Abstract
In contrast to arsenic (As) poisoning caused by naturally occurring inorganic arsenic-contaminated water consumption, coal arsenic poisoning (CAP) induced by elevated arsenic exposure from coal combustion has rarely been reported. In this study, the concentrations and distributions of urinary arsenic metabolites in 57 volunteers (36 subjects with skin lesions and 21 subjects without skin lesions), who had been exposed to elevated levels of arsenic present in coal in Changshapu village in the south of Shaanxi Province (China), were reported. The urinary arsenic species, including inorganic arsenic (iAs) [arsenite (iAsIII) and arsenate (iAsV)], monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV), were determined by high-performance liquid chromatography (HPLC) combined with inductively coupled plasma mass spectroscopy (ICP-MS). The relative distributions of arsenic species, the primary methylation index (PMI = MMAV/iAs) and the secondary methylation index (SMI = DMAV/MMAV) were calculated to assess the metabolism of arsenic. Subjects with skin lesions had a higher concentration of urinary arsenic and a lower arsenic methylation capability than subjects without skin lesions. Women had a significantly higher methylation capability of arsenic than men, as defined by a higher percent DMAV and SMI in urine among women, which was the one possible interpretation of women with a higher concentration of urinary arsenic but lower susceptibility to skin lesions. The findings suggested that not only the dose of arsenic exposure but also the arsenic methylation capability have an impact on the individual susceptibility to skin lesions induced by coal arsenic exposure.
1. Introduction
Arsenic (As) is a ubiquitous element in environment. An established association between human chronic arsenic exposure and a variety of health outcomes, including skin diseases, diabetes, peripheral vascular disease and kinds of cancers [1–5], has been known for many years. It was reported that there was a wide variation in susceptibility to arsenic health outcomes, which may be, to some extent, related to variations in arsenic metabolism [2].
The metabolism of arsenic in humans occurs through reduction and oxidative methylation catalyzed by reductases and methyltransferases [6]. First, pentavalent arsenate is reduced to trivalent arsenite in the blood, and then inorganic arsenic (iAs) is transformed into monomethylarsinic acid (MMAV) and dimethylarsinic acid (DMAV). Although MMAV and DMAV are less toxic than iAs [6,7], the existence of trivalent intermediates, monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII) during the arsenic metabolism process has been confirmed [8]. They are formed by the reduction of MMAV and DMAV and are more toxic than iAs. Petrick and his colleagues have proposed the following order of toxicity of arsenicals in Chang human hepatocytes: MMAIII > arsenite > arsenate > MMAV = DMAV [7]. Therefore, it is considered that methylation is the key to understanding the biotransformation of arsenic and may be related to the mechanism of arsenic toxicity.
Urinary arsenic is generally used as the main biomarker of exposure and is regarded as the most reliable indicator of recent exposure to iAs [9]. A positive correlation between total daily intake of arsenic from water and total arsenic concentration in urine has been found (r = 0.5, p < 0.001) in a study from Mexico [10]. The relative distributions of urinary iAs, MMA and DMA have been used as measures of human methylation capability [1,9,11,12]. Besides, primary methylation index (PMI, defined as MMA/iAs) and secondary methylation index (SMI, defined as DMA/MMA) has been applied to assess the first and second methylation step, respectively [1,9,11]. Several studies of arsenism induced by arsenic-contaminated water consumption from Bangladesh, Taiwan and Mexico, have shown an increasing prevalence of arsenic-associated toxic effects with increasing percentage of iAs and MMAV and decreasing percentage of DMAV and SMI [1–3,11].
Worldwide, to date coal arsenic poisoning (CAP) only has been found in China. Shaanxi Province is the second highest CAP-prevalent area next to Guizhou province in China. CAP is caused by arsenic exposure from coal confusion and the coal arsenic concentration is found to be 27.95–475.10 mg/kg (mean: 222.40 mg/kg) in the south of Shaanxi Province [13]. It is much higher than the average arsenic content in coal in the World (9.0 mg/kg) or in China (4.5 mg/kg) [14,15]. Coal is burnt indoors, in stoves without a chimney, for cooking and heating; therefore, indoor air and food were found to be polluted by smoke with high levels of arsenic. The arsenic content of indoor air was 6.32 ± 9.95 μg/m3 [13], being 2.3 times higher than the maximum levels of arsenic in the air (3 μg/m3) [16]. The average concentrations of arsenic in stove-dried corn and stove-dried chili were 0.76 ± 0.72 mg/kg and 0.96 ± 1.41 mg/kg [13], respectively, which are approximately 3 and 19 times higher than the maximum levels of arsenic allowed in foods in China (0.2 and 0.05 mg/kg, respectively) [17]. Moreover, arsenic concentrations in drinking water [13] were less than 10 μg/L the drinking water standard in China [18]. The CAP situation in Shaanxi Province is serious and the rate of prevalence of CAP ranges from 19.75–70.7% in different villages [19]. In contrast to arsenic (As) poisoning caused by naturally occurring inorganic arsenic-contaminated water consumption, CAP induced by elevated arsenic exposure from coal combustion has been rarely reported, especially in the field of evaluating the association between urinary arsenic profiles and the health outcomes of human.
Within the context of the perspectives mentioned above, the objectives of our study were: (1) to investigate the profile of urinary arsenic metabolites in residents exposed to coal arsenic in the south of Shaanxi Province (China); (2) to find out the relationship between urinary arsenic metabolites and skin lesions induced by chronic arsenic exposure; (3) to evaluate the effect of sex and age on urinary arsenic metabolites and arsenic methylation capability.
2. Experimental Section
2.1. Study Area
The study was conducted in a typical CAP epidemic village, namely Changshapu, in Pingli County of Ankang City located in the south of Shaanxi Province (China) (Figure 1).
Figure 1.
Map of the study area.
Changshapu is a poor small village with an agrarian economy. The climate is usually wet and humid. Therefore, for their daily cooking, heating and indoor drying of grains (corn and chili) the villagers usually burn stone-like coal, which contains a very low content of carbon and a high content of arsenic. The mean concentration of arsenic in the stone-like coal used in Changshapu village was 278 mg/kg [20]. In Changshapu village, the stone-like coal mainly originated from the same colliery near the village, which has been mined for 100 years. Therefore, all the residents had life-long exposures to arsenic polluted air and food.
2.2. Study Population
In Changshapu village, there were 335 registered permanent residents, 185 (55%) men and 150 (45%) women. Most of the young people (18 to 40 years old) worked far away from their hometown for most of the year. Therefore, 57 volunteers above eighteen years old participated in this study. They had always lived in Changshapu village since when they were born and had suffered life-long exposure to elevated arsenic concentrations in the environment. In order to identify the cases of skin lesions induced by arsenic exposure, detailed physical examinations were conducted by trained medical doctors according to the Standards of Diagnosis for Endemic Arsenism [21]. In the standards, palms of the hands, soles of the feet and parts of the body trunk were examined for symptoms of skin lesions, including pigmentation, hyperpigmentation (melanosis), hypopigmentation, keratosis, hyperkeratosis, skin ulceration and skin cancers (Bowen disease), and each category of clinical symptoms was assigned a rank. The arsenism patients were then classified as “suspected,” “mild,” “moderate,” “severe,” and “skin cancer” with increasing ranking order. There was only pigmentation and hypopigmentation cases but no keratosis and skin cancers cases among the 57 volunteers. The cases with “suspected”, “mild”, “moderate” and “severe” arsenism were classified as “subjects with skin lesions” and others not showing skin lesions were classified “subjects without skin lesions”. The volunteers included 36 subjects with skin lesions (12 females and 24 males) and 21 subjects without skin lesions (12 females and nine males).
According to the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects [22], all 57 of the volunteers were gave informed consent before participating. The data of personal information, including sex and age, were obtained by questionnaire.
