A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air
Abstract
:1. Introduction
2. Methods
2.1. Literature sources
2.2. Data analysis
3. Results
3.1. Emission sources of ambient and indoor naphthalene
3.1.1. Combustion sources
3.1.2. Pyrolysis sources
3.1.3. Off-gassing and volatilization
3.1.4. Emission inventories
3.2. Exposure concentrations
3.2.1. Previous Reviews
3.2.2. Indoor concentrations
3.2.3. Determinants of indoor concentrations
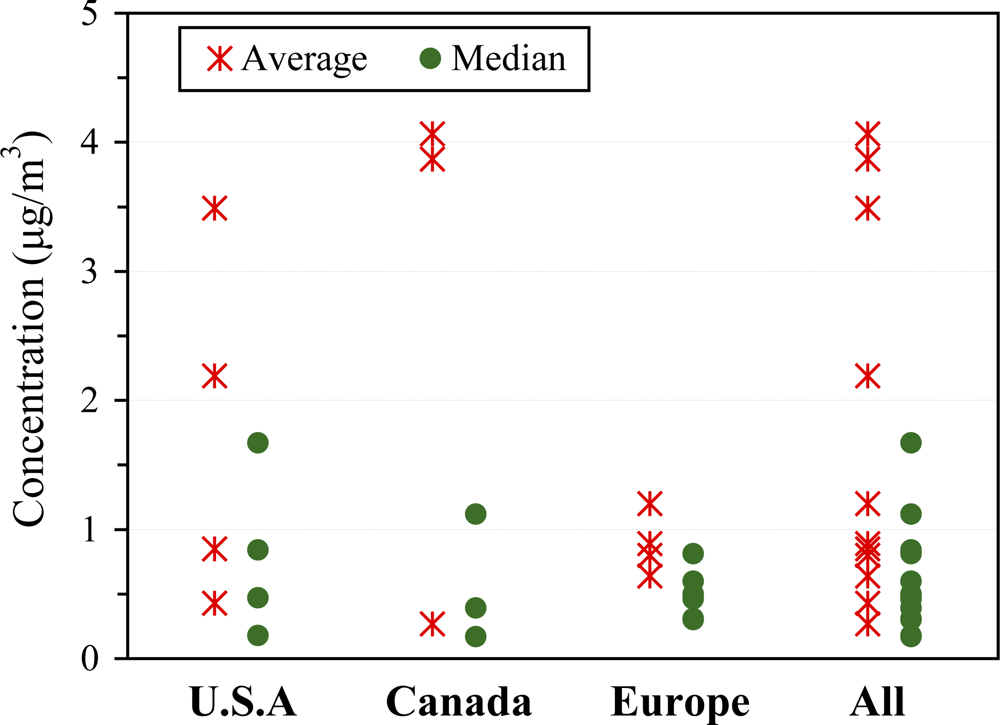
3.2.4. Outdoor concentrations
3.2.5. Determinants of outdoor concentrations
3.2.6. Personal exposures
3.3. Health risk assessment
4. Discussion
4.1. Information gaps
4.2. Measurement issues
5. Conclusion
Acknowledgments
References
- CEH. Chemical Economics Handbook, V. 27; SRI International: Menlo Park, CA, USA, 2000. [Google Scholar]
- ATSDR. Toxicological Profile for Naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005; pp. 1–291. [Google Scholar]
- NTP. Toxicology and Carcinogenesis Studies of Naphthalene (CAS No 91-20-3) in F344/N Rats (Inhalation Studies) Technical Report Series No 500; National Toxicology Program: Research Triangle Park, NC, USA, 2000; pp. 1–173. [Google Scholar]
- US EPA. Compendium Method TO-15, Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatograpy/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999; p. 1. [Google Scholar]
- Price, PS; Jayjock, MA. Available data on naphthalene exposures: Strengths and limitations. Regul. Toxicol. Pharm 2008, 51, S15–S21. [Google Scholar]
- Chuang, JC; Callahan, PJ; Lyu, CW; Wilson, NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J. Expo. Anal. Environ. Epidemiol 1999, 9, 85–98. [Google Scholar]
- Wilson, NK; Chuang, JC; Lyu, C; Menton, R; Morgan, MK. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J. Expo. Anal. Environ. Epidemiol 2003, 13, 187–202. [Google Scholar]
- Li, Z; Mulholland, JA; Romanoff, LC; Pittman, EN; Trinidad, DA; Lewin, MD; Sjodin, A. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. J. Environ. Monit 2010, 12, 1110–1118. [Google Scholar]
- Howard, PH. Handbook of Environmental Fate and Exposure Data for Organic Chemicals; Lewis Publishers: Chelsea, MI, USA, 1989; pp. 408–421. [Google Scholar]
- Nazaroff, WW; Singer, BC. Inhalation of hazardous air pollutants from environmental tobacco smoke in US residences. J. Expo. Anal. Environ. Epidemiol 2003, 14, S71–S77. [Google Scholar]
- Preuss, R; Angerer, J; Drexler, H. Naphthalene–an environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 2003, 76, 556–576. [Google Scholar]
- Robinson, MS; Anthony, TR; Littau, SR; Herckes, P; Nelson, X; Poplin, GS; Burgess, JL. Occupational PAH exposures during prescribed pile burns. Ann. Occup. Hyg 2008, 52, 497–508. [Google Scholar]
- Stohs, SJ; Ohia, S; Bagchi, D. Naphthalene toxicity and antioxidant nutrients. Toxicology 2002, 180, 97–105. [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans–Naphthalene; IARC: Lyon, France, 2002; pp. 367–435. [Google Scholar]
- US EPA. Toxicological Review of Naphthalene; U.S. Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–42. [Google Scholar]
- NTP. Report on Carcinogens - Naphthalene, Eleventh Edition; U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2004; pp. 1–2. [Google Scholar]
- US EPA. Integrated Risk Information System–Naphthalene. Available online: http://www.epa.gov/iris/subst/0436.htm#refinhal/ (accessed on 16 March 2010).
- US EPA. Toxicological Review of Naphthalene [External Review Draft]; U.S. Environmental Protection Agency: Washington, DC, USA, 2004; pp. 1–74. [Google Scholar]
- Belzer, RB; Bus, JS; Cavalieri, EL; Lewis, SC; North, DW; Pleus, RC. The naphthalene state of the science symposium: Objectives, organization, structure, and charge. Regul. Toxicol. Pharm 2008, 51, S1–S5. [Google Scholar]
- Griego, FY; Bogen, KT; Price, PS; Weed, DL. Exposure, epidemiology and human cancer incidence of naphthalene. Regul. Toxicol. Pharm 2008, 51, S22–S26. [Google Scholar]
- US EPA. Summary of Results for the 2002 National-Scale Assessment. Available online: http://www.epa.gov/ttn/atw/nata2002/risksum.html (accessed on 16 March 2010).
