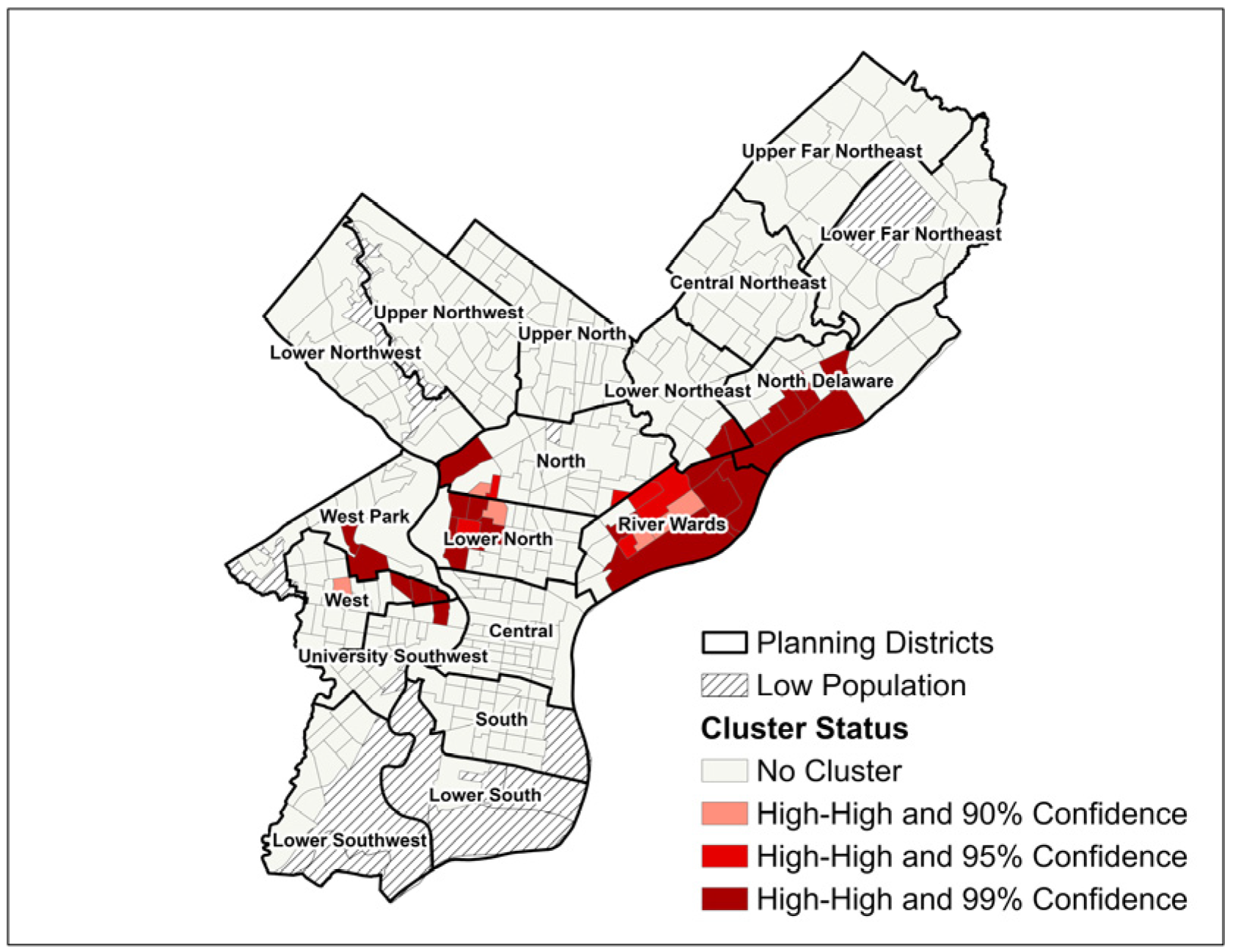
| Census Tract Characteristic | Mean (SD) | Pearson Correlation (SIR) | p-Value (SIR) | Pearson Correlation (SMR) | p-Value (SMR) |
|---|---|---|---|---|---|
| Lung cancer SIR a | 1.46 (0.55) | N/A | N/A | N/A | N/A |
| Lung cancer SMR a | 1.23 (0.54) | N/A | N/A | N/A | N/A |
| Median household income ($) b | 41,030.73 (20,864.95) | −0.325 | <0.001 * | −0.335 | <0.001 * |
| Black or African American (%) b | 44.44 (35.61) | 0.177 | 0.001 * | 0.176 | 0.001 * |
| White (%) b | 41.17 (33.00) | −0.149 | 0.004 * | −0.165 | 0.003 * |
| Asian (%) b | 6.01 (7.78) | −0.167 | 0.001 * | −0.153 | 0.033 * |
| Hispanic (%) b | 10.92 (16.34) | −0.021 | 0.688 | 0.015 | 0.769 |
| Male (%) b | 47.16 (4.08) | −0.001 | 0.984 | 0.007 | 0.896 |
| Homeownership (%) b | 52.71 (18.74) | 0.249 | <0.001 * | 0.244 | <0.001 * |
| Uninsured (%) c | 13.54 (6.17) | −0.023 | 0.662 | −0.032 | 0.544 |
| Adult smoking prevalence (%) d | 23.64 (6.22) | 0.457 | <0.001 * | 0.452 | <0.001 * |
| Diagnosed with COPD (%) d | 7.24 (2.29) | 0.341 | <0.001 * | 0.313 | <0.001 * |
| Obesity (%) e | 34.09 (5.55) | 0.198 | <0.001 * | 0.186 | <0.001 * |
| Achieved recommended physical activity (%) e | 20.58 (2.37) | −0.121 | 0.019 * | −0.105 | 0.042 * |
| Routine exam in past year (%) d | 73.85 (4.89) | 0.008 | 0.882 | −0.011 | 0.837 |
| Reported 14+ poor health days (%) d | 14.92 (4.37) | 0.321 | <0.001 * | 0.310 | <0.001 * |
| Residential addresses vacant (%) c | 13.37 (7.49) | 0.323 | <0.001 * | 0.305 | <0.001 * |
| Census Tract Characteristic | SIR β Coefficient (95% CI) | t-Statistic (SIR) | p-Value (SIR) | SMR β Coefficient (95% CI) | t-Statistic (SMR) | p-Value (SMR) |
|---|---|---|---|---|---|---|
| Median household income | −0.014 (−0.061, 0.034) | −0.568 | 0.570 | −0.021 (−0.069, 0.027) | −0.855 | 0.393 |
| African American (%) | 0.077 (−0.285, 0.439) | 0.417 | 0.677 | −0.113 (−0.481, 0.255) | −0.604 | 0.546 |
| White (%) | 0.086 (−0.253, 0.425) | 0.500 | 0.617 | −0.123 (−0.468, 0.221) | −0.703 | 0.482 |
| Asian (%) | 0.132 (−0.266, 0.530) | 0.654 | 0.513 | −0.077 (−0.482, 0.327) | −0.375 | 0.708 |
| Hispanic (%) | 0.041 (−0.231, 0.313) | 0.296 | 0.767 | −0.036 (−0.312, 0.240) | −0.255 | 0.799 |
| Male (%) | −0.112 (−0.294, 0.069) | −1.220 | 0.223 | −0.152 (−0.336, 0.032) | −1.620 | 0.106 |
| Homeownership (%) | 0.011 (−0.030, 0.052) | 0.520 | 0.603 | 0.018 (−0.023, 0.060) | 0.864 | 0.388 |