2.3. Sample Collection
The information about the heating period in Changshapu village was achieved by inquiry. The residents in Changshapu village started using stone-like coal for heating from November to March each year. Therefore, sample collection was taken in the middle of March, 2008, which was the end of the heating period of Changshapu. According to the IUPAC guidelines of sample collection for trace elements in blood and urine [23], 10 mL of first-morning void urine were obtained in polypropylene tubes and kept in a box with ice. The box was covered to avoid exposure to light. Within 8 h, the urine samples were transported to the local Center of Disease Control and stored at −20 °C. Within five days, all urine samples were transported to the Institute of Geographical Sciences and Natural Resources Research, Chinese Academy of Science for analysis.
2.4. Reagents and Standards
The standard solutions of arsenious acid (GBW08666, AsO33−), monomethylarsonic acid (GBW08668, CH3AsO32−) and dimethylarsinic acid (GBW08669, C2H7AsO2) were bought from the National Center of Standard Reference Materials (Beijing, China). The standard solution of arsenate (1,000-mg/L, Na3AsO4) was from Perkin Elmer Ltd Company (Beijing, China). All other reagents used in this study were guaranteed (the highest grade commercially available in China).
2.5. Determination of Arsenic Metabolites
Quantitative determination of urinary arsenic species was performed as described previously [24,25]. The arsenic species (iAsIII, iAsV, MMAV and DMAV) in urine were determined by using high-performance liquid chromatography (HPLC) (PerkinElmer 200 series, USA) combined with inductively coupled plasma mass spectroscopy (ICP-MS) (Elan DRC-e, PerkinElmer, USA). The parameters of the instruments for the chromatographic separation and detection were listed in Table 1. Separation was performed on a PRP-X100 anion exchange column (150 mm × 4.10 mm, 5 μm particle size, Hamilton) and a guard column (10 μm particle size, 20 mm length, 2.0 mm i.d., Hamilton). The mobile phase contained 20 mM Ammonium phosphate (dibasic). 15% ammonium hydroxide was used to adjust the pH to 6.0. The mobile phase was filtered through a 0.45 μm membrane and sonicated for 10 min before use in HPLC separation.

Table 1.
Operating conditions of the HPLC-ICP-MS for arsenic speciation in urine.
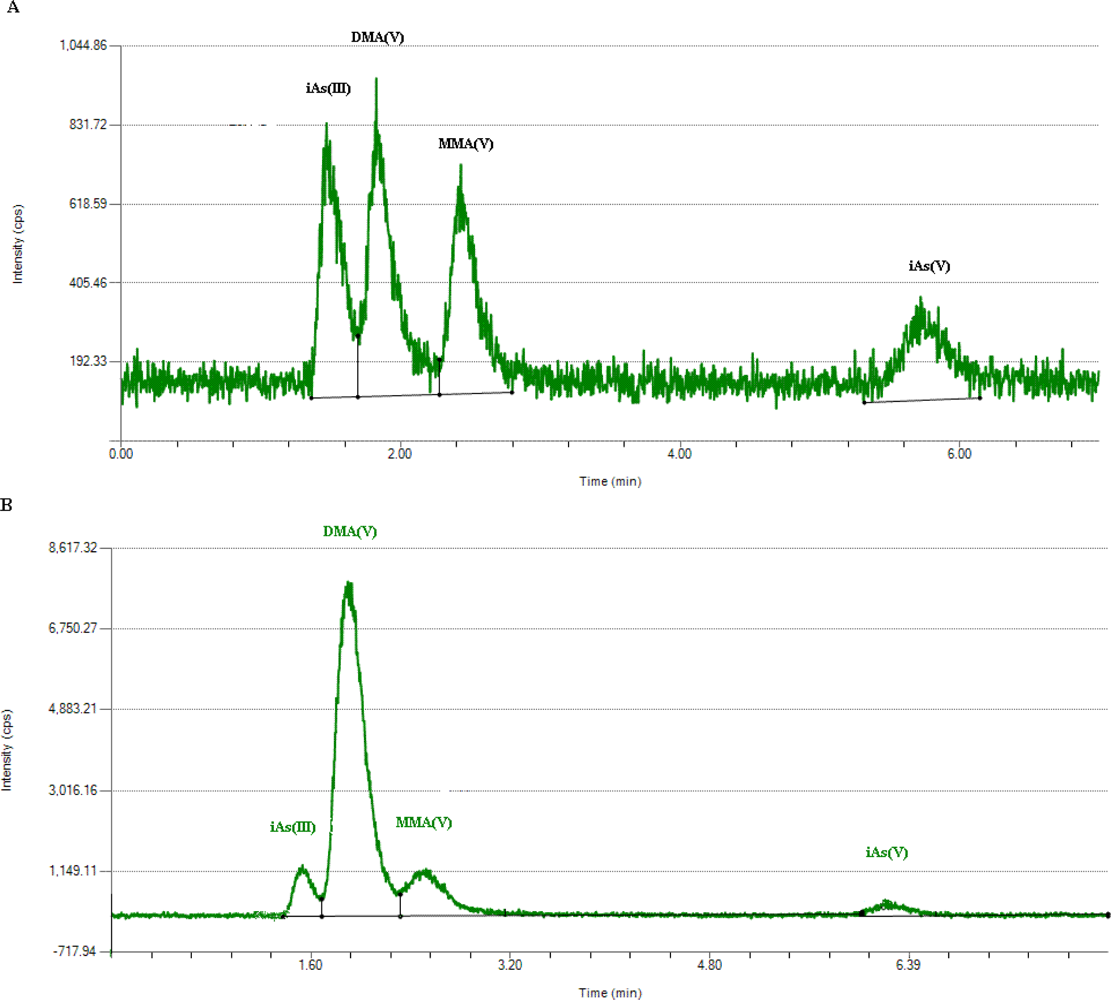
Briefly, 1-mL urine was passed through a filter with 0.22 μm pore size and 13 mm diameter. Then, each sample was determined by HPLC in triplicate. A typical chromatogram of four arsenic species in standard mixture in deionized water was shown in Figure 2(A) and in a urine sample in Figure 2(B). The retention times of iAsIII, iAsV, MMAV and DMAV were 1.5, 1.8, 2.4 and 5.7 min, respectively.
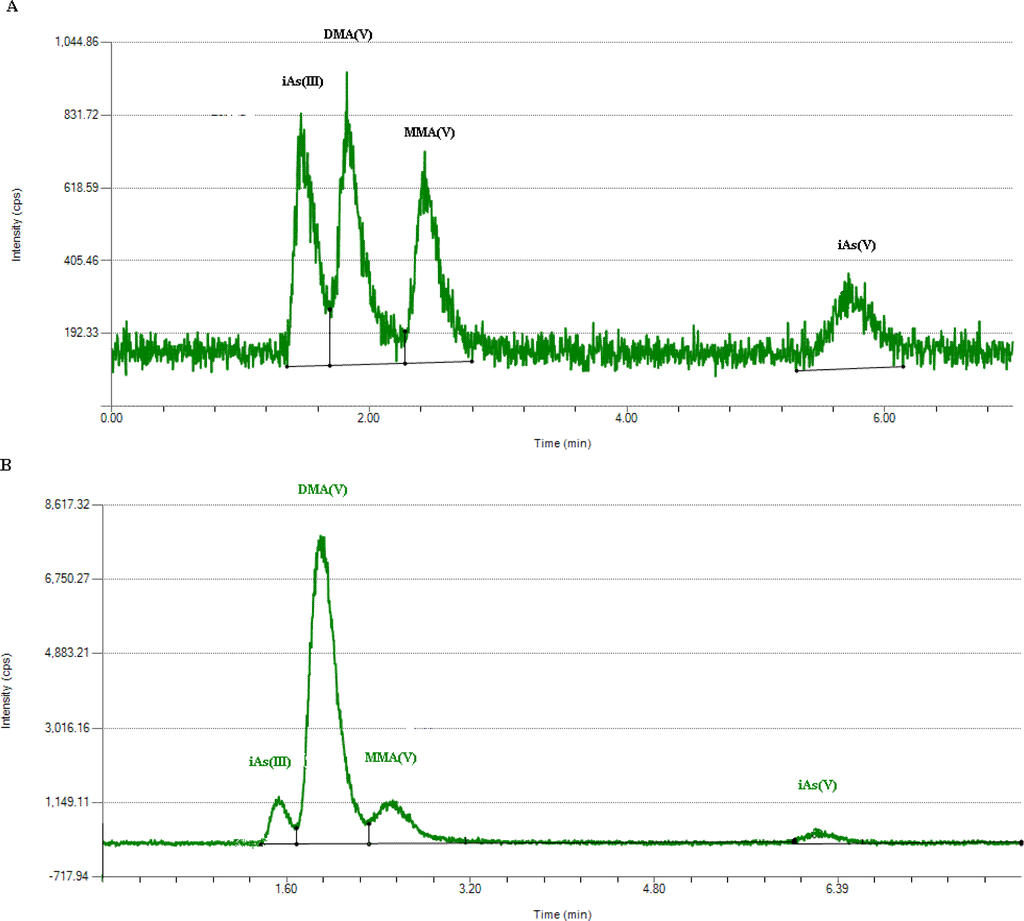
Figure 2.