- OEHHA. Air Toxics Hot Spots Program Risk Assessment Guidelines Part II: Technical Support Document for Cancer Potency Factors, Appendix B; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2009; p. B91. [Google Scholar]
- Magee, B; Samuelian, J; Haines, K; Chappel, M; Penn, I; Chin, D; Anders, D; Hinz, J. Screening-level population risk assessment of nasal tumors in the US due to naphthalene exposure. Regul. Toxicol. Pharm 2010, 57, 168–180. [Google Scholar]
- WHO. Development of WHO Guidelines for Indoor Air Quality; World Health Organization: Geneva, Switzerland, 2006; pp. 1–15. [Google Scholar]
- Klepeis, NE; Nelson, WC; Ott, WR; Robinson, JP; Tsang, AM; Switzer, P; Behar, JV; Hern, SC; Engelmann, WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol 2001, 11, 231–252. [Google Scholar]
- Leech, JA; Nelson, WC; Burnett, RT; Aaron, S; Raizenne, ME. It’s about time: A comparison of Canadian and American time-activity patterns. J. Expo. Anal. Environ. Epidemiol 2002, 12, 427–432. [Google Scholar]
- NIOSH National Occupational Exposure Survey, Estimated Numbers of Employees Potentially Exposed to Specific Agents by 2-Digit Standard Industrial Classification (SIC)–Naphthalene. Available online: http://www.cdc.gov/noes/noes1/49600sic.html/ (accessed on 16 March 2010).
- Preuss, R; Drexler, H; Bottcher, M; Wilhelm, M; Bruning, T; Angerer, J. Current external and internal exposure to naphthalene of workers occupationally exposed to polycyclic aromatic hydrocarbons in different industries. Int. Arch. Occup. Environ. Health 2005, 78, 355–362. [Google Scholar]
- Rappaport, SM; Waidyanatha, S; Serdar, B. Naphthalene and its biomarkers as measures of occupational exposure to polycyclic aromatic hydrocarbons. J. Environ. Monit 2004, 6, 413–416. [Google Scholar]
- ATSDR. ATSDR Minimal Risk Levels; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2009; p. 9. [Google Scholar]
- OEHHA. Air Toxics Hot Spots Program Risk Assessment Guidelines Part III. The Determination of Chronic Reference Exposure Levels for Airborne Toxicants; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2000. [Google Scholar]
- MDEQ. Michigan Air Toxic Program, Screening Level List; Michigan Department of Environmental Quality: Lansing, MI, USA, 2010. [Google Scholar]
- OSHA. 1910.1000 TABLE Z-1, Permissible Exposure Limits for Air Contaminants; Occupational Safety & Health Administration: Washington, DC, USA, 2001. [Google Scholar]
- NIOSH. NIOSH Pocket Guide to Chemical Hazards - Naphthalene, Publication Number 2005-149; National Institute for Occupational Safety and Health: Atlanta, GA, USA, 2005. [Google Scholar]
- ACGIH. ACGIH 2009 TLVs® and BEIs® Book; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2009. [Google Scholar]
- Loh, MM; Levy, JI; Spengler, JD; Houseman, EA; Bennett, DH. Ranking cancer risks of organic hazardous air pollutants in the United States. Environ. Health Perspect 2007, 115, 1160–1168. [Google Scholar]
- Brown, SK; Sim, MR; Abramson, MJ; Gray, CN. Concentrations of volatile organic-compounds in indoor air–A review. Indoor Air 1994, 4, 123–134. [Google Scholar]
- Jia, CR; D’Souza, J; Batterman, S. Distributions of personal VOC exposures: A population-based analysis. Environ. Int 2008, 34, 922–931. [Google Scholar]
- Ravindra, K; Sokhi, R; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ 2008, 42, 2895–2921. [Google Scholar] [Green Version]
- Lu, R; Wu, J; Turco, RP; Winer, AM; Atkinson, R; Arey, J; Paulson, SE; Lurmann, FW; Miguel, AH; Eiguren-Fernandez, A. Naphthalene distributions and human exposure in a Southern California. Atmos. Environ 2005, 39, 489–507. [Google Scholar]
- Jenkins, BM; Jones, AD; Turn, SQ; Williams, RB. Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environ. Sci. Technol 1996, 30, 2462–2469. [Google Scholar]
- Yang, HH; Lee, WJ; Chen, SJ; Lai, SO. PAH emission from various industrial stacks. J. Hazard. Mater 1998, 60, 159–174. [Google Scholar]
- Li, CT; Mi, HH; Lee, WJ; You, WC; Wang, YF. PAH emission from the industrial boilers. J. Hazard. Mater 1999, 69, 1–11. [Google Scholar]
- Charles, SM; Jia, C; Batterman, SA; Godwin, C. VOC and particulate emissions from commercial cigarettes: Analysis of 2,5-DMF as an ETS tracer. Environ. Sci. Technol 2008, 42, 1324–1331. [Google Scholar]
- Charles, SM; Batterman, SA; Jia, CR. Composition and emissions of VOCs in main- and side-stream smoke of research cigarettes. Atmos. Environ 2007, 41, 5371–5384. [Google Scholar]
- Yang, HH; Jung, RC; Wang, YF; Hsieh, LT. Polycyclic aromatic hydrocarbon emissions from joss paper furnaces. Atmos. Environ 2005, 39, 3305–3312. [Google Scholar]
- Singer, BC; Hodgson, AT; Guevarra, KS; Hawley, EL; Nazaroff, WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ. Sci. Technol 2002, 36, 846–853. [Google Scholar]
- Lee, RGM; Coleman, P; Jones, JL; Jones, KC; Lohmann, R. Emission factors and importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the domestic burning of coal and wood in the UK. Environ. Sci. Technol 2005, 39, 1436–1447. [Google Scholar]
- Oanh, NTK; Albina, DO; Ping, L; Wang, XK. Emission of particulate matter and polycyclic aromatic hydrocarbons from select cookstove-fuel systems in Asia. Biomass Bioenerg 2005, 28, 579–590. [Google Scholar]
