Abstract
Endocrine disrupting chemicals (EDC) are compounds that alter the normal functioning of the endocrine system of both wildlife and humans. A huge number of chemicals have been identified as endocrine disruptors, among them several pesticides. Pesticides are used to kill unwanted organisms in crops, public areas, homes and gardens, and parasites in medicine. Human are exposed to pesticides due to their occupations or through dietary and environmental exposure (water, soil, air). For several years, there have been enquiries about the impact of environmental factors on the occurrence of human pathologies. This paper reviews the current knowledge of the potential impacts of endocrine disruptor pesticides on human health.
1. Introduction
Since the discovery of DDT in 1939 [1], numerous pesticides (organochlorides, organophosphates, carbamates) have been developed and used extensively worldwide with few guidelines or restrictions. In industrialized countries, the Green Revolution of the 1960s significantly increased agricultural productivity by increasing the cultivated surfaces, mechanization, planting of hybrid crops with higher yields, and pest control [2]. This fight requires the massive use of pesticides, which are hazardous chemicals designed to repel or kill rodents, fungi, insects, and “weeds” that undermine intensive farming. The main effects of pesticides represent a great benefit for human health. Indeed, they help control agricultural pests (including diseases and weeds) and plant disease vectors, human and livestock disease vectors and nuisance organisms, and organisms that harm other human activities and structures (gardens, recreational areas, etc.). Moreover, they insure increased food production, a safe and secure food supply, and