Chromatogram of four arsenic species (A) in standard mixture in deionized water; (B) in a urine sample.
The Elan DRC-e ICP-MS system was operated in the dynamic reaction cell (DRC) mode. Oxygen was used as the reaction gas. Peak hopping scan mode was used to monitor AsO+ at 91 amu. The limits of detection were 0.163 μg/L for iAsIII, 0.33 μg/L for iAsV, 0.15μg/L for MMAV and 0.146 μg/L for DMAV. If the concentrations of iAsV in urinary were lower than the limits of detection, the values were recorded as half of the detection limit. The reliability of the arsenic species separation was evaluated by the analytical recoveries of added arsenic species. Spiking the recoveries of iAsIII, iAsV, MMAV and DMAV were 81–95%, 83–98%, 82–102% and 94–110%, respectively.
2.6. Creatinine in Urine
Creatinine was necessary for the correction of arsenic concentrations in urinary volume [26]. The concentrations of creatinine were determined by enzyme reaction with a commercial kit (Wako Pure Chemical Industries, Ltd., Japan).
2.7. Statistical Analysis
The total arsenic concentration (TAs), the percentages of the four arsenic species, the primary arsenic methylation index (PMI) and the secondary arsenic methylation index (SMI) are calculated as follows:
Data analysis was performed by using SPSS software (version 11.5, SPSS Inc., Chicago, IL, USA). The concentrations and percentages of arsenic species, as well as PMI and SMI, were first log-transformed to meet the requirement of equal variance and normal distribution of residuals. The values were transformed back to the arithmetic scale for reporting purposes. The independent samples t-test was used to reflect the difference of the log-transformed data of urinary arsenic species and arsenic methylation capability indexes. Spearman’s correlation was used to analyze the relationships between varies urinary arsenic species and age. The statistical significance was set at p < 0.01 and p < 0.05, respectively.
3. Results and Discussion
3.1. Urinary Arsenic Metabolites of the Study Population
The findings regarding the content of arsenic metabolites in urine and the arsenic methylation capability indexes of the 57 volunteers were presented in Table 2. The concentrations of urinary iAsIII, iAsV, MMAV, DMAV and TAs were 2.97, 0.9, 4.69, 23.41 and 31.97 μg/g Cr, respectively. DMAV was the major arsenic species in urine (72.38%), followed by MMAV (14.50%), iAsIII (9.17%) and iAsV (2.78%). Other two arsenic methylation capability indexes, namely PMI and SMI, were 1.32 and 4.99, respectively.

Table 2.
Concentrations and percentages of arsenic species in urine of the volunteers (N = 57).
3.2. Urinary Arsenic Metabolites between Subjects with and without Skin Lesions