- Schauer, JJ; Kleeman, MJ; Cass, GR; Simoneit, BRT. Measurement of emissions from air pollution sources. 5. C-1-C-32 organic compounds from gasoline-powered motor vehicles. Environ. Sci. Technol 2002, 36, 1169–1180. [Google Scholar]
- Kakareka, SV; Kukharchyk, TI; Khomich, VS. Study of PAH emission from the solid fuels combustion in residential furnaces. Environ. Pollut 2005, 133, 383–387. [Google Scholar]
- Schauer, JJ; Kleeman, MJ; Cass, GR; Simoneit, BRT. Measurement of emissions from air pollution sources. 3. C-1-C-29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol 2001, 35, 1716–1728. [Google Scholar]
- Shah, SD; Ogunyoku, TA; Miller, JW; Cocker, DR. On-road emission rates of PAH and n-alkane compounds from heavy-duty diesel vehicles. Environ. Sci. Technol 2005, 39, 5276–5284. [Google Scholar]
- Oanh, NTK; Reutergardh, LB; Dung, NT. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environ. Sci. Technol 1999, 33, 2703–2709. [Google Scholar]
- Chen, YC; Lee, WJ; Uang, SN; Lee, SH; Tsai, PJ. Characteristics of polycyclic aromatic hydrocarbon (PAH) emissions from a UH-1H helicopter engine and its impact on the ambient environment. Atmos. Environ 2006, 40, 7589–7597. [Google Scholar]
- Cooper, DA. Exhaust emissions from ships at berth. Atmos. Environ 2003, 37, 3817–3830. [Google Scholar]
- Cooper, DA. Exhaust emissions from high speed passenger ferries. Atmos. Environ 2001, 35, 4189–4200. [Google Scholar]
- Lu, H; Zhu, LZ; Zhu, N. Polycyclic aromatic hydrocarbon emission from straw burning and the influence of combustion parameters. Atmos. Environ 2009, 43, 978–983. [Google Scholar]
- Kakareka, SV; Kukharchyk, TI. PAH emission from the open burning of agricultural debris. Sci. Total Environ 2003, 308, 257–261. [Google Scholar]
- Won, D; Magee, RJ; Yang, W; Lusztyk, E; Nong, G; Shaw, CY. A Material Emission Database for 90 Target VOCs. In 10th International Conference on Indoor Air Quality and Climate (Indoor Air 2005); Tsinghua University Press: Beijing, China, 2005; pp. 2070–2075. [Google Scholar]
- CEH. Chemical Economics Handbook. File 359 on DIALOG; DIALOG Information Services, Inc: Palo Alto, CA, USA, 1993. [Google Scholar]
- US EPA. Reregistration Eligibility Decision for Naphthalene, EPA 738-R-07-010; U.S. Environmental Protection Agency: Washington, DC, USA, 2008. [Google Scholar]
- Lima, ALC; Farrington, JW; Reddy, CM. Combustion-derived polycyclic aromatic hydrocarbons in the environment–A review. Environ. Forensics 2005, 6, 109–131. [Google Scholar]
- Mastral, AM; Callen, MS. A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environ. Sci. Technol 2000, 34, 3051–3057. [Google Scholar]
- Lemieux, PM; Lutes, CC; Santoianni, DA. Emissions of organic air toxics from open burning: a comprehensive review. Prog. Energy Combust. Sci 2004, 30, 1–32. [Google Scholar]
- Yuan, HS; Tao, S; Li, BG; Lang, C; Cao, J; Coveney, RM. Emission and outflow of polycyclic aromatic hydrocarbons from wildfires in China. Atmos. Environ 2008, 42, 6828–6835. [Google Scholar]
- Masclet, P; Cachier, H; Liousse, C; Wortham, H. Emissions of polycyclic aromatic-hydrocarbons by savanna fires. J. Atmos. Chem 1995, 22, 41–54. [Google Scholar]
- Damoah, R; Spichtinger, N; Forster, C; James, P; Mattis, I; Wandinger, U; Beirle, S; Wagner, T; Stohl, A. Around the world in 17 days–hemispheric-scale transport of forest fire smoke from Russia in May 2003. Atmos. Chem. Phys 2004, 4, 1311–1321. [Google Scholar]
- Forster, C; Wandinger, U; Wotawa, G; James, P; Mattis, I; Althausen, D; Simmonds, P; O’Doherty, S; Jennings, SG; Kleefeld, C; Schneider, J; Trickl, T; Kreipl, S; Jager, H; Stohl, A. Transport of boreal forest fire emissions from Canada to Europe. J. Geophys. Res. Atmos 2001, 106, 22887–22906. [Google Scholar]
- Moir, D; Rickert, WS; Levasseur, G; Larose, Y; Maertens, R; White, P; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol 2008, 21, 494–502. [Google Scholar]
- Zhang, L; Jiang, Z; Tong, J; Wang, Z; Han, Z; Zhang, J. Using charcoal as base material reduces mosquito coil emissions of toxins. Indoor Air 2010, 20, 176–184. [Google Scholar]
- Chen, SJ; Su, HB; Chang, JE; Lee, WJ; Huang, KL; Hsieh, LT; Huang, YC; Lin, WY; Lin, CC. Emissions of polycyclic aromatic hydrocarbons (PAHs) from the pyrolysis of scrap tires. Atmos. Environ 2007, 41, 1209–1220. [Google Scholar]
- Chien, YC; Liang, CP; Shih, PH. Emission of polycyclic aromatic hydrocarbons from the pyrolysis of liquid crystal wastes. J. Hazard. Mater 2009, 170, 910–914. [Google Scholar]
- Zhu, LZ; Wang, J. Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air, China. Chemosphere 2003, 50, 611–618. [Google Scholar]
- Marr, LC; Kirchstetter, TW; Harley, RA; Miguel, AH; Hering, SV; Hammond, SK. Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environ. Sci. Technol 1999, 33, 3091–3099. [Google Scholar]
- Harley, RA; Coulter-Burke, SC; Yeung, TS. Relating liquid fuel and headspace vapor composition for California reformulated gasoline samples containing ethanol. Environ. Sci. Technol 2000, 34, 4088–4094. [Google Scholar]
- McDougal, JN; Pollard, DL; Weisman, W; Garrett, CM; Miller, TE. Assessment of skin absorption and penetration of JP-8 jet fuel and its components. Toxicol. Sci 2000, 55, 247–255. [Google Scholar]
- HSDB Hazardous Substances Data Bank–Naphthalene. Available online: http://sis.nlm.nih.gov/ (accessed on 16 March 2010).