other secondary benefits [3]. However, many first generation pesticides have been found to be harmful to the environment. Some of them can persist in soils and aquatic sediments, bioconcentrate in the tissues of invertebrates and vertebrates, move up trophic chains, and affect top predators.
Rachel Carson’s book “Silent Spring”, published in 1962 [4], first drew attention to the hazard of the widespread extensive use of pesticides for the environment (namely birds) and also for human health. The book resulted in big modifications to the US national policy on pesticides, leading to a national ban on DDT and certain other pesticides.
Worldwide consumption of pesticides for agricultural use is constantly increasing, rising from 0.49 kg/ha in 1961 to 2 kg/ha in 2004 (see various web sources, such as for example http://ec.europa.eu/agriculture/envir/report/fr/pest_fr/report.htm#fig6; http://faostat.fao.org/site/424/default.aspx#ancor; http://www.goodplanet.info/eng/Food-Agriculture/Pesticides/Pesticides/(theme/266)), and humans and wildlife are today continuously exposed to a number of pesticides via the environment (surface water, ground water, soil), food and drinking water [5].
The World Health Organization (WHO) has reported that roughly three million pesticide poisonings occur annually, resulting in 220,000 deaths worldwide [6]. In some cases, it has been suggested that diseases such as cancer, allergies, neurological disorders and reproductive disorders may be connected to pesticide exposure.
This article focuses on pesticides that act as endocrine disruptors, and reviews the available information about the exposure and effects of such pesticides, as well as human biomonitoring for human health risk assessment.
2. Effects of Endocrine Disruptor Pesticides