The concentrations of all of the urinary arsenic species and TAs of subjects with skin lesions were significantly higher than that of subjects without skin lesions (Table 2). It was shown that the subjects with skin lesions excreted more arsenic in urine than subjects without skin lesions, which may indicate a higher recent arsenic exposure in subjects with skin lesions.
The percent DMAV and the SMI were significantly lower, while the percent iAsV was significantly higher of the subjects with skin lesions than that of the subjects without skin lesions. A higher percent iAsIII and percent MMAV and a lower PMI were also observed in subjects with skin lesions; however, the difference did not reach statistical significance. These data suggested that the arsenic methylation capability, especially the secondary methylation capability in subjects with skin lesions were lower than in subjects without skin lesions.
3.3. Urinary Arsenic Metabolites among Men and Women
The results in Table 3 show that the concentrations of TAs of women were significantly higher than that of men for the subjects without skin lesions. DMAV of women were significantly higher than that of men, whether for the subjects with or without skin lesions. Women may have a higher recent arsenic exposure due to their roles as housewives in families. For the subjects without skin lesions, women had significantly lower percent iAsIII and percent MMAV, but significantly higher percent DMAV and SMI than men. On the other hand, for the subjects with skin lesions, significantly higher percent DMAV and SMI were observed in women than in men. These differences suggested that arsenic methylation capability, especially the secondary methylation capability of women was higher than that of men.

Table 3.
Concentrations and percentages of arsenic species in urine of the men and women (N = 57).
3.4. Relationship between Age and Urinary Arsenic Metabolites
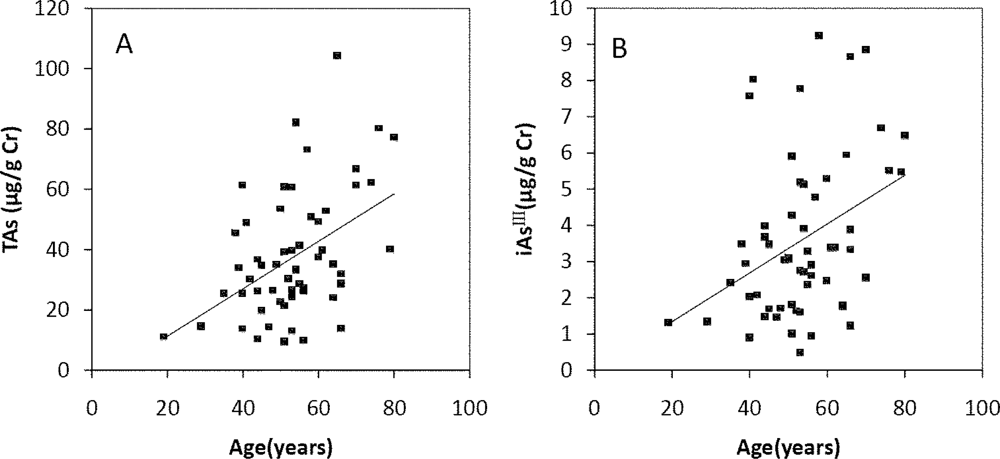
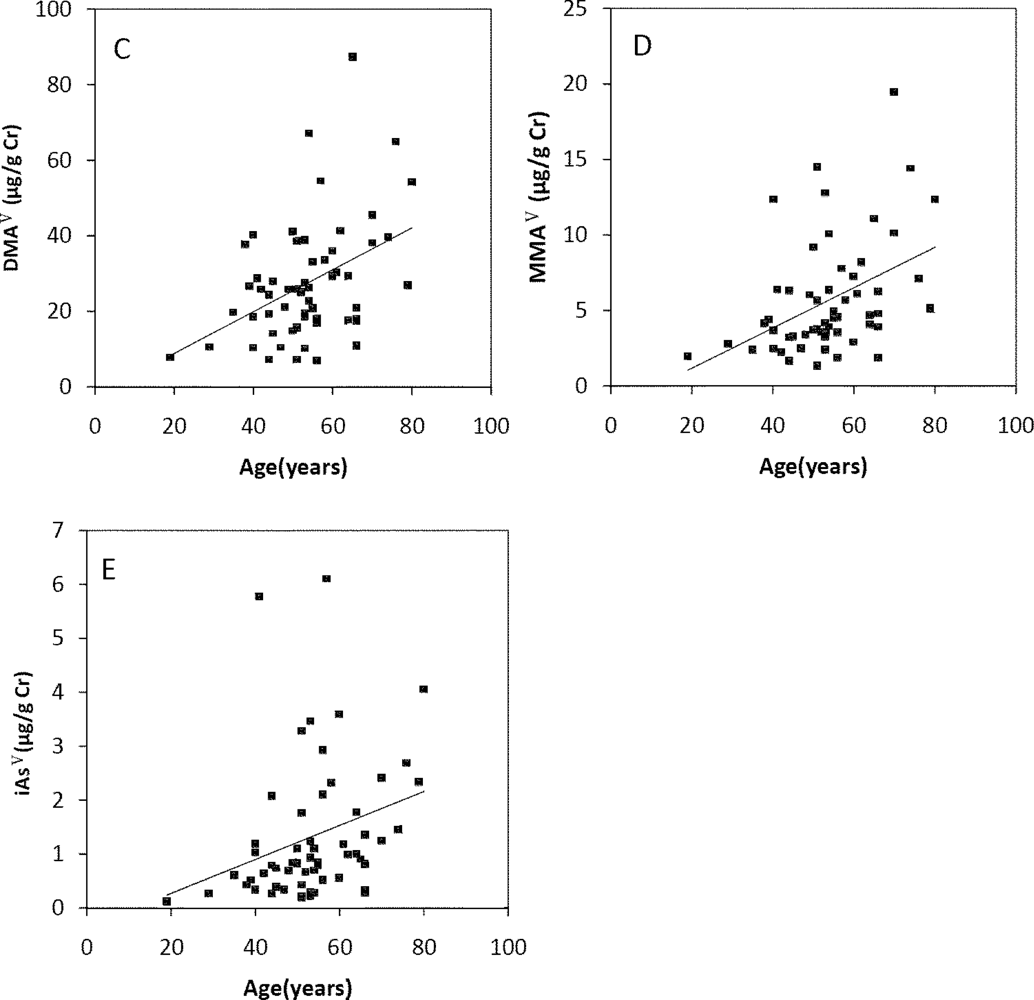
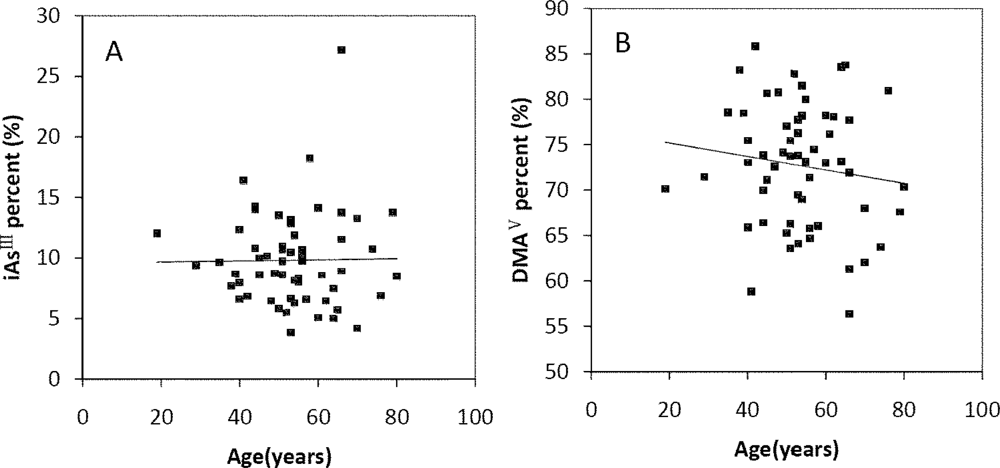
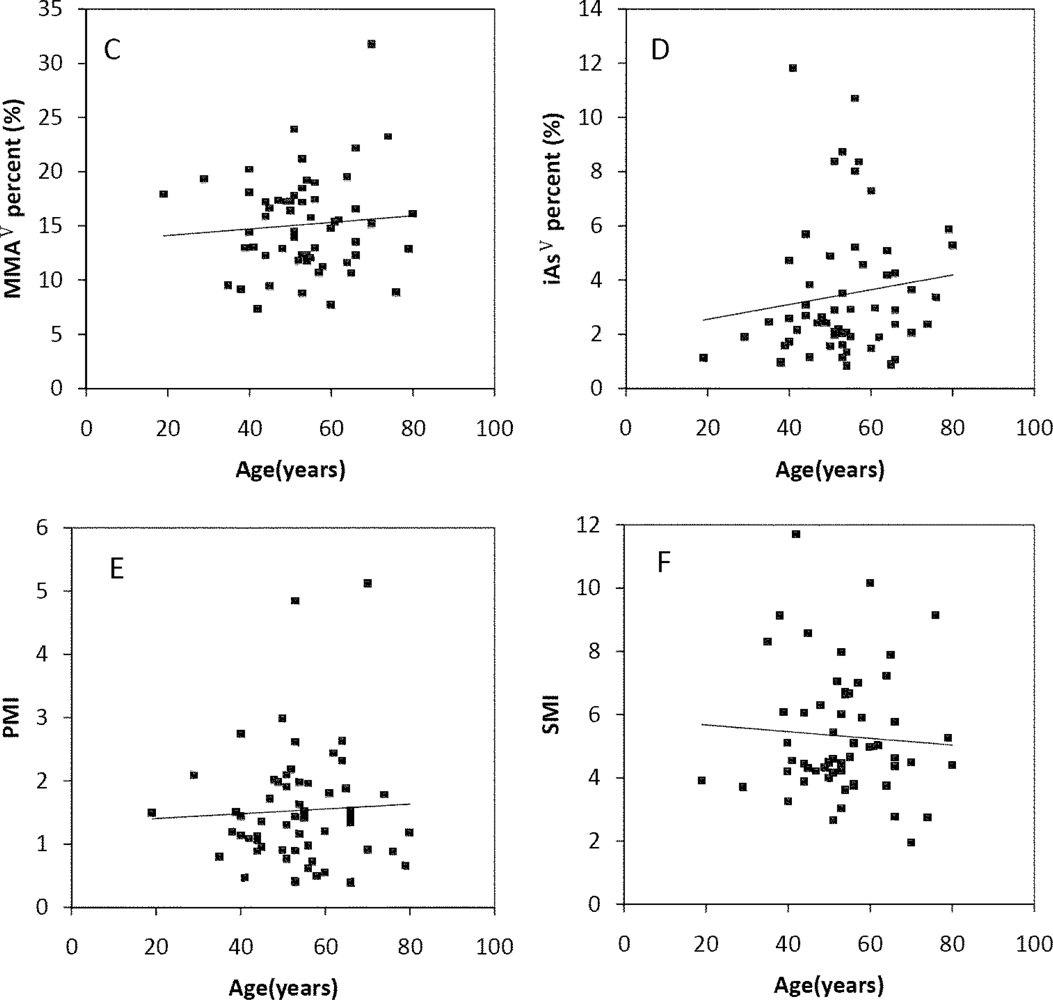
The Spearman’s correlation coefficients of Age/TAs (0.411), Age/iAsIII (0.343), Age/DMAV (0.353), Age/MMAV (0.439) and Age/iAsV (0.422) were shown in Figure 3A–E. The figures shown that age was positive correlated with the concentrations of TAs, iAsIII, DMAV, MMAV and iAsV. These suggested that more arsenic may have been accumulated in the elder than in the younger. As regards arsenic methylation capability, the iAsIII percent, iAsV percent, MMAV percent, DMAV percent, PMI and SMI did not reach statistical significance with age. (Figure 4). These suggested that the arsenic methylation capability may not correlated with age.