| Uninsured (%) | 0.027 (−0.079, 0.133) | 0.500 | 0.618 | −0.002 (−0.110, 0.106) | −0.035 | 0.972 |
| Adult smoking prevalence (%) | 0.645 (0.163, 1.128) | 2.632 | 0.009 | 0.673 (0.183, 1.163) | 2.703 | 0.007 |
| Diagnosed with COPD (%) | 2.805 (0.580, 5.030) | 2.479 | 0.014 | 2.995 (0.734, 5.255) | 2.605 | 0.010 |
| Obesity (%) | 0.613 (0.012, 1.214) | 2.006 | 0.046 | 0.499 (−0.112, 1.109) | 1.606 | 0.109 |
| Achieved recommended physical activity (%) | 0.450 (−0.400, 1.299) | 1.041 | 0.299 | 0.530 (−0.333, 1.393) | 1.208 | 0.228 |
| Routine exam in past year (%) | −0.603 (−1.271, 0.064) | −1.777 | 0.076 | −0.525 (−1.203, 0.154) | −1.521 | 0.129 |
| Reported 14+ poor health days (%) | −1.918 (−3.499, −0.337) | −2.386 | 0.018 | −2.144 (−3.750, −0.538) | −2.625 | 0.009 |
| Residential addresses vacant (%) | 0.130 (0.050, 0.209) | 3.206 | 0.001 | 0.112 (0.031, 0.192) | 2.716 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntire, R.K.; Senter, K.; Shusted, C.; Yearwood, R.; Barta, J.; Keith, S.W.; Zeigler-Johnson, C. A Geospatial Analysis of the Lung Cancer Burden in Philadelphia, Using Pennsylvania Cancer Registry Data from 2008–2017. Int. J. Environ. Res. Public Health 2025, 22, 455. https://doi.org/10.3390/ijerph22030455
McIntire RK, Senter K, Shusted C, Yearwood R, Barta J, Keith SW, Zeigler-Johnson C. A Geospatial Analysis of the Lung Cancer Burden in Philadelphia, Using Pennsylvania Cancer Registry Data from 2008–2017. International Journal of Environmental Research and Public Health. 2025; 22(3):455. https://doi.org/10.3390/ijerph22030455
Chicago/Turabian StyleMcIntire, Russell K., Katherine Senter, Christine Shusted, Rickisa Yearwood, Julie Barta, Scott W. Keith, and Charnita Zeigler-Johnson. 2025. "A Geospatial Analysis of the Lung Cancer Burden in Philadelphia, Using Pennsylvania Cancer Registry Data from 2008–2017" International Journal of Environmental Research and Public Health 22, no. 3: 455. https://doi.org/10.3390/ijerph22030455
APA StyleMcIntire, R. K., Senter, K., Shusted, C., Yearwood, R., Barta, J., Keith, S. W., & Zeigler-Johnson, C. (2025). A Geospatial Analysis of the Lung Cancer Burden in Philadelphia, Using Pennsylvania Cancer Registry Data from 2008–2017. International Journal of Environmental Research and Public Health, 22(3), 455. https://doi.org/10.3390/ijerph22030455