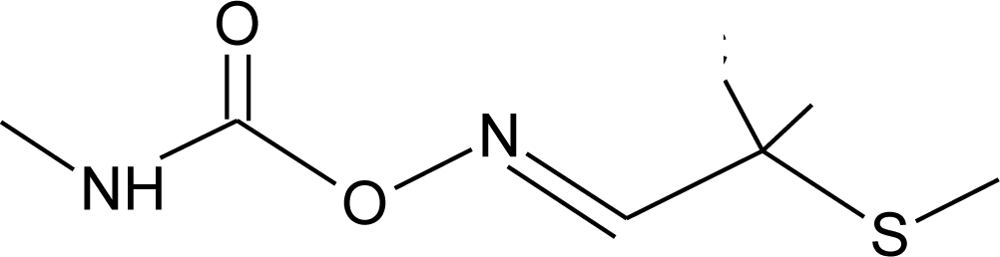
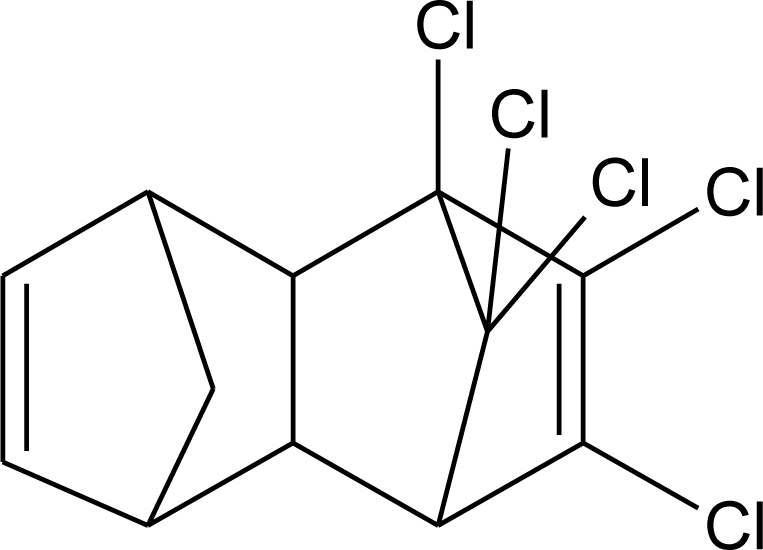
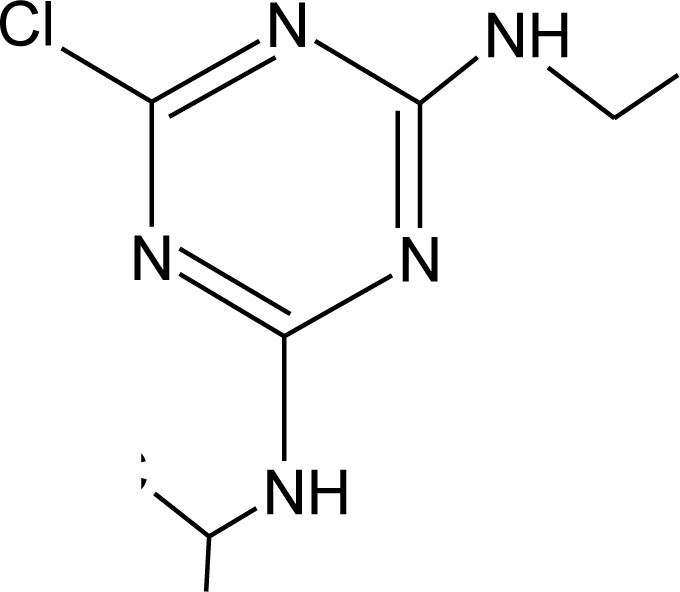
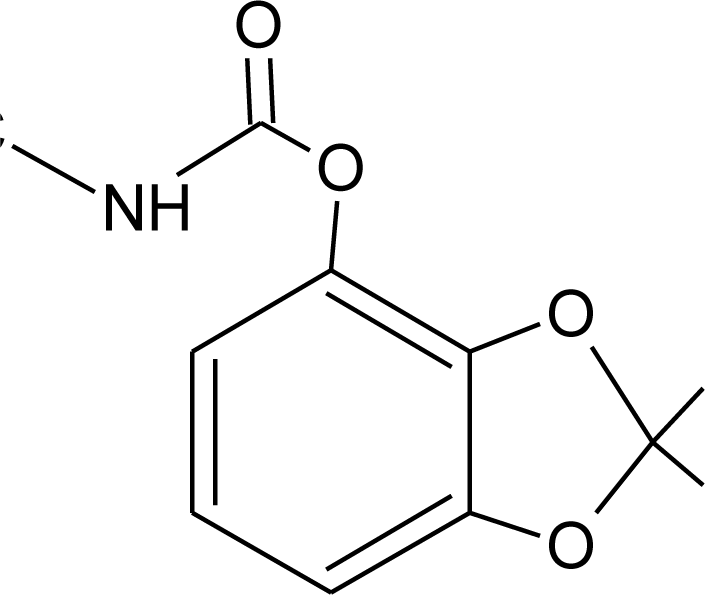
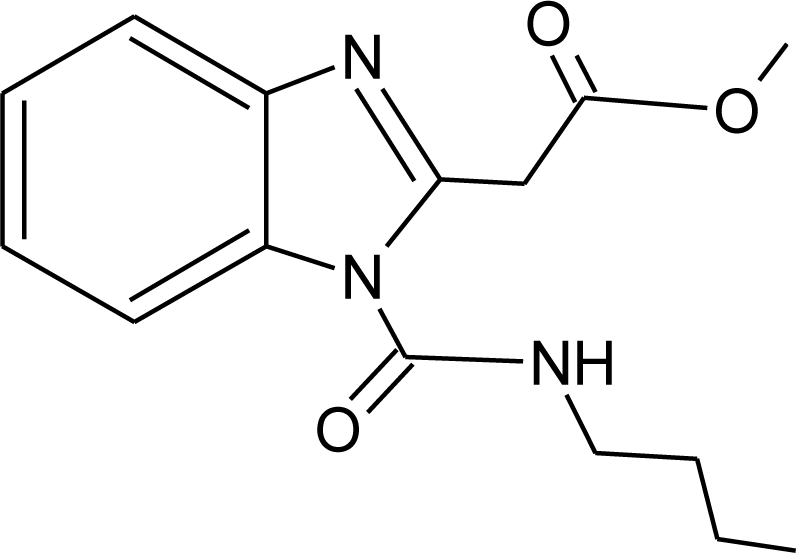
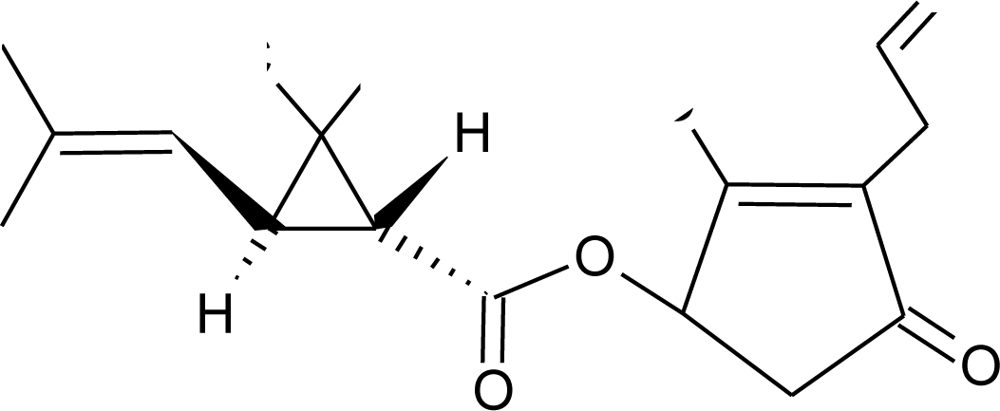
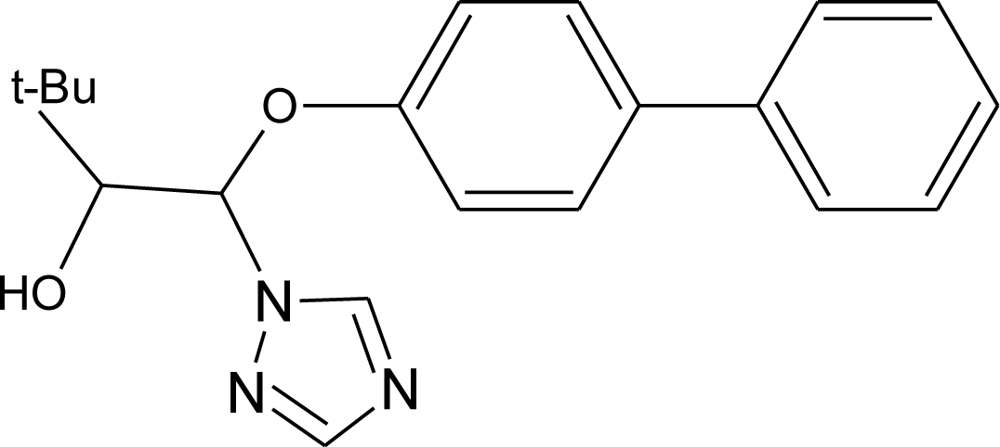
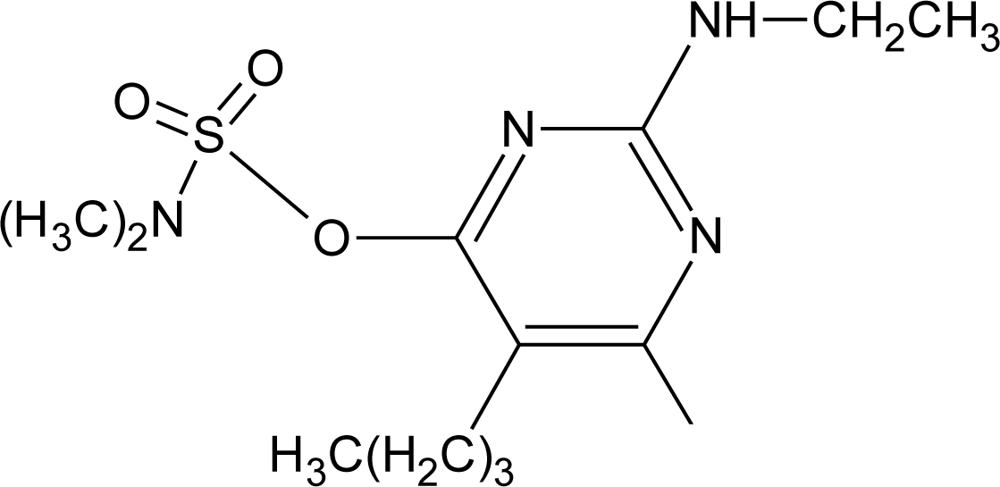
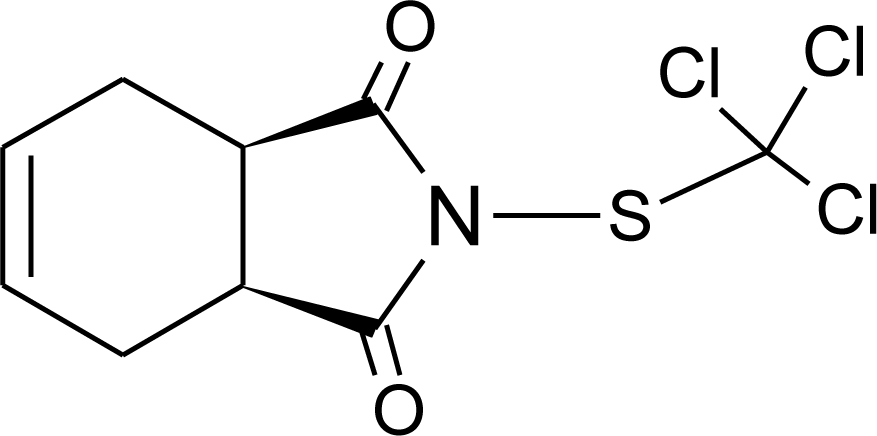
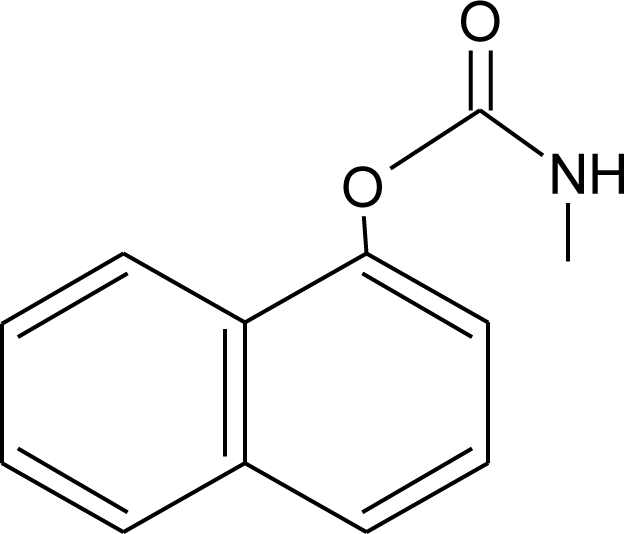
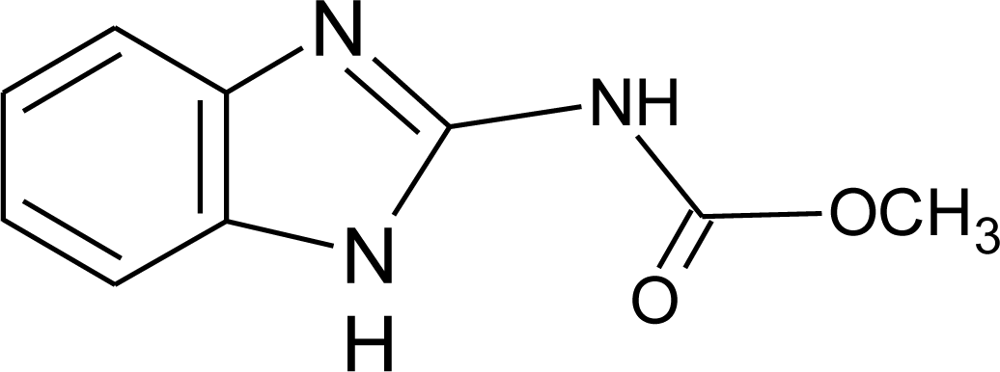
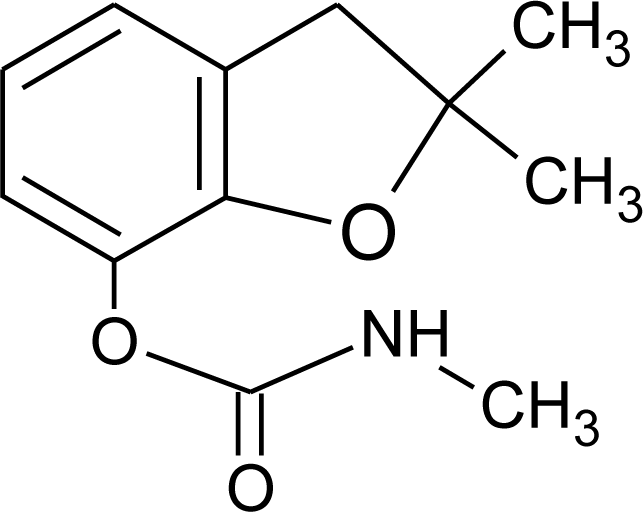
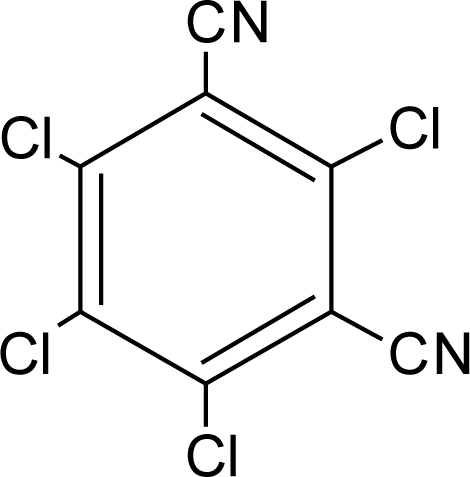
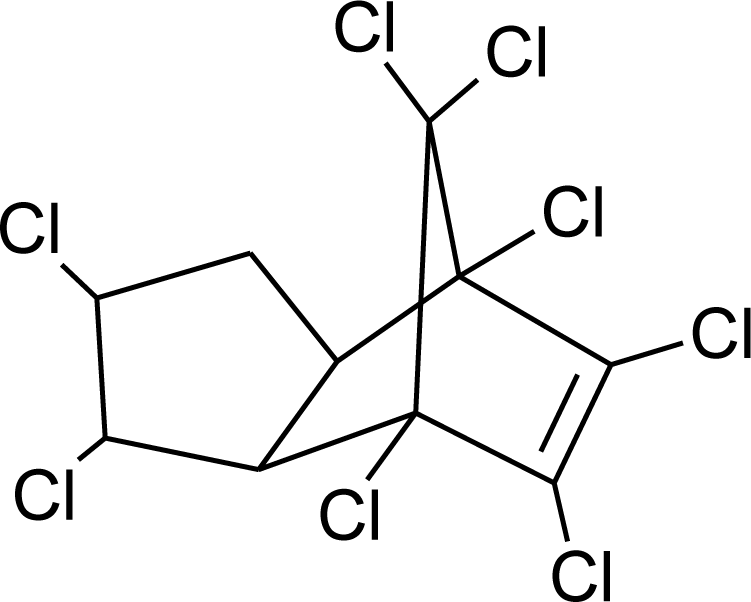
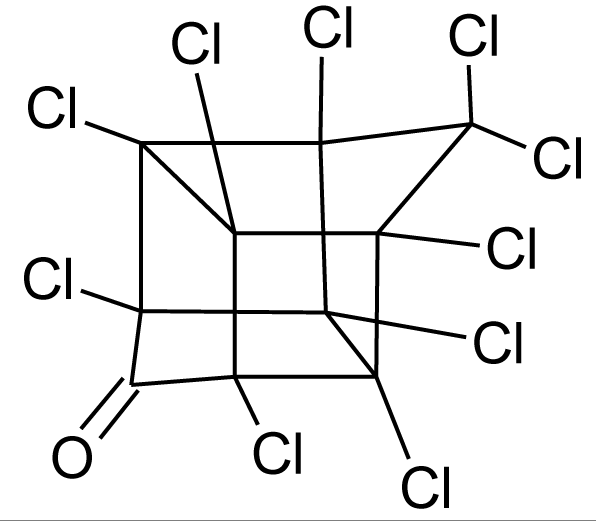
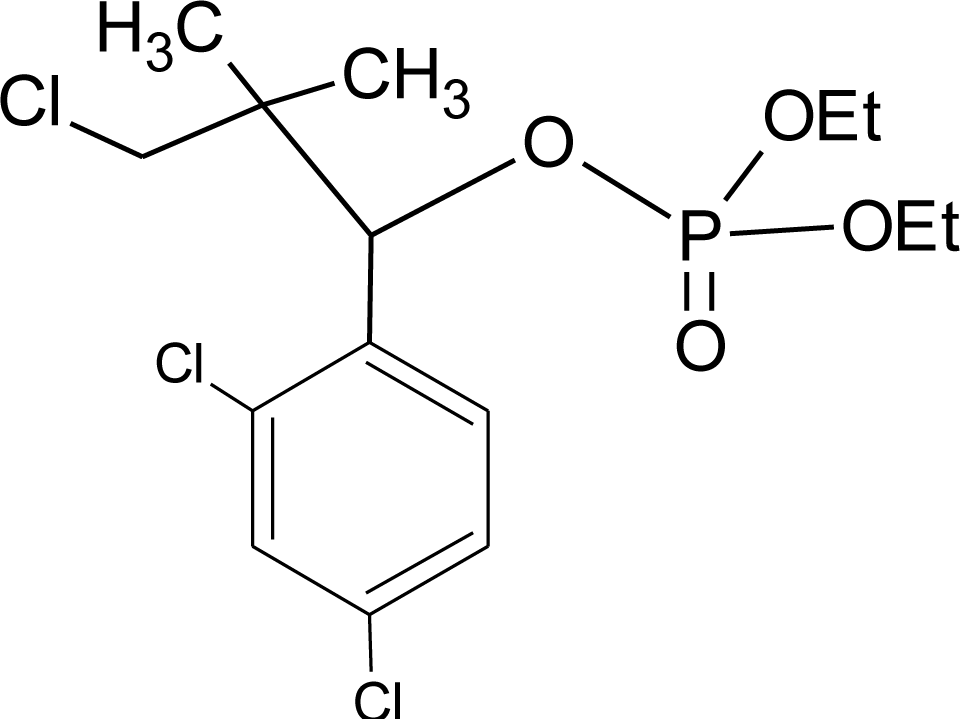
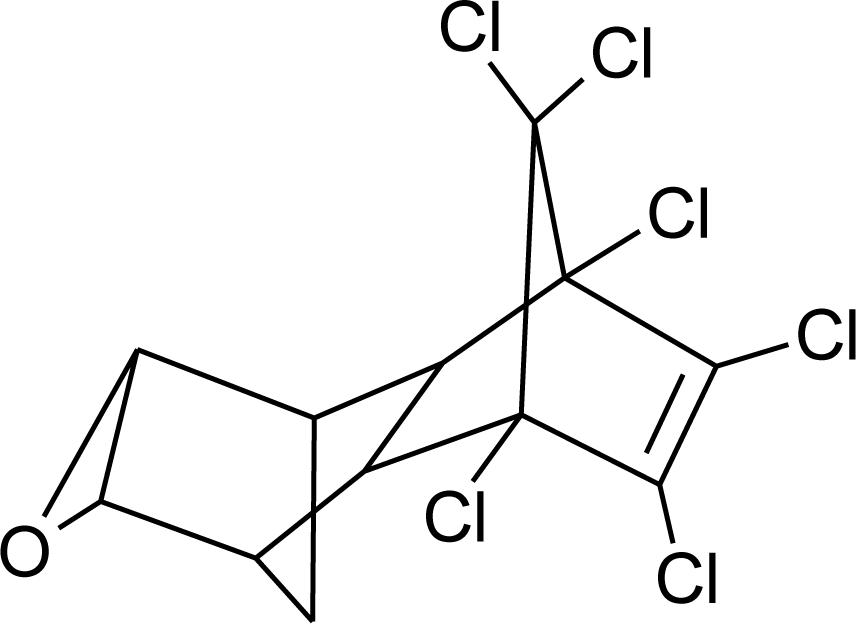
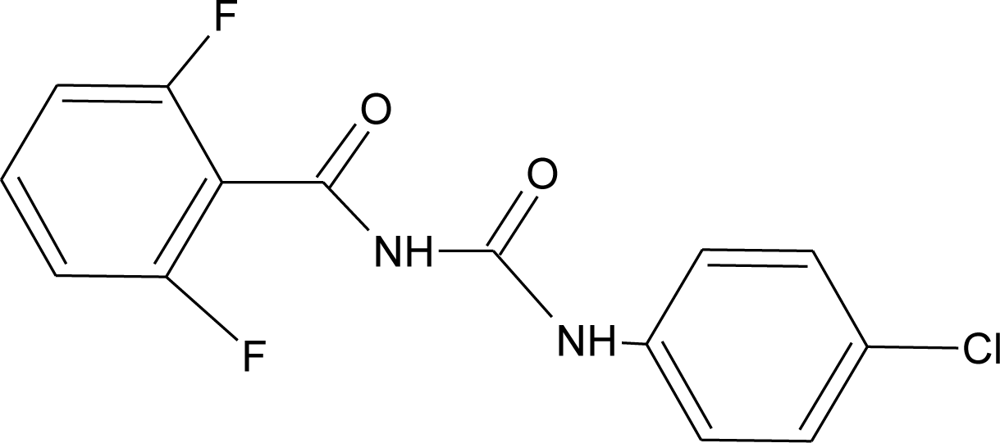
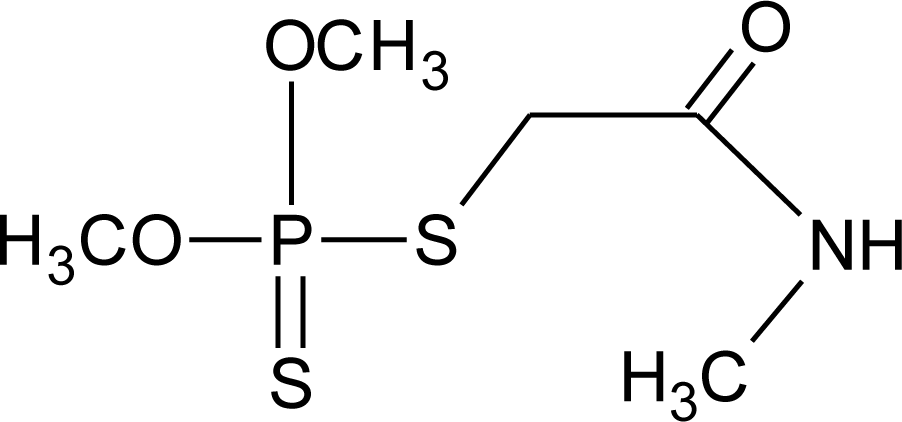
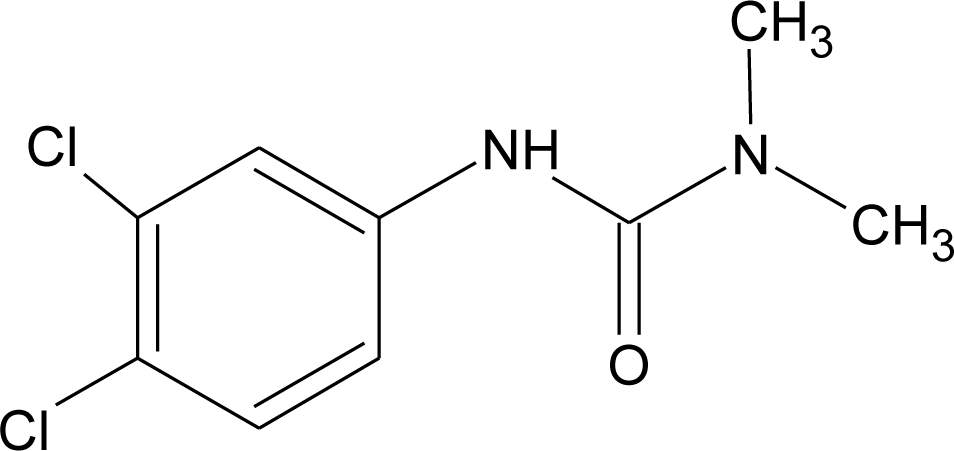
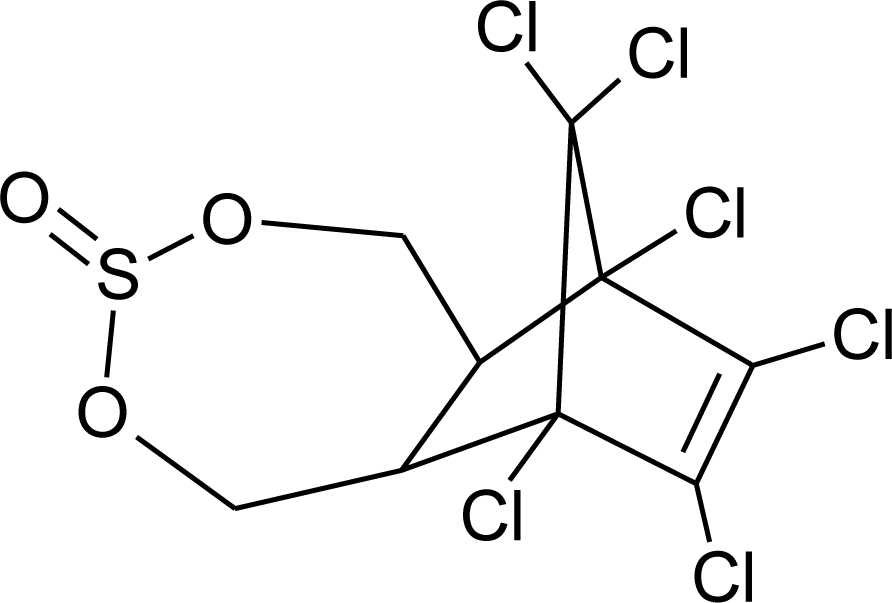