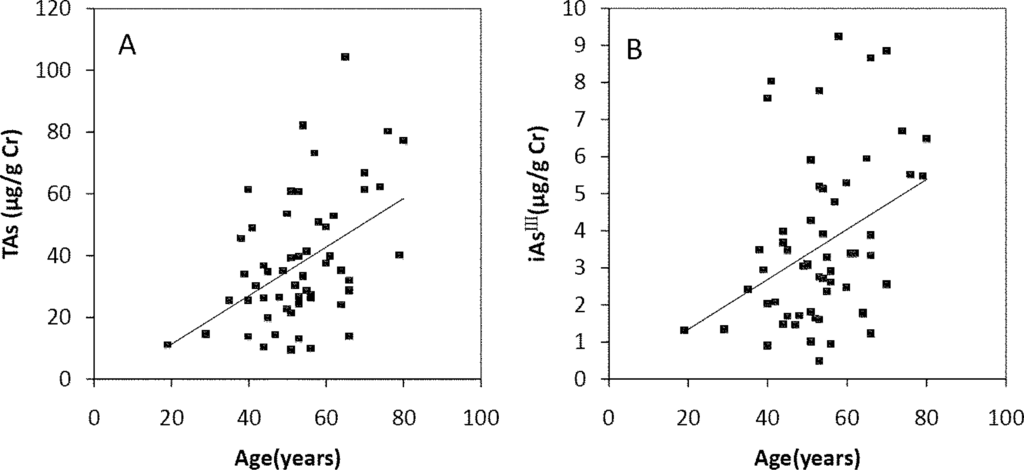
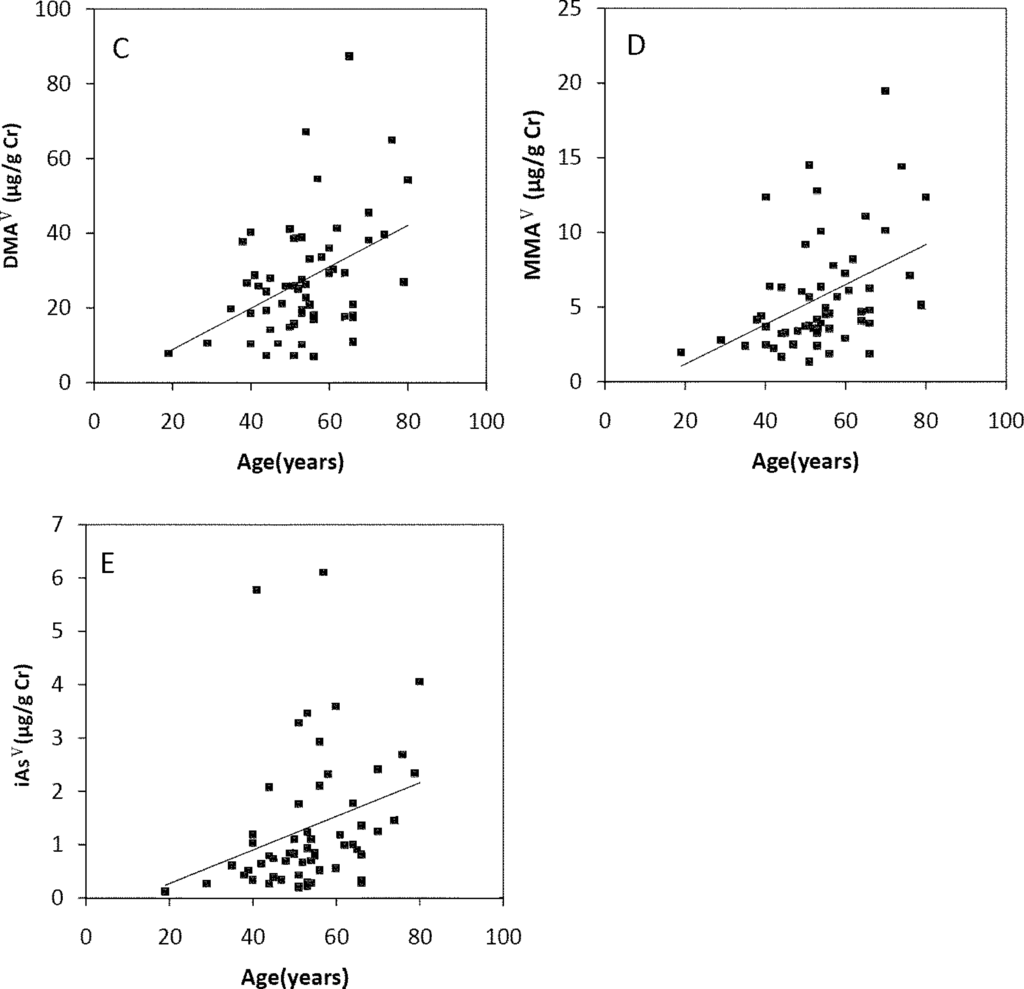
Figure 3.
Correlation of age with (A) TAs (R = 0.411, p = 0.001); (B) iAsIII (R = 0.343, p = 0.009); (C) iAsV (R = 0.422, p = 0.001); (D) MMAV (R = 0.439, p = 0.001); (E) DMAV (R = 0.353, p = 0.007) in the urine.
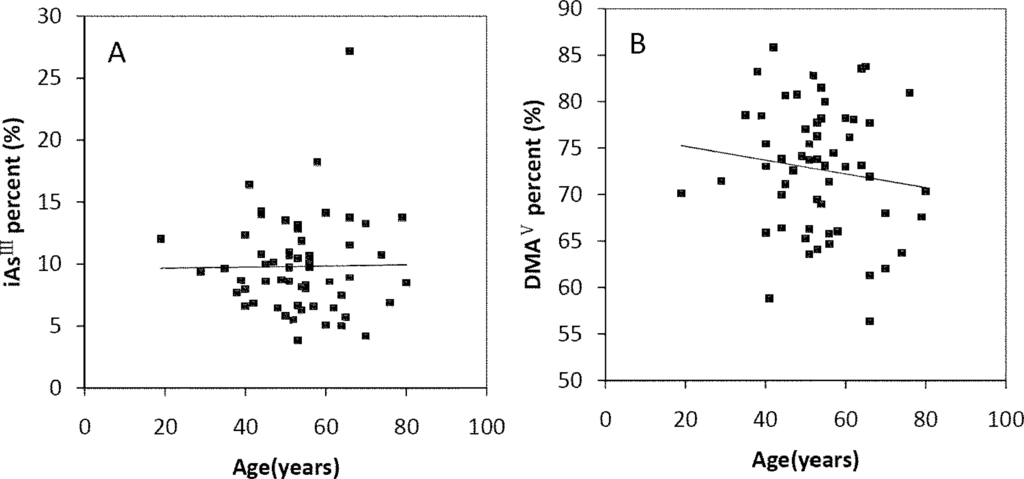
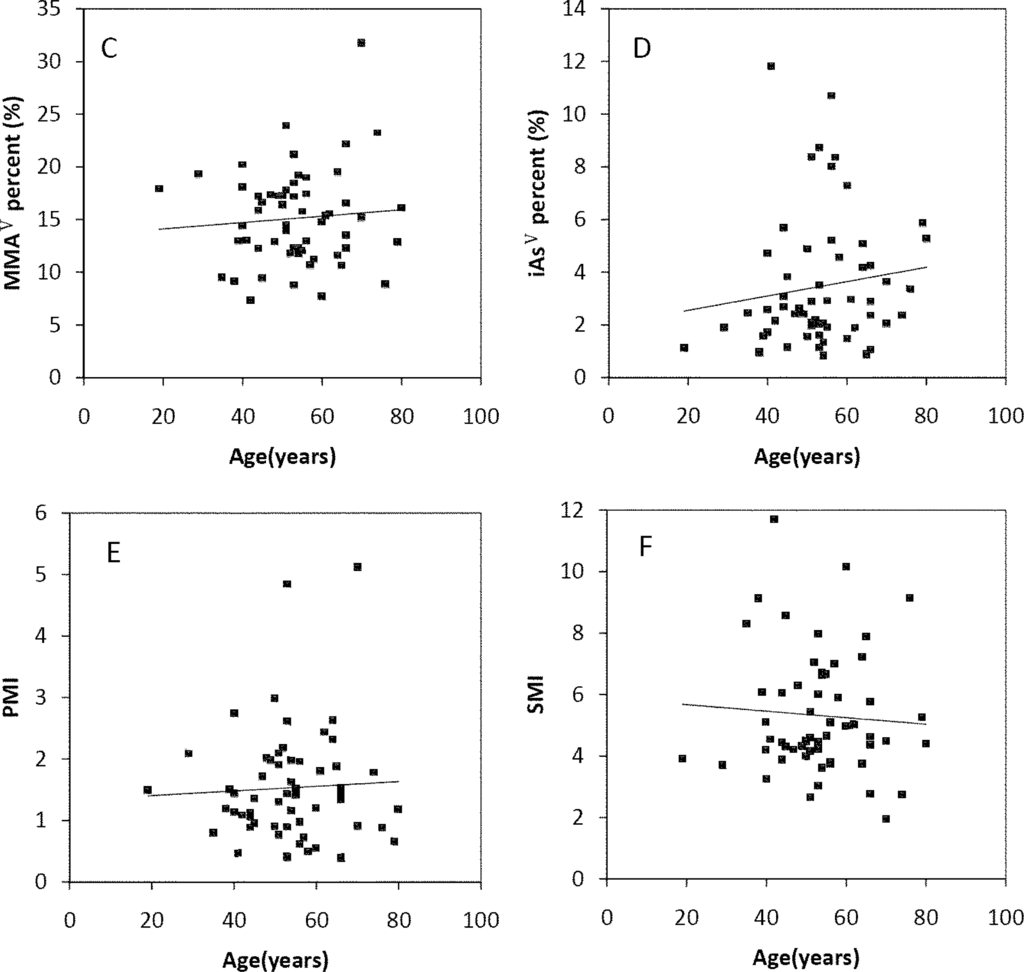
Figure 4.
Correlation of age with (A) iAsIII percent (R = −0.06, p > 0.05); (B) iAsV percent (R = 0.195, p > 0.05); (C) MMAV percent (R = −0.17, p > 0.05); (D) DMAV percent (R = −0.133, p > 0.05); (E) PMI (R = −0.021, p > 0.05); (F) SMI (R = −0.007, p > 0.05) in the urine.
3.5. Profiles of Urinary Arsenic Metabolites
Based on population studies, the relative distributions of iAs, MMAV and DMAV in urine of various population groups seems to be fairly constant and in the range of 10–30%, 10–20% and 60–80%, respectively [9,27]. This study clearly showed that the percentages of arsenic species in urine were consistent with previously studies. However, it was documented that there were variations in arsenic methylation between population groups. A lower percent MMA in urine (in the range of 0.0–11%) of Atacamenos living in the Andes [28], whereas an unusual higher percent MMA in urine (20–30% on average) of people living in certain areas of Taiwan were also found [3,29]. The variation of arsenic metabolism between different population groups may be modulated by genetic polymorphism [29–31].
3.6. Arsenic Metabolism and Arsenic-Induced Skin Lesions
Urinary arsenic is generally used as the main biomarker of exposure and is regarded as the most reliable indicator of recent exposure to inorganic arsenic [9]. However, it has been reported that dietary intake of seafood, containing high-level organic arsenicals such as arsenobetaine (AsB), arsenocholine (AsC) and arsenosugars, which are regarded as non-toxic, can account for increases of DMA in urine [32]. In this study area, seafood is rare in the diet because of the poor economic conditions and long distance from the sea. Therefore, it was feasible to take total urinary arsenic as a biomarker of exposure to inorganic arsenic. It was shown that the subjects with skin lesions had a higher urinary arsenic excretion (Table 2), indicating a higher recent arsenic exposure than subjects without skin lesions. Dose of arsenic exposure was an important factor being association with its health outcomes. A case-control study from the Black-foot-disease area in Taiwan found that cases with skin cancer had higher total urinary arsenic [33].
The mechanisms of arsenic toxicity are not fully understood. In consideration of the end products in inorganic arsenic methylation being MMAV and DMAV, which are less reactive with tissue constituents and are more readily excreted in urine than inorganic arsenic [34], the methylation acts as a detoxification mechanism. However, the trivalent intermediates formed by reduction of MMAV and DMAV, namely MMAIII and DMAIII, are more toxic than arsenite [7], and have been found in human urine [35,36]. Because trivalent arsenic species are stronger protein-binders than the pentavalent species [37], MMAIII and DMAIII generated during the metabolism of arsenic in the cell are the species that interact with cytosolic proteins and may