- Nielsen. Brand Rank Report for Total Moth Preventatives, 52 Weeks Ending 12/29/07; AC Nielsen: New York, NY, USA, 2008. [Google Scholar]
- Jo, WK; Lee, JH; Lim, HJ; Jeong, WS. Naphthalene emissions from moth repellents or toilet deodorant blocks determined using head-space and small-chamber tests. J. Environ. Sci. (China) 2008, 20, 1012–1017. [Google Scholar]
- De Coensel, N; Desmet, K; Sandra, P; Gorecki, T. Domestic sampling: Exposure assessment to moth repellent products using ultrasonic extraction and capillary GC-MS. Chemosphere 2008, 71, 711–716. [Google Scholar]
- Wilke, O; Jann, O; Brödner, D. VOC- and SVOC-emissions from adhesives, floor coverings and complete floor structures. Indoor Air 2004, 14, 98–107. [Google Scholar]
- Van Winkle, MR; Scheff, PA. Volatile organic compounds, polycyclic aromatic hydrocarbons and elements in the air of ten urban homes. Indoor Air-Int. J. Indoor Air Qual. Clim 2001, 11, 49–64. [Google Scholar]
- Baldasano, JM; Guereca, LP; Lopez, E; Gasso, S; Jimenez-Guerrero, P. Development of a high-resolution (1 km x 1 km, 1 h) emission model for Spain: The High-Elective Resolution Modelling Emission System (HERMES). Atmos. Environ 2008, 42, 7215–7233. [Google Scholar]
- US EPA. Toxic Release Inventory (TRI). Available online: http://www.epa.gov/triexplorer/chemical.htm/ (accessed on 30 November 2009).
- US EPA. National Emissions Inventory (NEI). Available online: http://www.epa.gov/ttn/chief/eiinformation.html/ (accessed on 30 November 2009).
- .
- .
- Scottish EPA.
- .
- Eiguren-Fernandez, A; Miguel, AH; Froines, JR; Thurairatnam, S; Avol, EL. Seasonal and spatial variation of polycyclic aromatic hydrocarbons in vapor-phase and PM 2.5 in Southern California urban and rural communities. Aerosol Sci. Technol 2004, 38, 447–455. [Google Scholar]
- Harrison, RM; Smith, DJT; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ. Sci. Technol 1996, 30, 825–832. [Google Scholar]
- Lai, CH; Chen, KS; Wang, HK. Influence of rice straw burning on the levels of polycyclic aromatic hydrocarbons in agricultural county of Taiwan. J. Environ. Sci. (China) 2009, 21, 1200–1207. [Google Scholar]
- Fang, GC; Chang, KF; Lu, CS; Bai, HL. Estimation of PAHs dry deposition and BaP toxic equivalency factors (TEFs) study at Urban, Industry Park and rural sampling sites in central Taiwan, Taichung. Chemosphere 2004, 55, 787–796. [Google Scholar]
- Atkinson, R; Arey, J. Atmospheric chemistry of gas-phase polycyclic aromatic-hydrocarbons–formation of atmospheric mutagens. Environ. Health Perspect 1994, 102, 117–126. [Google Scholar]
- US EPA. Indoor Concentrations of Environmental Carcinogens; Environmental Criteria and Assessment Office: Research Triangle Park, NC, USA, 1991. [Google Scholar]
- Holcomb, LC; Seabrook, BS. Review: Indoor concentrations of volatile organic compounds: Implications for comfort, health and regulation. Indoor Built. Environ 1995, 4, 7–26. [Google Scholar]
- Hodgson, AT; Levin, H. Volatile Organic Compounds in Indoor Air: A Review of Concentrations Measured in North America since 1990; LBNL-51715; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2003. [Google Scholar]
- Wang, SB; Ang, HM; Tade, MO. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int 2007, 33, 694–705. [Google Scholar]
- Shah, JJ; Singh, HB. Distribution of volatile organic chemicals in outdoor and indoor air–A national VOCs data base. Environ. Sci. Technol 1988, 22, 1381–1388. [Google Scholar]
- Dawson, HE; McAlary, T. A compilation of statistics for VOCs from post-1990 indoor air concentration studies in North American residences unaffected by subsurface vapor intrusion. Ground Water Monit. Remediat 2009, 29, 60–69. [Google Scholar]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ. Chem. Lett 2007, 5, 169–195. [Google Scholar]
- Chang, KF; Fang, GC; Chen, JC; Wu, YS. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: A review from 1999 to 2004. Environ. Pollut 2006, 142, 388–396. [Google Scholar]
- Zhang, Z; Guo, B; Zhang, JS. Determination of volatile organic compounds in residential buildings. International Conderence on Indoor Air Quality Problems and Engineering Solutions, Research Triangle Park, NC, USA, 21–23 July 2003.
- Brown, SK. Volatile organic pollutants in new and established buildings in Melbourne, Australia. Indoor Air 2002, 12, 55–63. [Google Scholar]
- Ward, TJ; Underberg, H; Jones, D; Hamilton, RF; Adams, E. Indoor/ambient residential air toxics results in rural western Montana. Environ. Monit. Assess 2009, 153, 119–126. [Google Scholar]
- Jia, C; Batterman, S; Godwin, C. VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ 2008, 42, 2083–2100. [Google Scholar]
- Jia, C; Batterman, S; Godwin, C. VOCs in industrial, urban and suburban neighborhoods, Part 2: Factors affecting indoor and outdoor concentrations. Atmos. Environ 2008, 42, 2101–2116. [Google Scholar]
- Li, A; Schoonover, TM; Zou, Q; Norlock, F; Conroy, LM; Scheff, PA; Wadden, RA. Polycyclic aromatic hydrocarbons in residential air of ten Chicago area homes: Concentrations and influencing factors. Atmos. Environ 2005, 39, 3491–3501. [Google Scholar]
- Van Winkle, MR; Scheff, PA. Volatile organic compounds, polycyclic aromatic hydrocarbons and elements in the air of ten urban homes. Indoor Air 2001, 11, 49–64. [Google Scholar]
- Chuang, JC; Mack, GA; Kuhlman, MR; Wilson, NK. Polycyclic aromatic-hydrocarbons and their derivatives in indoor and outdoor air in an 8-home study. Atmos. Environ. Part B-Urban Atmosphere 1991, 25, 369–380. [Google Scholar]