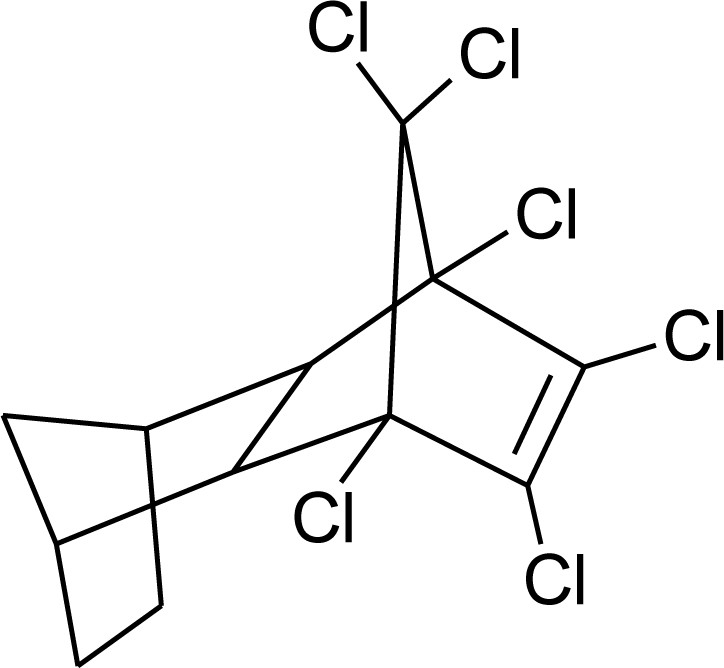
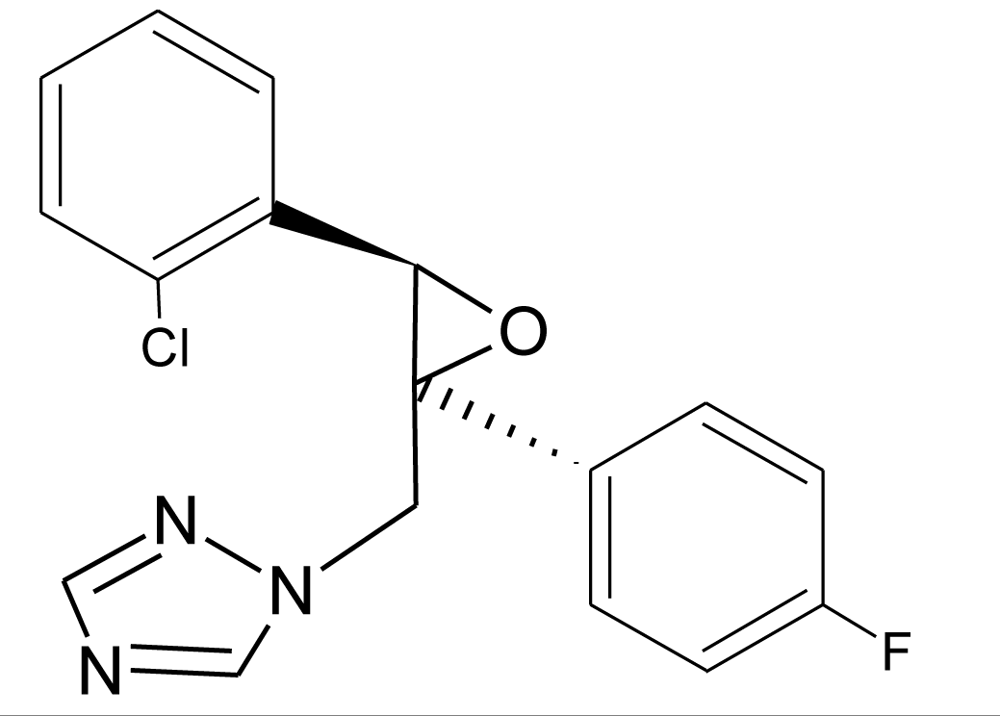
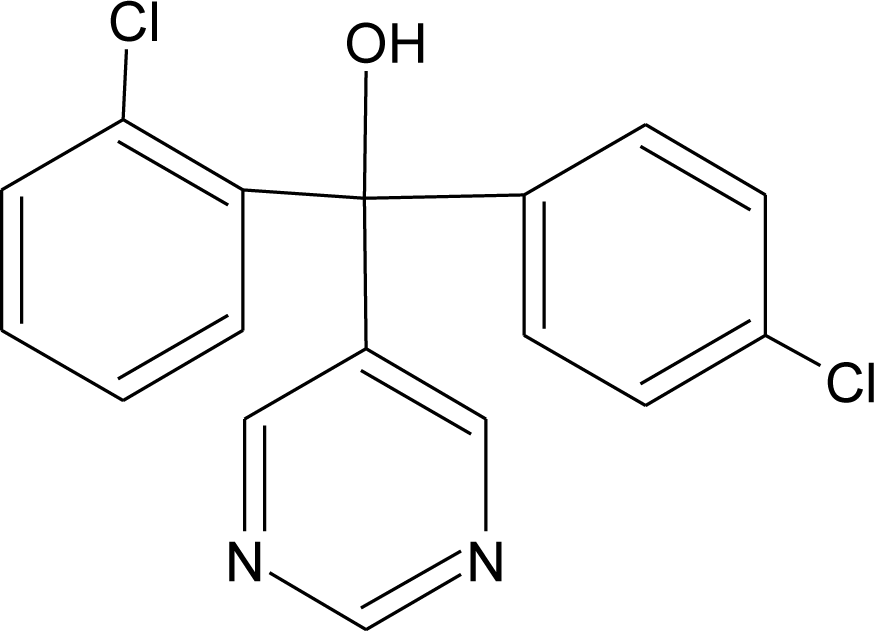
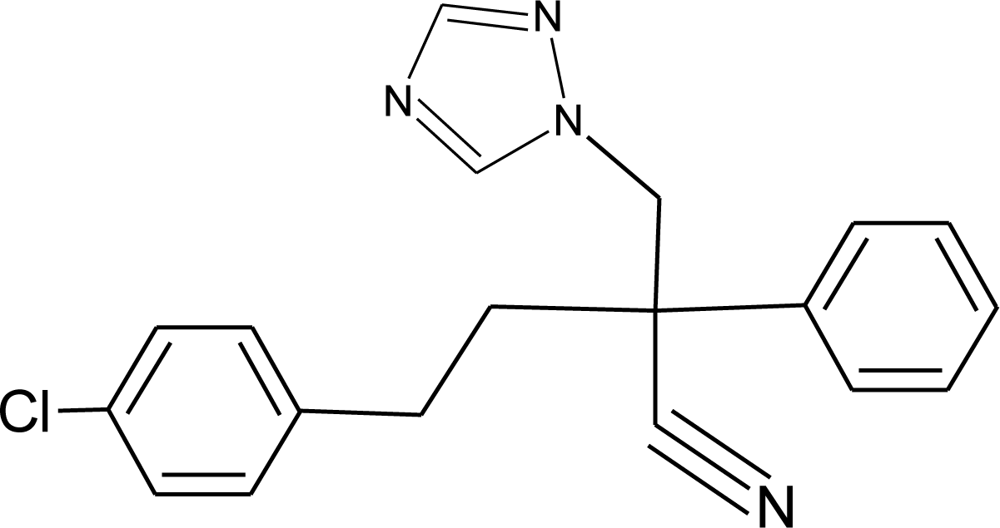
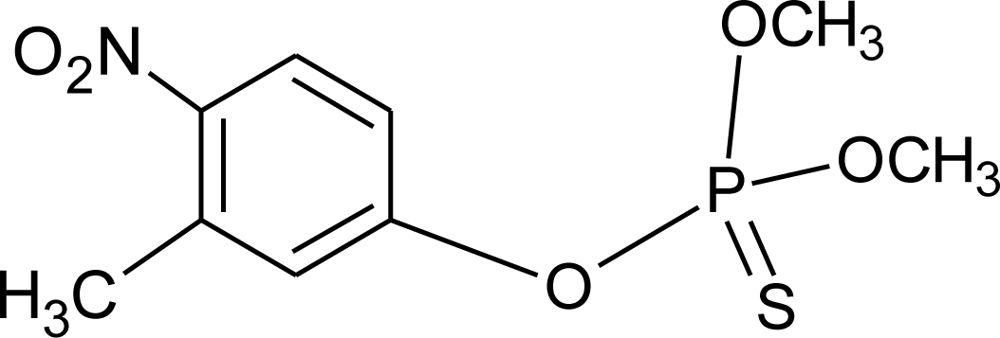
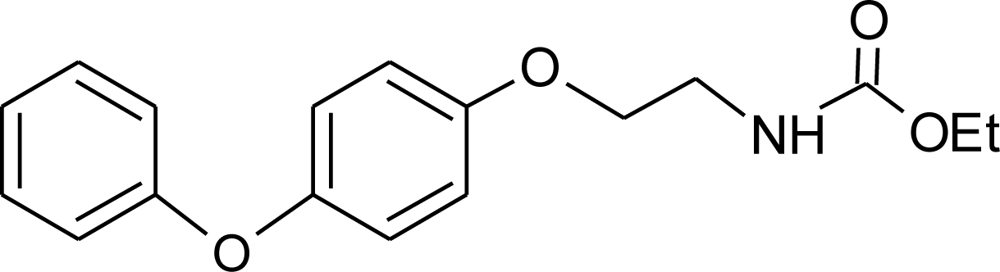
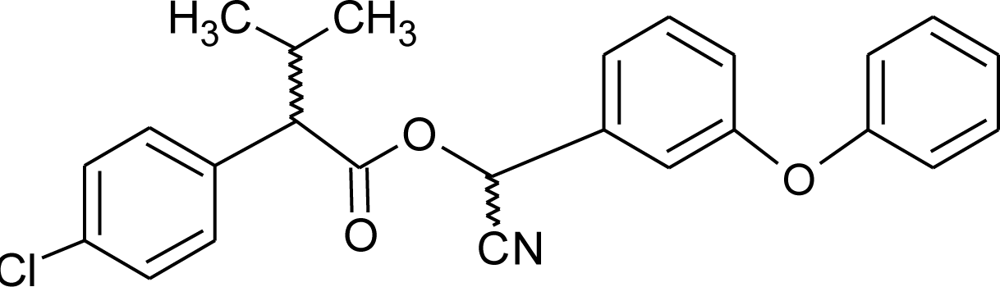
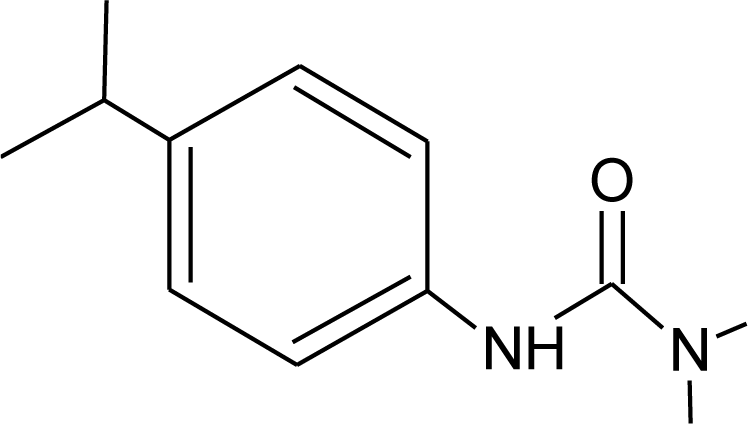
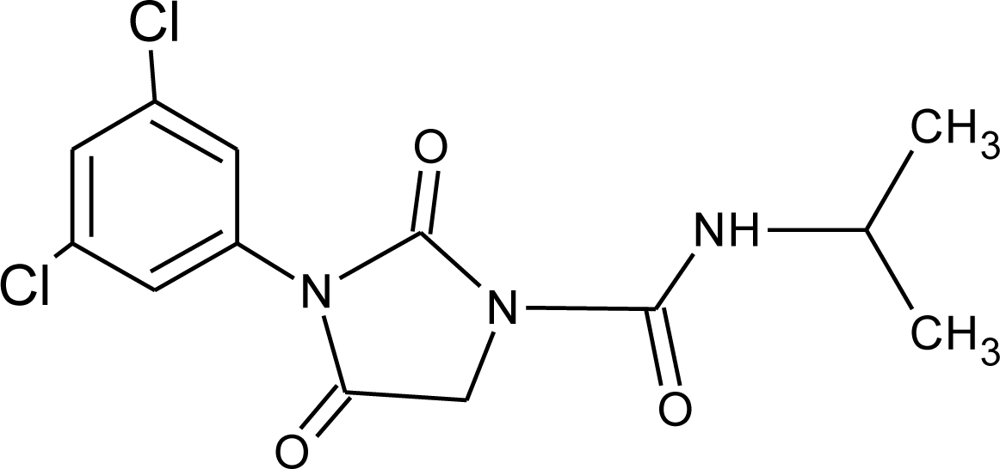
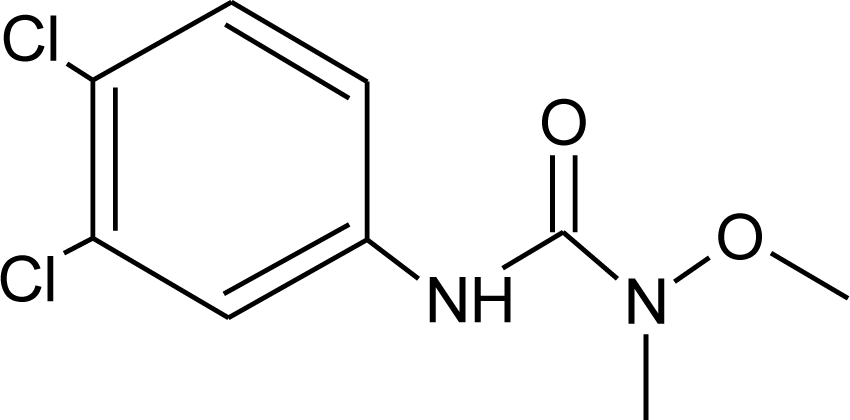
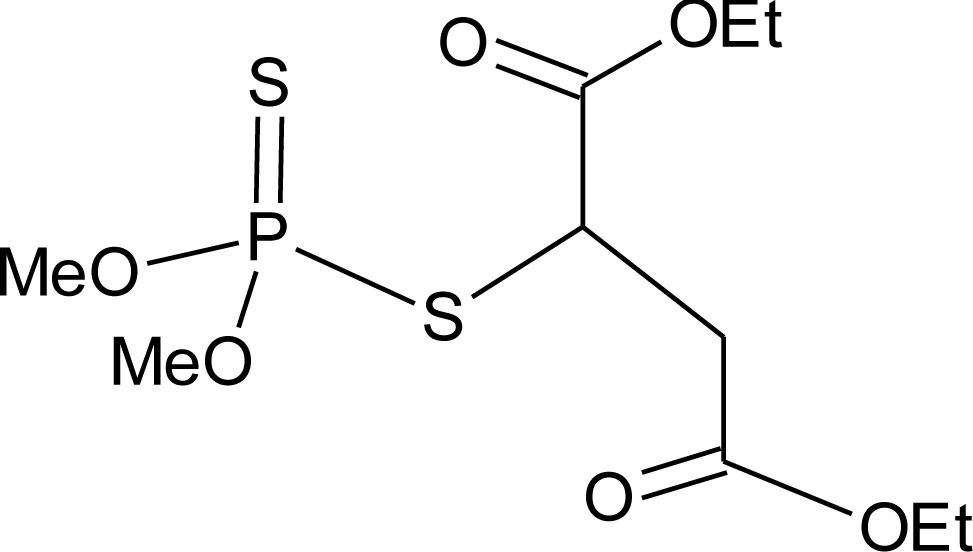
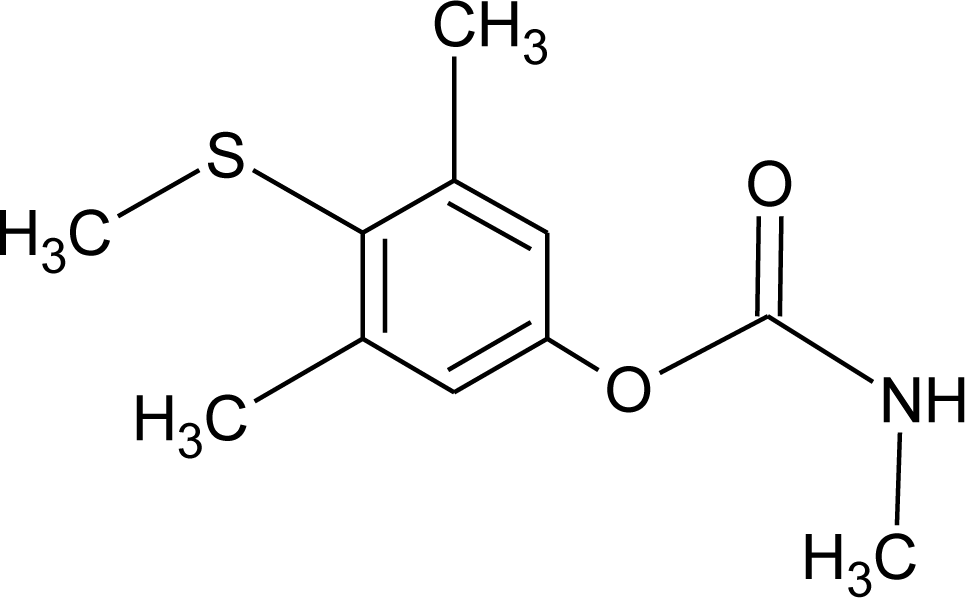
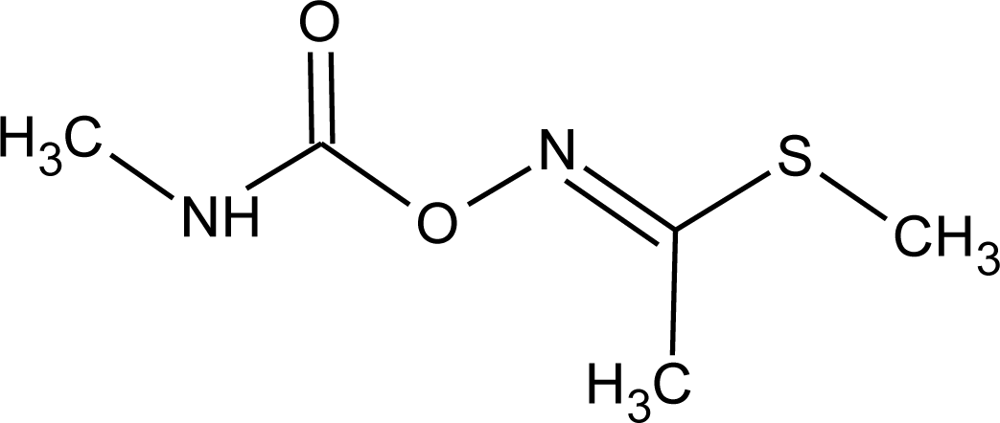
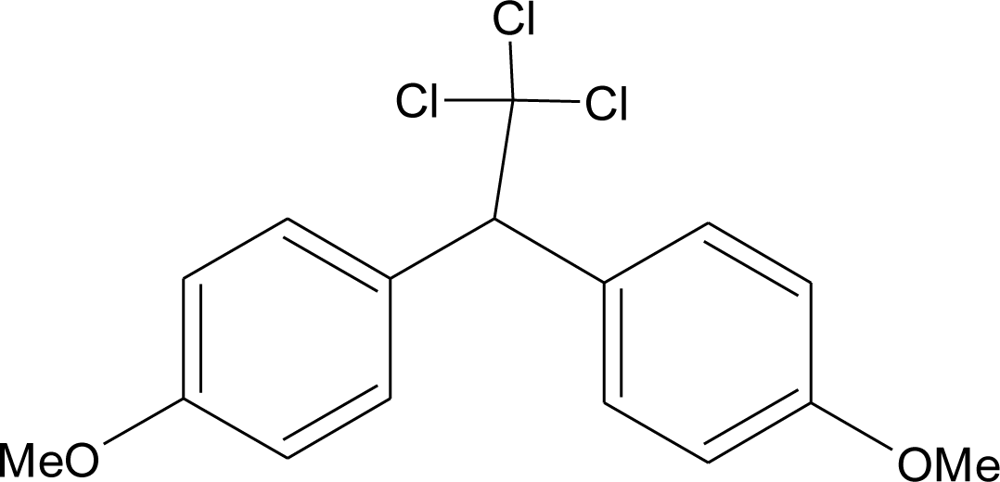
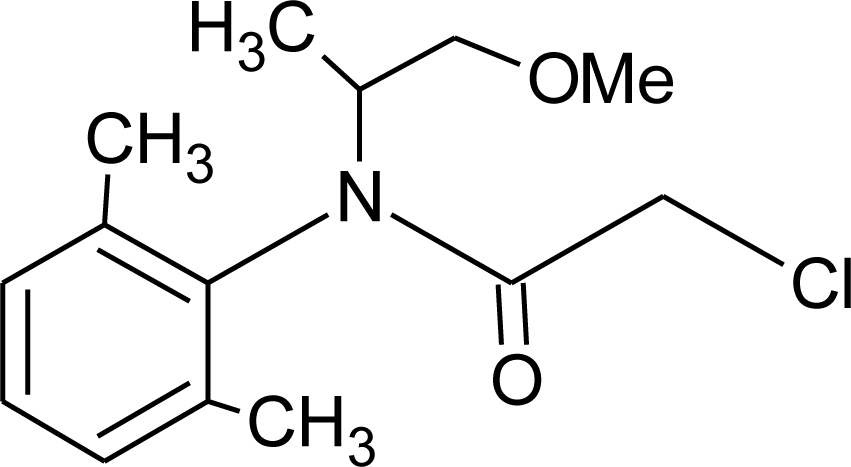
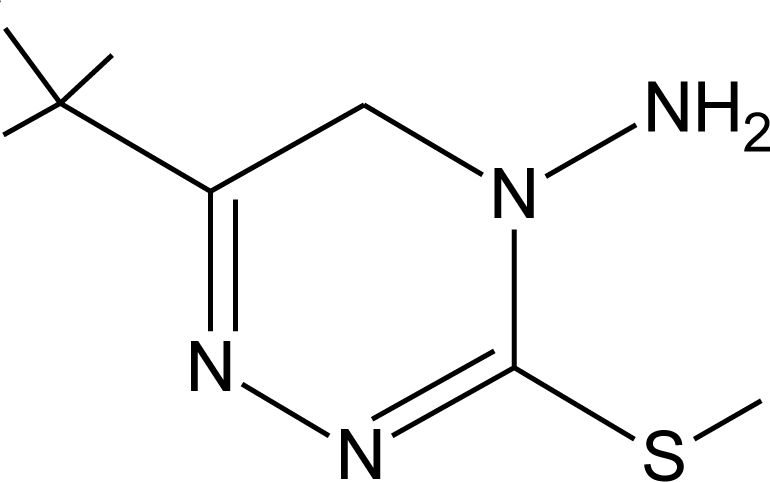
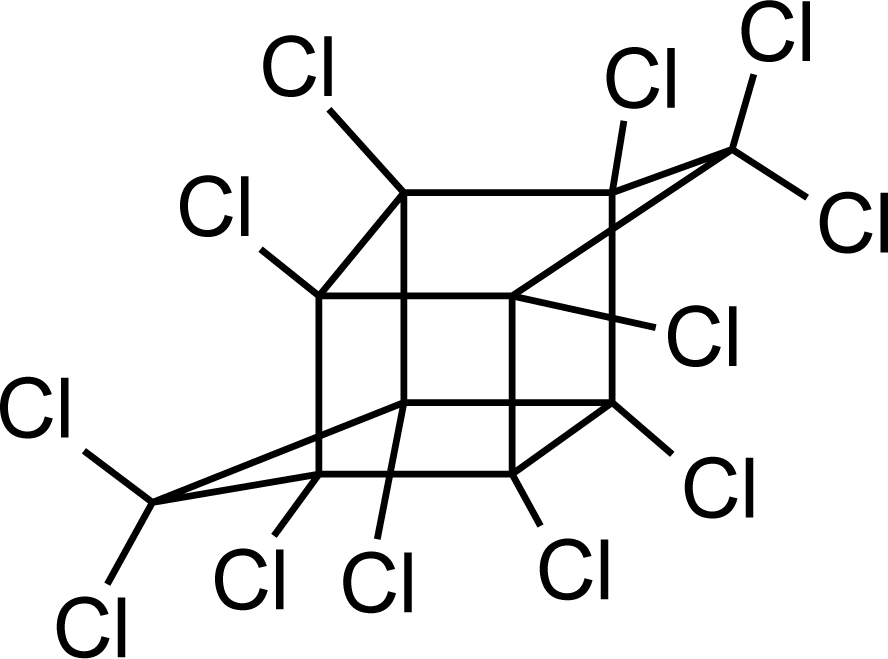
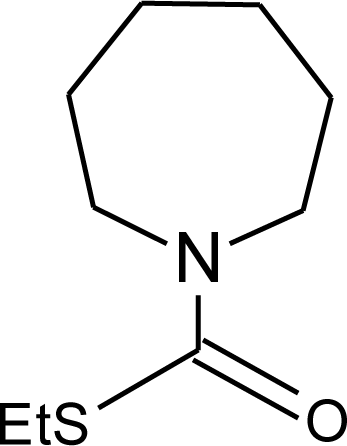
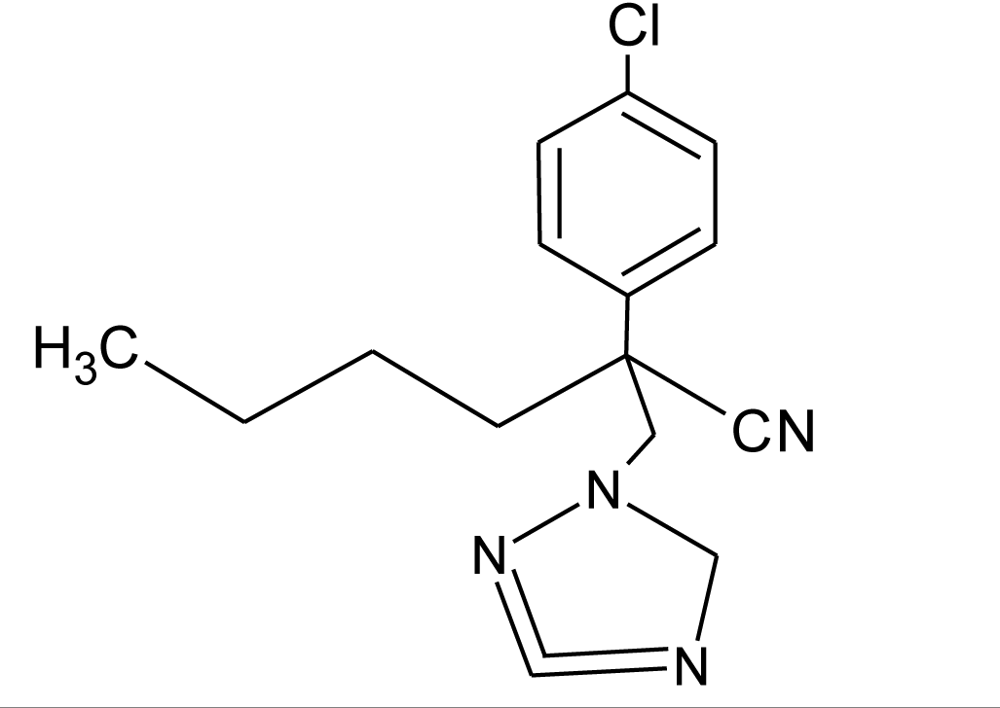
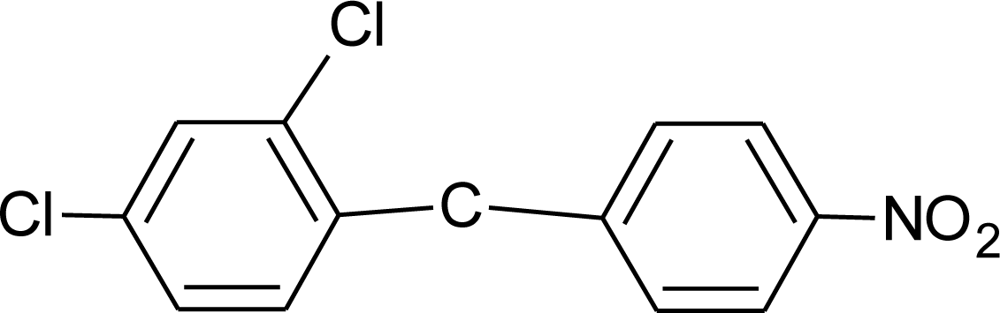
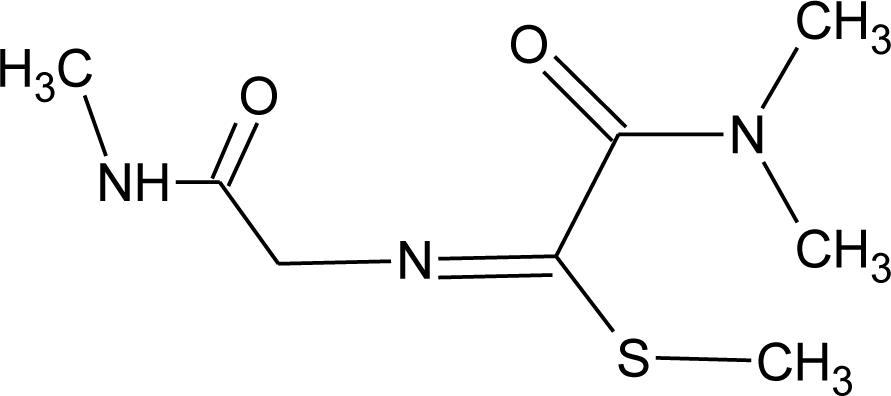
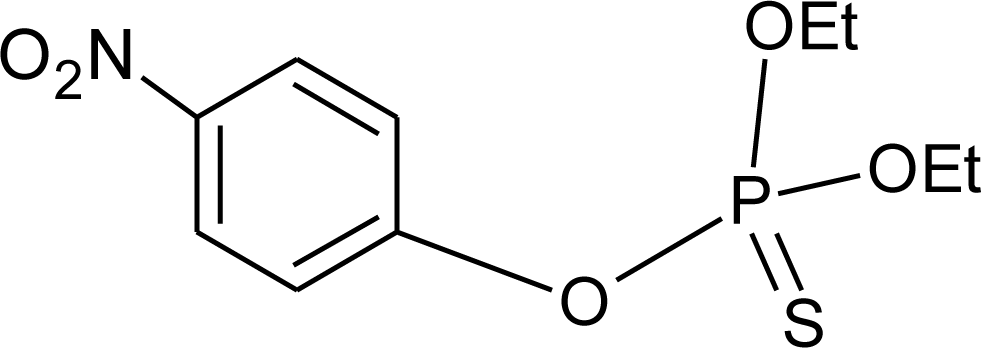