produce some of the toxic effects that accompany exposure to arsenic. Some scholars believe that various urinary arsenic metabolites may be associated with the individual susceptibility to clinical outcomes or diseases induced by arsenic exposure [2,34,37]. A pilot study conducted in Mexico found that the percentages of iAs and MMAV in individuals with cutaneous symptoms were higher than those individuals with normal skin, while the percentage of DMAV was lower [11]. In Bangladesh, a higher proportion of both inorganic arsenic and MMAV and a lower proportion of DMAV in urine were associated with a higher risk of skin lesions after adjusting for age, sex, socioeconomic status, tobacco use and cumulative arsenic exposure [1]. In Taiwan, an elevated urinary MMAV proportion was associated with an increased risk of skin cancer [33]. These literatures from arsenism induced by arsenic polluted water consumption suggested that reduced methylation capability with increased percent MMAV, decreased DMAV, or decreased DMAV/MMAV (SMI) is associated with health outcomes of arsenic exposure. The result of this study conducted in a CAP area of China was consistent with these previous studies. The subjects with skin lesions had a lower arsenic methylation capability, as defined by a higher percent iAs and percent MMAV and a lower percent DMAV and SMI in urine than subjects without skin lesions, which suggested that arsenic methylation capability obviously have an effect on susceptibility to arsenic-induced skin lesions.
It was documented that subjects with a lower percent MMAV in urine may tend to have a lower retention and a faster elimination of ingested arsenic than those with a higher percent MMAV [34]. Higher percent MMAV and lower percent DMAV in urine may be a result of inhibition of the second methylation step, which may increase the chances of cells to be exposed to the more toxic forms of MMAIII. It is possible that the higher MMAV and lower DMAV in urine are only a marker of higher MMAIII in the blood or inside the cells [37]. Besides, MMAIII could be oxidized into MMAV rapidly under room temperature conditions [38]. Therefore, it is highly possible that a significant proportion of the measured MMAV is actually derived from MMAIII. These could be possibly interpretations of people with a lower MMAV and a higher percent DMAV or a higher SMI tending to have a lower risk of developing arsenic induced disease.
3.7. Differences of Arsenic Metabolism between Men and Women
In this study, urinary arsenic in men and in women was compared (Table 3). A higher arsenic excretion in urine of women was observed and that may indicate a higher recent arsenic exposure of women. From the results of the face to face questionnaire, it was found that in Changshapu village women usually do housework and spend more time in the kitchen, while men usually cultivate the land and spend more time outdoors. In a study from Shaanxi Province, it was observed that the concentrations of inorganic arsenic in the air of kitchen are higher than in outdoor air [13]. It was possible that women might have more chances to be exposed to arsenic than men do. Therefore, different roles in family taken by men and women might be the reason why the urinary arsenic of women was significantly higher than that of men.