- Heroux, M-E; Gauvin, D; Gilbert, NL; Guay, M; Dupuis, G; Legris, M; Levesque, B. Housing characteristics and indoor concentrations of selected volatile organic compounds (VOCs) in Quebec City, Canada. Indoor Built. Environ 2008, 17, 128–137. [Google Scholar]
- Zhu, JP; Newhook, R; Marro, L; Chan, CC. Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environ. Sci. Technol 2005, 39, 3964–3971. [Google Scholar]
- Sanderson, EG; Farant, JP. Indoor and outdoor polycyclic aromatic hydrocarbons in residences surrounding a Soderberg aluminum smelter in Canada. Environ. Sci. Technol 2004, 38, 5350–5356. [Google Scholar]
- Fellin, P; Otson, R. Assessment of the influence of climatic factors on concentration levels of volatile organic compounds (Vocs) in Canadian homes. Atmos. Environ 1994, 28, 3581–3586. [Google Scholar]
- Kim, YM; Harrad, S; Harrison, RM. Concentrations and sources of VOCs in urban domestic and public microenvironments. Environ. Sci. Technol 2001, 35, 997–1004. [Google Scholar]
- Schlink, U; Rehwagen, M; Damm, M; Richter, M; Borte, M; Herbarth, O. Seasonal cycle of indoor-VOCs: comparison of apartments and cities. Atmos. Environ 2004, 38, 1181–1190. [Google Scholar]
- Rehwagen, M; Schlink, U; Herbarth, O. Seasonal cycle of VOCs in apartments. Indoor Air 2003, 13, 283–291. [Google Scholar]
- Zorn, C; Kohler, M; Weis, N; Scharenberg, W. Proposal for assessment of indoor air polycyclic aromatic hydrocarbon (PAH). In 10th International Conference on Indoor Air Quality and Climate (Indoor Air 2005); Yang, X, Zhao, B, Zhao, R, Eds.; Tsinghua University Press: Beijing, China, 2005; pp. 2535–2540. [Google Scholar]
- Hippelein, M. Background concentrations of individual and total volatile organic compounds in residential indoor air of Schleswig-Holstein, Germany. J. Environ. Monit 2004, 6, 745–752. [Google Scholar]
- Edwards, RD; Jurvelin, J; Saarela, K; Jantunen, M. VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland. Atmos. Environ 2001, 35, 4531–4543. [Google Scholar]
- Kostiainen, R. Volatile organic compounds in the indoor air of normal and sick houses. Atmos. Environ 1995, 29, 693–702. [Google Scholar]
- Liu, YJ; Zhu, LZ; Shen, XY. Polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air of Hangzhou, China. Environ. Sci. Technol 2001, 35, 840–844. [Google Scholar]
- Viau, C; Hakizimana, G; Bouchard, M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int. Arch. Occup. Environ. Health 2000, 73, 331–338. [Google Scholar]
- Batterman, S; Hatzvasilis, G; Jia, CR. Concentrations and emissions of gasoline and other vapors from residential vehicle garages. Atmos. Environ 2006, 40, 1828–1844. [Google Scholar]
- Batterman, S; Jia, CR; Hatzivasilis, G. Migration of volatile organic compounds from attached garages to residences: A major exposure source. Environ. Res 2007, 104, 224–240. [Google Scholar]
- Gustafson, P; Ostman, C; Sallsten, G. Indoor levels of polycyclic aromatic hydrocarbons in homes with or without wood burning for heating. Environ. Sci. Technol 2008, 42, 5074–5080. [Google Scholar]
- Li, CS; Ro, YS. Indoor characteristics of polycyclic aromatic hydrocarbons in the urban atmosphere of Taipei. Atmos. Environ 2000, 34, 611–620. [Google Scholar]
- Zou, SC; Lee, SC; Chan, CY; Ho, KF; Wang, XM; Chan, LY; Zhang, ZX. Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China. Chemosphere 2003, 51, 1015–1022. [Google Scholar]
- Zielinska, B; Fujita, E; Sagebiel, J; Harshfield, G; Uberna, E; Hayes, T; Keene, F. Arizona hazardous air pollutants monitoring program. J. Air Waste Manage. Assoc 1998, 48, 1038–1050. [Google Scholar]
- Reisen, F; Arey, J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ. Sci. Technol 2005, 39, 64–73. [Google Scholar]
- White, DH; Hardy, JW. Ambient air concentrations of PCDDs, PCDFs, coplanar PCBs, and PAHs at the Mississippi Sandhill Crane National-wildlife-refuge, Jackson county, Mississippi. Environ. Monit. Assess 1994, 33, 247–256. [Google Scholar]
- Miller, L; Xu, XH; Luginaah, I. Spatial Variability of Volatile Organic Compound Concentrations in Sarnia, Ontario, Canada. J. Toxicol. Env. Heal. A–Current Issues 2009, 72, 610–624. [Google Scholar]
- You, XQ; Senthilselvan, A; Cherry, NM; Kim, HM; Burstyn, I. Determinants of airborne concentrations of volatile organic compounds in rural areas of Western Canada. J. Expo. Sci. Environ. Epidemiol 2008, 18, 117–128. [Google Scholar]
- Srivastava, A. Variability in VOC concentrations in an urban area of Delhi. Environ. Monit. Assess 2005, 107, 363–373. [Google Scholar]
- Srivastava, A; Joseph, AE; Devotta, S. Volatile organic compounds in ambient air of Mumbai–India. Atmos. Environ 2006, 40, 892–903. [Google Scholar]
- Park, SS; Kim, YJ; Kang, CH. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ 2002, 36, 2917–2924. [Google Scholar]
- McCarthy, MC; Hafner, HR; Chinkin, LR; Charrier, JG. Temporal variability of selected air toxics in the United States. Atmos. Environ 2007, 41, 7180–7194. [Google Scholar]
- Prevedouros, K; Brorstrom-Lunden, E; Halsall, CJ; Jones, KC; Lee, RGM; Sweetman, AJ. Seasonal and long-term trends in atmospheric PAH concentrations: Evidence and implications. Environ. Pollut 2004, 128, 17–27. [Google Scholar]
- Mohamed, MF; Kang, DW; Aneja, VP. Volatile organic compounds in some urban locations in United States. Chemosphere 2002, 47, 863–882. [Google Scholar]
- Cheng, L; Fu, L; Angle, RP; Sandhu, HS. Seasonal variations of volatile organic compounds in Edmonton, Alberta. Atmos. Environ 1997, 31, 239–246. [Google Scholar]