The results had shown that the distributions of arsenic species varied widely among men and women (Table 3). Women had a higher arsenic methylation capability than men in Changshapu village, as defined by significantly higher percent DMAV and SMI in women’s urine. Many other studies have also suggested that the methylation capability of women was better than that of men [1,30,39,40]. This finding was consistent with the results from a study in Guizhou Province, another CAP epidemical area of China [41]. Shraim and his colleagues found that among 51 individuals exposed to arsenic through coal-burning, more than 30% of them had symptoms of arsenism and women had a higher percent DMA but a lower percent iAs in urine. This difference of arsenic methylation capability may be explained by the effect of estrogen. It was believed that estrogens involved in arsenic methylation protect against the skin effects of arsenic [1]. Compared to men, the efficient methylation of arsenic in women might be related to the higher production of choline, which was then oxidized into betaine, the sole alternate methyl group to folate for the remethylation of homocysteine to methionine [42,43]. Elevated homocysteine levels, indicative of a lower one-carbon metabolism, were associated with less efficient methylation of arsenic [40]. Another possible reason for less efficient arsenic methylation in men might be attributed to that men might be exposed to more hazard factors such as smoking [44,45].
According to an epidemiologic investigation conducted in the CAP area in Shaanxi Province, the prevalence of skin lesions in women was lower than men (women vs. men: 16.57% and 21.61%, p < 0.05) [19]. In this study, it seemed paradoxical but interesting that women had higher urinary arsenic concentrations but lower susceptibility to skin lesions than men. It was shown in Table 3 that similar TAs concentrations were observed in men with skin lesions and women without skin lesions (men with skin lesions: 32.3 μg/g Cr, women without skin lesions: 31.92 μg/g Cr; p > 0.1), suggesting that the two groups had well-matched arsenic exposures; but arsenic exposure induced skin lesions in men but did not in women. These data suggested that men showed symptoms of poisoning before women under the same arsenic exposure and that men were more susceptible than women to arsenism. Studies from Bangladesh, Taiwan and India had all reported that men were more susceptible to arsenic-related skin effects than women [46–49]; however, the interpretations were not very clear. Lindberg and his colleagues investigated the interaction between gender and arsenic metabolism for the risk of developing skin lesions and established a hypothesis for the first time that the higher prevalence of arsenic-related skin lesions among men may be mainly attributed to the less efficient of arsenic metabolism among men [1]. In Table 3, women without skin lesions had lower percent MMAV and percent iAsV, but higher percent DMAV and SMI than men with skin lesions, indicating that the arsenic methylation capability, particularly the second arsenic methylation capability of women was markedly higher than that of men. These findings approved the hypothesis of Lindberg that women with higher arsenic exposure being less susceptible to skin lesions induced by coal arsenic exposure than men may be explained by the more efficient arsenic methylation of women, as defined by their higher percent DMAVand SMI in the urine of women.
3.8. Arsenic Excretion and Metabolism in Different Age
In this study, the content of arsenic in urine increased with age, and the arsenic methylation capability was not correlated with age. Age may be associated with arsenic metabolism. A study in Taiwan suggested that a decrease in the second methylation capability may be associated with the increasing of age [50]. A similar diminishing capability with age was also observed by other investigators [3,33]. On the other hand, a case-control study conducted in Taiwan to evaluate the association between exposure to low arsenic levels and urothelial cancer observed no association between age and any of the urinary arsenic indices in patients with urothelial cancer [39]. Aging was negatively associated with SMI in residents of northern Chile, but was not significant when the effect of length of residence in the contaminated area was adjusted [9]. In this study, the residents living in Changshapu had been exposed to arsenic since they were born. The time of duration of arsenic exposure significantly affected the amount of cumulative arsenic exposure. However, the cumulative arsenic exposure did not directly affect arsenic methylation metabolism. Therefore, we could not determine whether there was relationship between age and arsenic methylation interfered by the duration time of arsenic exposure. Furthermore, aging may be associated with a variety of functional changes (such as the liver and kidney) in the organs involved in the metabolism or retention of the metabolites of arsenic [51].
3.9. Limitations to the Present Study
Limitations to the present study include the small sample size. Both the use of spot urine samples and the storage of urine samples limited the size of the present sample, and this raises the possibility that findings may have been attributed to chance and hence need to be replicated. However, our sample size is comparable to those of numerous other studies of urinary arsenic metabolism [52,53]. Second, the other important risk factors related with skin lesions such as assets and education were not mentioned in present study, because each person’s assets and education are similar in this poor small village. In future work, we will solve these limitations.
4. Conclusions
Subjects with skin lesions had a higher concentration of urinary arsenic and a lower arsenic methylation capability than subjects without skin lesions. Not only the dose of arsenic exposure but also the arsenic methylation capability had an impact on the individual susceptibility to skin lesions induced by coal arsenic exposure. It was found that the capability for inorganic arsenic methylation is modified by sex. The lower susceptibility to skin lesions in women than in men was mainly explained by the more efficient methylation of arsenic, as defined by a higher percent DMAV and SMI in urine among women. Further studies with more larger sample sizes, study protocols and consideration of potential confounders need to be done in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (40571009).
- Conflict of InterestThe authors declare no conflict of interest.
References and Notes
- Lindberg, AL; Rahman, M; Persson, LA; Vahter, M. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol. Appl. Pharmacol 2008, 230, 9–16. [Google Scholar]
- Ahsan, H; Chen, Y; Kibriya, MG; Slavkovich, V; Parvez, F; Jasmine, F; Gamble, MV; Graziano, JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomarkers Prev 2007, 16, 1270–1278. [Google Scholar]
- Hsueh, YM; Huang, YL; Huang, CC; Wu, WL; Chen, HM; Yang, MH; Lue, LC; Chen, CJ. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J. Toxicol. Environ. Health Part A 1998, 54, 431–444. [Google Scholar]
- Walton, FS; Harmon, AW; Paul, DS; Drobna, Z; Patel, YM; Styblo, M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: Possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol 2004, 198, 424–433. [Google Scholar]
- Mushak, P; Crocetti, AF. Risk and revisionism in arsenic cancer risk assessment. Environ. Health Perspect 1995, 103, 684–689. [Google Scholar]
- Vahter, M; Concha, G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol 2001, 89, 1–5. [Google Scholar]
- Petrick, JS; Ayala-Fierro, F; Cullen, WR; Carter, DE; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol 2000, 163, 203–207. [Google Scholar]
- Mandal, BK; Ogra, Y; Suzuki, KT. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem. Res. Toxicol 2001, 14, 371–378. [Google Scholar]
- Hopenhayn-Rich, C; Biggs, ML; Kalman, DA; Moore, LE; Smith, AH. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ. Health Perspect 1996, 104, 1200–1207. [Google Scholar]
- Meza, MM; Kopplin, MJ; Burgess, JL; Gandolfi, AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res 2004, 96, 119–126. [Google Scholar]
- Del Razo, LM; Garcia-Vargas, GG; Vargas, H; Albores, A; Gonsebatt, ME; Montero, R; Ostrosky-Wegman, P; Kelsh, M; Cebrian, ME. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch. Toxicol 1997, 71, 211–217. [Google Scholar]
- Sun, G; Xu, Y; Li, X; Jin, Y; Li, B; Sun, X. Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ. Health Perspect 2007, 115, 648–652. [Google Scholar]
- Li, Y. Relation between arsenic in environment and coal burning-born endemic arsenism in the south of Shaanxi province. Chin J Endemiol 2004, 23, 562–565. (in Chinese). [Google Scholar]
- Chen, P; Tang, XY. Arsenic in coal of China. Coal Geol Chin 2002, 14, 18–24. (in Chinese). [Google Scholar]
- Yudovich, YE. Arsenic in coal: A review. Int. J. Coal Geol 2005, 61, 141–196. [Google Scholar]
- Ministry of Health. Hygienic Standards for the Design of Industrial Enterprises, TJ 36–1979; Ministry of Health of the People’s Republic of China: Beijing, China, 1979. [Google Scholar]
- Ministry of Health. Maximum Levels of Contaminants in Foods, GB 2762-2005; Ministry of Health of the People’s Republic of China: Beijing, China, 2005. [Google Scholar]
- Ministry of Health. Standards for Drinking Water Quality, GB 5749-2006; Ministry of Health of the People’s Republic of China: Beijing, China, 2006. [Google Scholar]
- Bai, GL; Liu, XL; Fan, ZX; Li, XQ. Epldemiologic survey on coal-burning endemic arsenism in Shaanxi province. Chin J Endemiol 2006, 25, 57–60. (in Chinese). [Google Scholar]
- Tang, L. The Transport and Transformation of Airborne Arsenic from Burning Stone-Like Coal in Shaanxi; Master dissertation; China Capital Normal University: Beijing, China, 2009. (in Chinese) [Google Scholar]
- Ministry of Health. Stnadards of Diagnosis for Endemic Arsenism, WS/T 211-2001; Ministry of Health of the People’s Republic of China: Beijing, China, 2001. [Google Scholar]
- World Medical Association Declaration Of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed on 30 May 2011).