- Lewis, AC; Bartle, KD; Pilling, MJ. Formation and lifetime of polycyclic aromatic compounds during a national-scale transient pollution episode. Polycyclic Aromat. Compd 2002, 22, 175–196. [Google Scholar]
- Afroz, R; Hassan, MN; Ibrahim, NA. Review of air pollution and health impacts in Malaysia. Environ. Res 2003, 92, 71–77. [Google Scholar]
- Khalili, NR; Scheff, PA; Holsen, TM. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ 1995, 29, 533–542. [Google Scholar]
- Collins, MJ; Williams, PL; McIntosh, DL. Ambient air quality at the site of a former manufactured gas plant. Environ. Monit. Assess 2001, 68, 137–152. [Google Scholar]
- Saborit, JMD; Aquilina, NJ; Meddings, C; Baker, S; Vardoulakis, S; Harrison, RM. Measurement of Personal Exposure to Volatile Organic Compounds and Particle Associated PAH in Three UK Regions. Environ. Sci. Technol 2009, 43, 4582–4588. [Google Scholar]
- Hoffmann, K; Krause, C; Seifert, B; Ullrich, D. The German Environmental Survey 1990/92 (GerES II): Sources of personal exposure to volatile organic compounds. J. Expo. Anal. Environ. Epidemiol 2000, 10, 115–125. [Google Scholar]
- Callen, MS; de la Cruz, MT; Lopez, JM; Murillo, R; Navarro, MV; Mastral, AM. Long-range atmospheric transport and local pollution sources on PAH concentrations in a South European urban area. Fulfilling of the european directive. Water Air Soil Pollut 2008, 190, 271–285. [Google Scholar]
- NRC. Human Exposure Assessment of Airborne Pollutants: Advances and Opportunities; National Research Council, National Academy of Science: Washington, DC, USA, 1991. [Google Scholar]
- US EPA. Compendium Method TO-14A. Determination of Volatile Organic Compounds in Ambient Air Using Specially Prepared Canisters with Subsequent Analysis by Gas Chromatography. January 1999. Report No EPA/625/R-96/010b; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999. [Google Scholar]
- Ras, MR; Borrull, F; Marce, RM. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. Trac-Trend. Anal. Chem 2009, 28, 347–361. [Google Scholar]
- US EPA. Compendium Method TO-17, Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999. [Google Scholar]
- Chung, CW; Morandi, MT; Stock, TH; Afshar, M. Evaluation of a passive sampler for volatile organic compounds at ppb concentrations, varying temperatures, and humidities with 24-h exposures. 2. Sampler performance. Environ. Sci. Technol 1999, 33, 3666–3671. [Google Scholar]
- Pennequin-Cardinal, A; Plaisance, H; Locoge, N; Ramalho, O; Kirchner, S; Galloo, JC. Performances of the Radiello® diffusive sampler for BTEX measurements: Influence of environmental conditions and determination of modelled sampling rates. Atmos. Environ 2005, 39, 2535–2544. [Google Scholar]
- Peng, CY; Batterman, S. Performance evaluation of a sorbent tube sampling method using short path thermal desorption for volatile organic compounds. J. Environ. Monit 2000, 2, 313–324. [Google Scholar]
- US EPA. Compendium Method TO-13A, Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999. [Google Scholar]
- Naumova, YY; Eisenreich, SJ; Turpin, BJ; Weisel, CP; Morandi, MT; Colome, SD; Totten, LA; Stock, TH; Winer, AM; Alimokhtari, S; Kwon, J; Shendell, D; Jones, J; Maberti, S; Wall, SJ. Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the US. Environ. Sci. Technol 2002, 36, 2552–2559. [Google Scholar]
- Fortune, A; Gendron, L; Tuday, M. Comparison of naphthalene ambient air sampling & analysis methods at former manufactured gas plant (MGP) remediation sites. Int J Soil Sediment Water 2009, 3. Article 1. [Google Scholar]
- MacIntosh, DL; Spengler, JD. Human Exposure Assessment; Wolrd Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Barro, R; Regueiro, J; Llompart, M; Garcia-Jares, C. Analysis of industrial contaminants in indoor air: Part 1. Volatile organic compounds, carbonyl compounds, polycyclic aromatic hydrocarbons and polychlorinated biphenyls. J. Chromatogr. A 2009, 1216, 540–566. [Google Scholar]



| Organization | Reference Level | Unit | Interpretation | Year | Ref |
|---|---|---|---|---|---|
| Environmental | |||||
| Environmental Protection Agency (EPA) | 3 | μg m−3 | Inhalation RfC | 1998 | [17] |
| Agency for Toxic Substances and Disease Registry (ATSDR) | 3.6 | μg m−3 | Inhalation MRL (Chronic) | 2005 | [30] |
| Office of Environmental Health Hazard Assessment (OEHHA), California | 9 | μg m−3 | Inhalation REL (Chronic) | 2000 | [31] |
| Office of Environmental Health Hazard Assessment (OEHHA), California | 3.4 × 10−5 | per μg m−3 | Inhalation Unit Risk | 2009 | [22] |
| Michigan Department of Environmental Quality (MDEQ) | 3 0.08 0.8 | μg m−3 | ITSL (24 hr) IRSL (cancer risk of 10−6) SRSL (cancer risk of 10−5) | 2004 | [32] |
| Occupational | |||||
| Occupational Safety & Health Administration (OSHA) | 50 | μg m−3 | PEL (TWA) | 2001 | [33] |
| National Institute for Occupational Safety and Health (NIOSH) | 50 75 | μg m−3 | REL (TWA) REL (STEL, 15 min) | 2005 | [34] |
| American Conference of Governmental Industrial Hygienists (ACGIH) | 50 75 | μg m−3 | TLV (TWA) TLV (STEL, 15 min) | 2009 | [35] |
| Emission source | Emission factor | Unit | Ref |
|---|---|---|---|
| Industrial stacks, furnaces, and boilers | |||
| Industrial stacks | 69–2707 | μg/kg | [42] |
| Fueled-boilers | 10900 | μg/kg | [43] |
| Diesel fueled-boiler | 1263 | μg/kg | |
| HO-NG fueled-boiler | 1835 | μg/kg | |
| COG-BFG fueled-boiler | 37.3 | μg/kg | |