- Cornelis, R; Heinzow, B; Herber, RF; Christensen, JM; Poulsen, OM; Sabbioni, E; Templeton, DM; Thomassen, Y; Vahter, M; Vesterberg, O. Sample collection guidelines for trace elements in blood and urine. J. Trace Elem. Med. Biol 1996, 10, 103–127. [Google Scholar]
- Chen, LW; Lu, X; Le, XC. Complementary chromatography separation combined with hydride generation-inductively coupled plasma mass spectrometry for arsenic speciation in human urine. Anal. Chim. Acta 2010, 675, 71–75. [Google Scholar]
- Xie, R; Johnson, W; Spayd, S; Hall, GS; Buckley, B. Arsenic speciation analysis of human urine using ion exchange chromatography coupled to inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2006, 578, 186–194. [Google Scholar]
- Lin, TH; Huang, YL. Chemical speciation of arsenic in urine of patients with blackfoot disease. Biol. Trace Elem. Res 1995, 48, 251–261. [Google Scholar]
- Vahter, M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci. Prog 1999, 82, 69–88. [Google Scholar]
- Vahter, M; Concha, G; Nermell, B; Nilsson, R; Dulout, F; Natarajan, AT. A unique metabolism of inorganic arsenic in native Andean women. Eur. J. Pharmacol 1995, 293, 455–462. [Google Scholar]
- Chiou, HY; Hsueh, YM; Hsieh, LL; Hsu, LI; Hsu, YH; Hsieh, FI; Wei, ML; Chen, HC; Yang, HT; Leu, LC; et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat. Res 1997, 386, 197–207. [Google Scholar]
- Lindberg, AL; Ekstrom, EC; Nermell, B; Rahman, M; Lonnerdal, B; Persson, LA; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res 2008, 106, 110–120. [Google Scholar]
- Vahter, M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 2000, 112–113, 209–217. [Google Scholar]
- Choi, BS; Choi, SJ; Kim, DW; Huang, M; Kim, NY; Park, KS; Kim, CY; Lee, HM; Yum, YN; Han, ES; et al. Effects of repeated seafood consumption on urinary excretion of arsenic species by volunteers. Arch. Environ. Contam. Toxicol 2010, 58, 222–229. [Google Scholar]
- Hsueh, YM; Chiou, HY; Huang, YL; Wu, WL; Huang, CC; Yang, MH; Lue, LC; Chen, GS; Chen, CJ. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol. Biomarkers Prev 1997, 6, 589–596. [Google Scholar]
- Vahter, M. Mechanisms of arsenic biotransformation. Toxicology 2002, 181–182, 211–217. [Google Scholar]
- Thomas, DJ; Styblo, M; Lin, S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol 2001, 176, 127–144. [Google Scholar]
- Styblo, M; Drobna, Z; Jaspers, I; Lin, S; Thomas, DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ. Health Perspect 2002, 110, 767–771. [Google Scholar]
- Tseng, CH. Arsenic methylation, urinary arsenic metabolites and human diseases: Current perspective. J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev 2007, 25, 1–22. [Google Scholar]
- Gong, Z. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. At. Spectrom 2001, 16, 1409–1413. [Google Scholar]
- Chung, CJ; Huang, CJ; Pu, YS; Su, CT; Huang, YK; Chen, YT; Hsueh, YM. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol. Appl. Pharmacol 2008, 226, 14–21. [Google Scholar]
- Gamble, MV; Liu, X; Ahsan, H; Pilsner, R; Ilievski, V; Slavkovich, V; Parvez, F; Levy, D; Factor-Litvak, P; Graziano, JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect 2005, 113, 1683–1688. [Google Scholar]
- Shraim, A; Cui, X; Li, S; Ng, JC; Wang, J; Jin, Y; Liu, Y; Guo, L; Li, D; Wang, S; et al. Arsenic speciation in the urine and hair of individuals exposed to airborne arsenic through coal-burning in Guizhou, PR China. Toxicol. Lett 2003, 137, 35–48. [Google Scholar]
- Zeisel, SH. Betaine supplementation and blood lipids: Fact or artifact? Nutr. Rev 2006, 64, 77–79. [Google Scholar]
- Chiuve, SE; Giovannucci, EL; Hankinson, SE; Zeisel, SH; Dougherty, LW; Willett, WC; Rimm, EB. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr 2007, 86, 1073–1081. [Google Scholar]
- Chen, YC; Su, HJ; Guo, YL; Hsueh, YM; Smith, TJ; Ryan, LM; Lee, MS; Christiani, DC. Arsenic methylation and bladder cancer risk in Taiwan. Canc. Causes Contr 2003, 14, 303–310. [Google Scholar]
- Lindberg, AL; Sohel, N; Rahman, M; Persson, LA; Vahter, M. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Environ. Health Perspect 2010, 118, 533–538. [Google Scholar]
- Vahter, M; Akesson, A; Liden, C; Ceccatelli, S; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res 2007, 104, 85–95. [Google Scholar]
- Chen, CJ; Hsu, LI; Wang, CH; Shih, WL; Hsu, YH; Tseng, MP; Lin, YC; Chou, WL; Chen, CY; Lee, CY; et al. Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol. Appl. Pharmacol 2005, 206, 198–206. [Google Scholar]
- Guha Mazumder, DN; Haque, R; Ghosh, N; De, BK; Santra, A; Chakraborty, D; Smith, AH. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int. J. Epidemiol 1998, 27, 871–877. [Google Scholar]
- Watanabe, C; Inaoka, T; Kadono, T; Nagano, M; Nakamura, S; Ushijima, K; Murayama, N; Miyazaki, K; Ohtsuka, R. Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: Analyses based on urinary arsenic. Environ. Health Perspect 2001, 109, 1265–1270. [Google Scholar]
- Huang, YK; Huang, YL; Hsueh, YM; Yang, MH; Wu, MM; Chen, SY; Hsu, LI; Chen, CJ. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: A twelve-year follow-up study. Canc. Causes Contr 2008, 19, 829–839. [Google Scholar]
- Tseng, CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol 2009, 235, 338–350. [Google Scholar]
- Cascio, C; Raab, A; Jenkins, RO; Feldmann, J; Meharg, AA; Haris, PI. The impact of a rice based diet on urinary arsenic. J. Environ. Monit 2011, 13, 257–265. [Google Scholar]
- Navas-Acien, A; Umans, JG; Howard, BV; Goessler, W; Francesconi, KA; Crainiceanu, CM; Silbergeld, EK; Guallar, E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The Strong Heart Study. Environ. Health Perspect 2009, 117, 1428–1433. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).