| Joss paper furnaces | 41.2 | mg/kg | [46] |
| Combustion of wood and coals | |||
| House coal | 19 | mg/kg | [48] |
| Hardwood | 8.2 | mg/kg | |
| Pine wood | 4–27.67 | mg/kg | [49] |
| Rice husk briquettes | 18.06 | mg/kg | |
| Anthracite coal | Nd | mg/kg | |
| Birchwood | 52.8 | mg/kg | [51] |
| Pinewood | 71.4 | mg/kg | |
| Wood waste | 9.1 | mg/kg | |
| Peat briquette | 71.4 | mg/kg | |
| Domestic Waste | 331.5 | mg/kg | |
| Pine | 227 | mg/kg | [52] |
| Wood | 39.1 | mg/kg | [54] |
| Coal briquette | 44.5 | mg/kg | |
| Charcoal | 7.48 | mg/kg | |
| Almond | 7.3 | mg/kg | [41] |
| Walnut | 14.6 | mg/kg | |
| Fir | 13.6 | mg/kg | |
| Pine | 17.0 | mg/kg | |
| Burning of agricultural residue | |||
| Rice straw | 5.0–5.7* | mg/kg | [58] |
| Bean straw | 1.8–3.6* | mg/kg | |
| Agricultural debris | 25.2 | mg/kg | [59] |
| Barley | 11.1–149.5 | mg/kg | [41] |
| Corn | 1.3–7.6 | mg/kg | |
| Rice | 7.3–9.6 | mg/kg | [41] |
| Wheat | 44.4–348 | mg/kg | |
| Tobacco smoke** | |||
| Commercial cigarette | 13.2 | μg/ciga | [44] |
| Research cigarette | 15.1–18.1 | μg/ciga | [45] |
| In wallboard only room | 26–54 | μg/ciga | [47] |
| In wallboard/carpet room | 28–42 | μg/ciga | |
| In fully furnished room | 17–34 | μg/ciga | |
| Mobile | |||
| Catalyst-equipped gasoline-powered vehicle | 1 | mg/km | [50] |
| Non-catalyst-equipped gasoline-powered vehicle | 50 | mg/km | |
| Heavy-duty diesel vehicles-Idle | 10.2 | μg/mile | [53] |
| Heavy-duty diesel vehicles-Creep | 505 | μg/mile | |
| Heavy-duty diesel vehicles-Transient | 276 | μg/mile | |
| Heavy-duty diesel vehicles-Cruise | 20.1 | μg/mile | |
| Helicopter | 503 | μg/m3 | [55] |
| Ship auxiliary engine | 72–5850 | μg/kWh | [56] |
| Ship | 6.5–244 | μg/m3 | [57] |
| Household materials | |||
| Caulking | 310.0 | g/(m2h) | [60] |
| Adhesive | 1 | g/(m2h) | |
| Flooring materials | 0.001–57.7 | g/(m2h) | |
| Wood materials | 0.02–0.2 | g/(m2h) | |
| Year | US Industry (ton) | US Mobile (ton) | Canada (ton) | Netherlands (ton) | Scotland (kg) | Switzerland (kg) |
|---|---|---|---|---|---|---|
| 2008 | 2,913 | 58 | 560 | |||
| 2007 | 1,290 | 332 | 115 | 294 | 30 | |
| 2006 | 1,521 | 504 | 115 | 19 | ||
| 2005 | 1,755 | 3,761 | 656 | 118 | 18 | |
| 2004 | 1,560 | 294 | 35 | |||
| 2003 | 1,646 | 190 | ||||
| 2002 | 1,368 | 5,151 | 358 | |||
| 2001 | 1,205 | 168 | ||||
| 2000 | 1,400 | 221 | 133 | |||
| 1999 | 1,747 | 253 | ||||
| 1998 | 2,729 | 201 | ||||
| 1997 | 1,504 | 613 | ||||
| 1996 | 1,837 | 100 | ||||
| 1995 | 1,510 | 69 | 196 | |||
| 1994 | 1,624 | 113 | ||||
| 1993 | 1,470 | |||||
| 1992 | 2,299 | |||||
| 1991 | 1,831 | |||||
| 1990 | 2,286 | 263 | ||||
| 1989 | 2,215 | |||||
| 1988 | 3,049 | |||||
| Reference | [85] | [86] | [87] | [88] | [89] | [90] |
| Country | Location | Setting | Sampling period | No. of residences | Sampling method | DF | Concentration (μg m−3) | Rep | VOC/PAH | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | SD | GM | Median | Max | ||||||||||
| US | Missoula, MT | Rural | 2005–2006 | 51 high school students’ homes | 12-h active sorbent | - | - | - | 0.3 | 1.4 | N | VOC | [106] | |
| US | Southeast MI | Urban and suburban | 2004–2005 | 159 homes | 4-d passive sorbent | 100% | 3.49 | - | - | 0.84 | 91.75 | Y | VOC | [107, 108] |
| US | Syracuse, NY | Urban | 2001–2003 | 150 residential buildings | 24-h active sorbent | 9.52 | - | - | 2.84 | 44.7 | N | VOC | [104] | |
| US | Chicago, IL | Urban and suburban | 2000–2001 | 10 homes | 48-h active PUF | - | - | - | 0.18 | 2.34 | Y | PAH | [109] | |
| US | Raleigh-Durham-Chapel Hill Area, NC | Urban | 1997 | 9 children’s homes | 48-h active PUF | 0.43 | - | - | - | 1.24 | Y | PAH | [7] | |
| US | Five cities, NC | Urban and rural | 1995 | 24 low-income families | 24-h active resin | 2.19 | 1.87 | - | 1.67 | 9.7 | Y | PAH | [6] | |
| US | Southeast Chicago, IL | Urban | 1994–1995 | 10 homes | 24-h active PUF | 89% | 0.85 | 0.95 | - | 0.47 | 50 | Y | PAH | [110] |
| US | Columbus, OH | Urban | 1986–1987 | 8 homes | 8-h active resin | 1.4 | - | - | - | 4.2 | N | PAH | [111] | |
| Canada | Quebec City, Quebec | Urban | 2005 | 96 dwellings | 7-d passive sorbent | 100% | - | - | 1.45 | 1.12 | 23.02 | Y | VOC | [112] |
| Canada | Ottawa, Ontario | Urban | 2002–2003 | 75 residences | 100-min active sorbent | 83% | 3.87 | 17.25 | 0.33 | 0.39 | 144.44 | Y | VOC | [113] |
| Canada | Montreal, Quebec | Urban | 1991–1994 | 18 residences | 24-h active resin | 100% | 0.27 | - | 0.17 | 0.17 | - | Y | PAH | [114] |
| Canada | Canada nationwide | 1991 | 754 homes | 24-h passive sorbent | 4.07 | - | - | - | - | Y | VOC | [115] | ||
| UK | Birmingham | Urban | 1999–2000 | 12 homes | Active sorbent | 0.8 | 1 | - | 0.5 | 6 | Y | VOC | [116] | |
| Germany | Leipzig, Munchen, and Koln | Urban | 1994–2001 | 2103 measurements | 4-week OVM passive | 0.8 | - | - | 0.3 | 1.8 | Y | VOC | [117] | |
| Germany | Leipzig | Urban | 1994–2001 | 222 measurements | 4-week OVM passive | 0.89 | - | - | 0.31 | 40.79 | Y | VOC | [118] | |
| Germany | Bremer | Urban | NA | 182 measurements | Active PUP | 100% | - | - | 0.81 | 30.91 | N | PAH | [119] | |
| Germany | Schleswig-Holstein | Urban | 2000–2001 | 39 dwellings and houses | Active sorbent | 1.2 | 2.8 | 0.31 | 0.46 | 14 | Y | VOC | [120] | |
| Finland | Helsinki | Urban | 1996–1997 | 201 homes | 48-h active sorbent | 24% | 0.64 | 0.53 | 0.55 | 0.6 | 3.89 | Y | VOC | [121] |
| Finland | NA | NA | NA | 50 normal houses | Active sorbent | 0.44 | 0.46 | - | 0.31 | 1.63 | N | VOC | [122] | |
| Australia | Melbourne | Urban | N/A | 22 non-complaint homes 5 complaint homes | 30–50 min active sorbent | 30% | 3.2 6.9 | - | 1.6 4.1 | 1.6 4.1 | - | N | VOC | [105] |
| China | Hangzhou | Urban | 1999 | 8 nonsmoking and smoking homes | XAD-2 resin | 100% | 6.77 | 6.90 | 3.94 | 4.59 | 20.57 | N | PAH | [123] |
| Country | Location | Setting | Sampling period | No. of sampling locations | Sampling method | DF | Concentration (μg m−3) | Rep | VOC/PAH | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | SD | GM | Median | Max | ||||||||||
| US | Missoula, MT | Rural | 2005–2006 | Outside of 51 high school students’ homes | 12-h active sorbent | - | - | - | 0.1 | 0.4 | N | VOC | [106] | |
| US | Southeast Michigan | Urban and suburban | 2004–2005 | Outside of 159 homes | 4-d passive sorbent | 94% | 0.28 | - | - | 0.18 | 4.72 | Y | VOC | [107] |
| US | Chicago, IL | Urban and suburban | 2000–2001 | Outside of 10 homes | 48-h active PUF | - | - | - | 0.17 | 1.87 | Y | PAH | [109] | |
| US | Raleigh–Durham–Chapel Hill, NC | Urban | 1997 | 4 sites | 48-h active PUF | 0.06 | - | - | - | 0.076 | N | PAH | [7] | |
| US | Five cities in NC | Urban and rural | 1995 | Outside of 24 low-income families | 24-h active resin | 0.43 | 0.51 | - | 0.28 | 1.82 | Y | PAH | [6] | |
| US | Columbus, OH | Urban | 1986–1987 | Outside of 8 homes | 8-h active resin | 0.17 | - | - | - | 0.33 | N | PAH | [111] | |
| US | Phoenix and Tucson, AZ | Urban | 1994–1996 | 5 sites, 305 samples | 6L canister | 0.01–0.82 | - | - | - | 1.96 | Y | VOC | [130] | |
| US | San Dimas, Upland, Mira Loma, Riverside, CA | Urban | 2001–2002 | 4 schools | 24-h active PUF | 0.21–0.58 | - | - | - | 1.04 | Y | PAH | [91] | |
| US | Los Angeles, CA Riverside, CA | Urban | 2002–2003 | 2 sites | 5-day active sorbent | 0.7 0.23 | - | - | - | 2.54 0.77 | Y | PAH | [131] | |
| US | Wildlife Refuge, MS | Remote | 1991 | 2 sites, 80 samples | 4-day active PUF | 0.0001 | - | - | - | - | N | PAH | [132] | |
| Canada | Sarnia, Ontario | Urban | 2005 | 37 sites | 2-week OVM passive | 0.12 | 0.05 | - | 0.11 | - | Y | VOC | [133] | |
| Canada | Ottawa, Ontario | Urban | 2002–2003 | Outside of 74 homes | 100-min active sorbent | 54% | 0.18 | - | - | 0.02 | 3.9 | Y | VOC | [113] |
| Canada | Western Canada | Rural | 2004 | 11399 samples | 1-month OVM passive | 70% | 0.008 | - | 0.003 | 0.003 | 1.7 | N | VOC | [134] |
| UK | Birmingham | Urban | 1999–2000 | Outside of 12 homes | Active sorbent | 0.3 | 0.2 | - | 0.2 | 0.9 | Y | VOC | [116] | |
| UK | Birmingham | Urban | 1992 | 1site, 55 samples | 24-h active PUF | 0.002–0.012 | - | - | - | N | PAH | [92] | ||
| Germany | Leipzig | Urban | 1994–2001 | 222 measurements | 4-week OVM passive | 0.1 | - | - | 0.1 | 1.5 | Y | VOC | [118] | |
| Germany | Germany | Urban | NA | 47 measurements | Active PUP | 100% | - | - | - | 0.1 | 1.4 | Y | PAH | [119] |
| Finland | Helsinki, Finland | Urban | 1996–1997 | Outside of 183 homes | 48-h active sorbent | <20% | - | - | - | - | 1.3 | N | VOC | [121] |
| Australia | Melbourne | Urban | N/A | 27 sites | 30–50 min active sorbent | 30% | <MDL | - | - | <MDL | - | N | VOC | [105] |
| India | Delhi | Urban | 2001 | Multiple sites | 4-h active sorbent | 0.39 | 0.30 | - | 0.31 | - | Y | VOC | [135] | |
| India | Mumbai | Urban | 2001–2002 | Multiple sites | 4-h active sorbent | 0.10 | 0.12 | - | 0.06 | - | Y | VOC | [136] | |
| Korea | Seoul | Urban | 1999 | 1 site | 24-h active PUF | 0.01 | 0.01 | - | 0.01 | - | Y | PAH | [137] | |
| China | Hangzhou | Urban | 1999 | Outside of 8 homes | XAD-2 resin | 100% | 6.31 | 6.82 | 3.15 | 4.15 | 19.83 | N | PAH | [123] |
| Industrial | 0.41 | - | - | - | - | N | ||||||||
| Taiwan | Taichung | Urban | 2002 | 1 site | 3-day active PUF | 0.28 | - | - | - | - | Y | PAH | [94] | |
| Rural | 0.22 | - | - | - | - | N | ||||||||
| Country | Location | Setting | Sampling period | Sample size | Sampling method | DF | Concentration (μg m−3) | Rep | VOC/PAH | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | SD | GM | Median | Max | ||||||||||
| UK | London and Birmingham | Urban | 191 | 0.78 | 1.49 | 0.49 | 0.49 | 12.67 | Y | |||||
| Birmingham | Suburban | 2005–2007 | 209 | 5-day sorbent active | 0.72 | 0.75 | 0.55 | 0.55 | 6.35 | Y | VOC | [146] | ||
| Midlands and Wales | Rural | 100 | 0.71 | 0.54 | 0.58 | 0.58 | 2.84 | N | ||||||
| Germany | West Germany | Urban | 1990–1991 | 113 | 7-day OVM passive | 96% | 2.3 | 2.1 | 2.0 | 4.0 | Y | VOC | [147] | |
| Finland | Helsinki | Urban | 1996–1997 | 183 | 2-day sorbent active | 10% | na | na | na | 2.7 | N | VOC | [121] | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jia, C.; Batterman, S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. Int. J. Environ. Res. Public Health 2010, 7, 2903-2939. https://doi.org/10.3390/ijerph7072903
Jia C, Batterman S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. International Journal of Environmental Research and Public Health. 2010; 7(7):2903-2939. https://doi.org/10.3390/ijerph7072903
Chicago/Turabian StyleJia, Chunrong, and Stuart Batterman. 2010. "A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air" International Journal of Environmental Research and Public Health 7, no. 7: 2903-2939. https://doi.org/10.3390/ijerph7072903
APA StyleJia, C., & Batterman, S. (2010). A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. International Journal of Environmental Research and Public Health, 7(7), 2903-2939. https://doi.org/10.3390/ijerph7072903