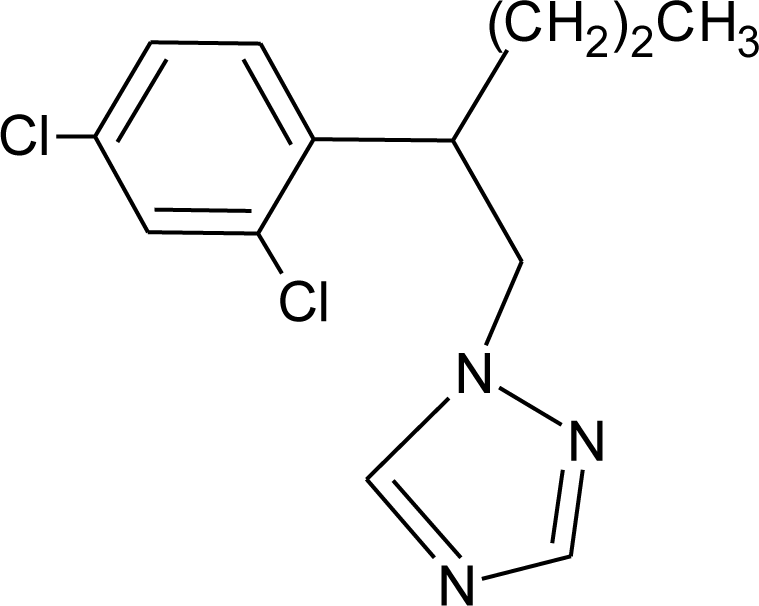
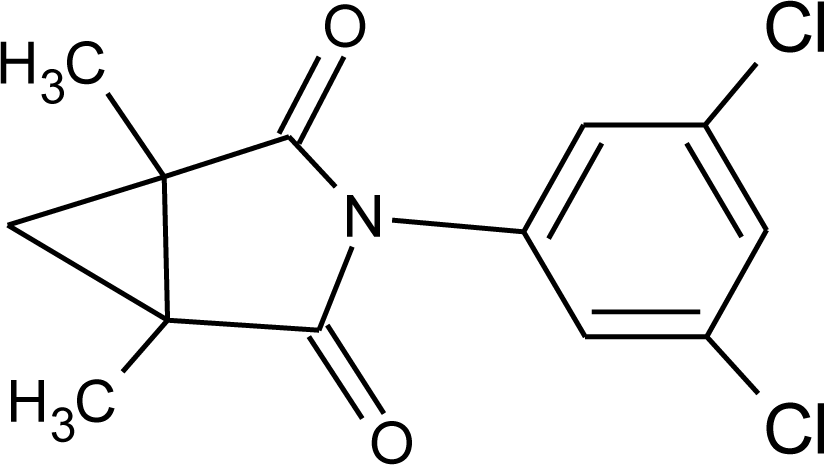
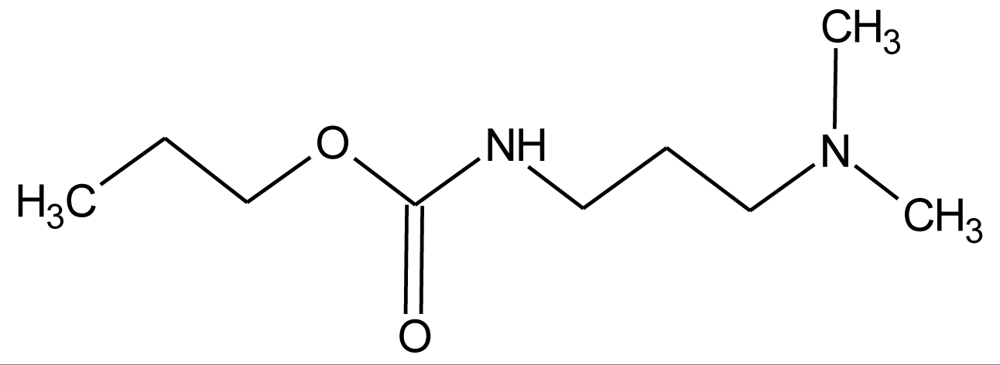
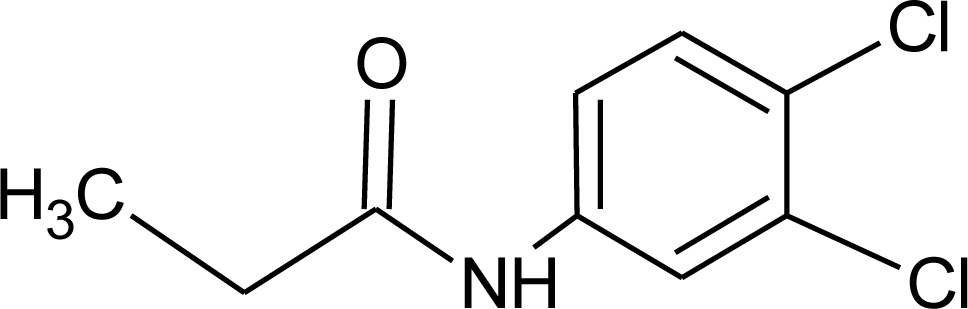
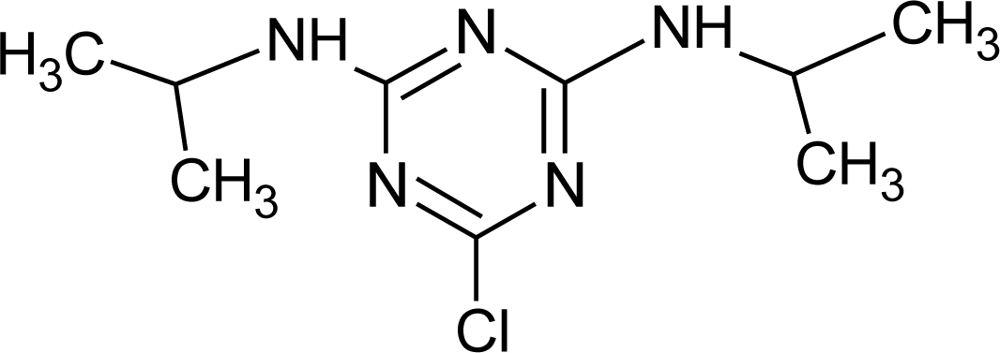
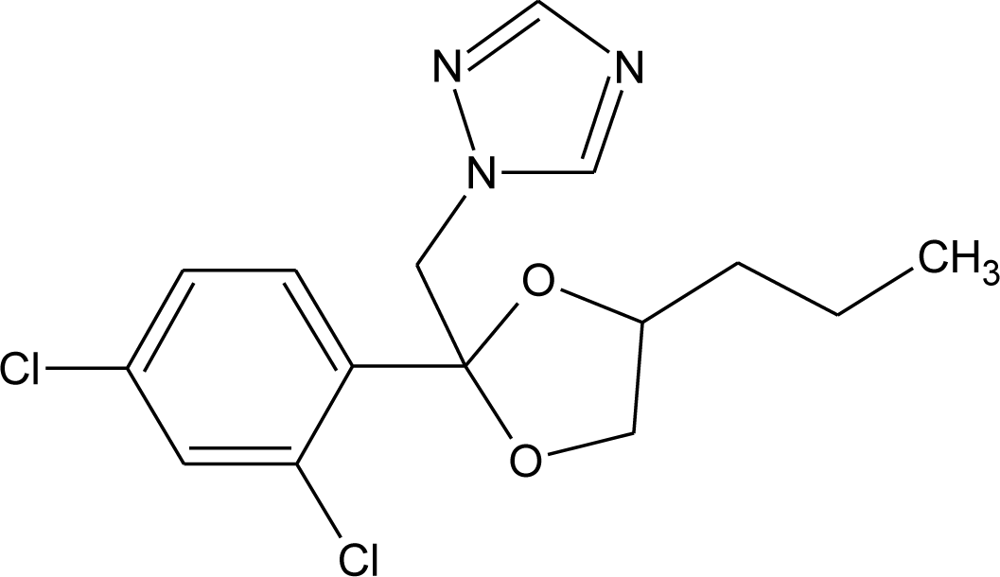
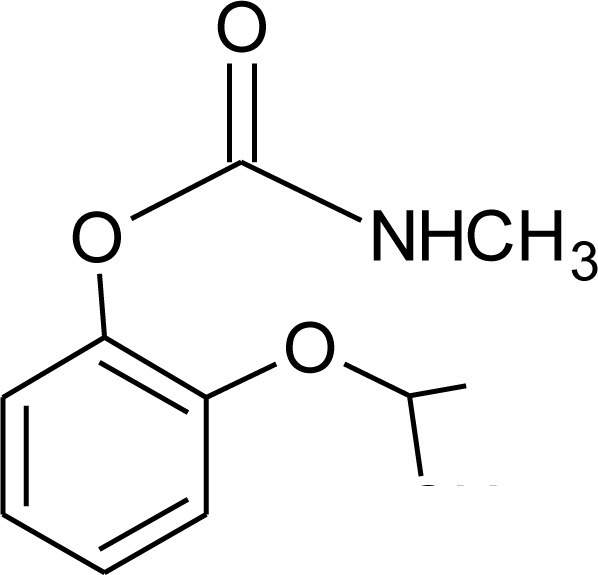
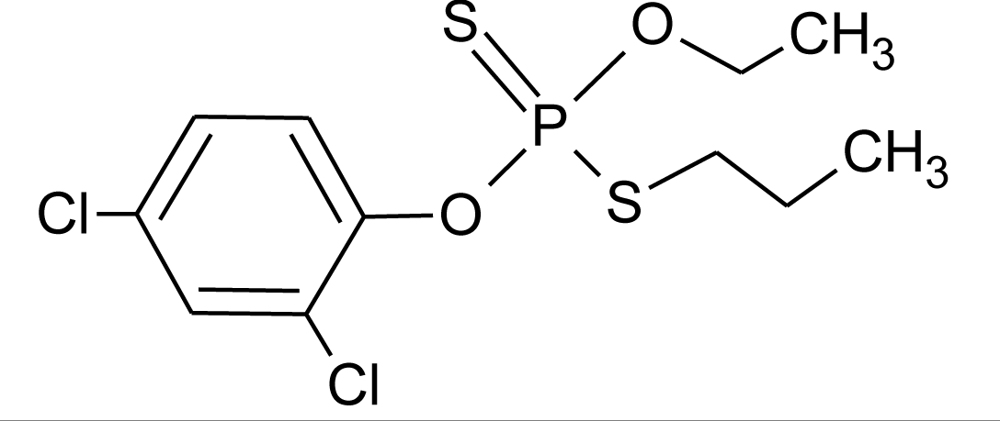
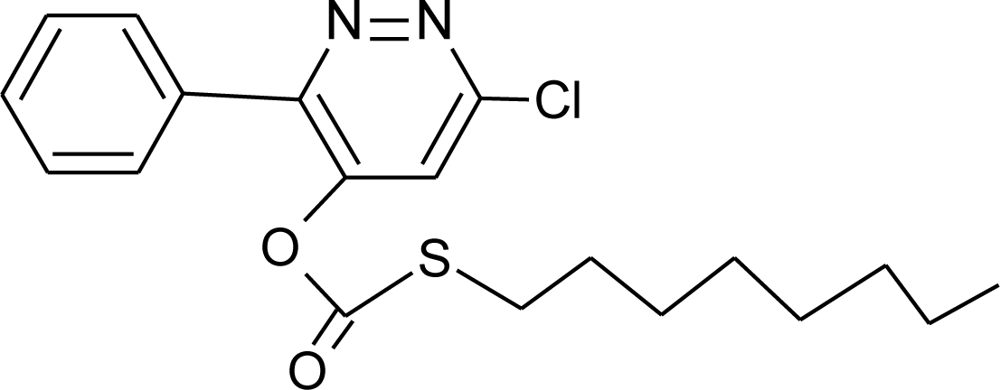
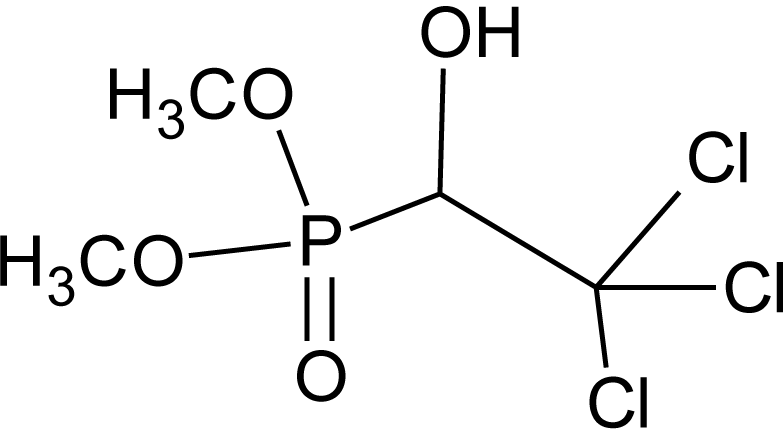
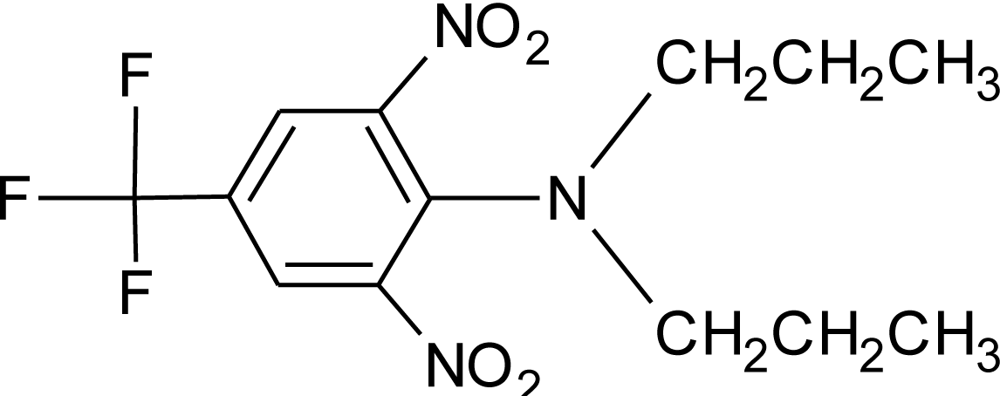
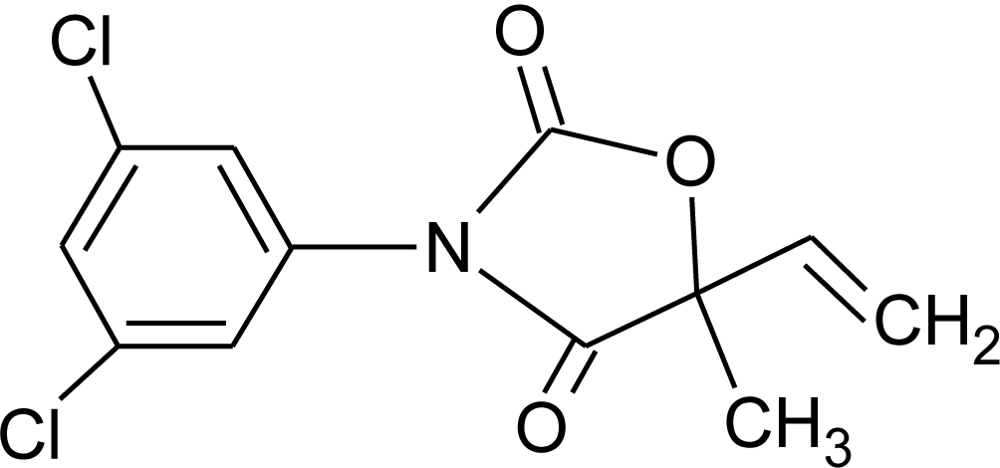
Many chemicals that have been identified as endocrine disruptors are pesticides [7–11]. About 105 substances can be listed, and most of them are shown in Table 1. Of these, 46% are insecticides, 21% herbicides and 31% fungicides; some of them were withdrawn from general use many years ago but are still found in the environment (ex. DDT and atrazine in several countries).

Table 1.
Effects of different groups of endocrine disruptorpesticides and their chemical structures (adapted from [65]).
EDCs act mainly by interfering with natural hormones because of their strong potential to bind to estrogen or androgen receptors [12] as shown in Table 1. In particular, EDCs can bind to and activate various hormone receptors (AR, ER, AhR, PXR, CAR, ERR) and then mimic the natural hormone’s action (agonist action). EDCs may also bind to these receptors without activating them. This antagonist action blocks the receptors and inhibits their action. Finally, EDCs may also interfere with the synthesis, transport, metabolism and elimination of hormones, thereby decreasing the concentration of natural hormones. For example, thyroid hormone production can be inhibited by some ten endocrine disruptor pesticides (amitrole, cyhalothrin, fipronil, ioxynil, maneb, mancozeb, pentachloronitro-benzene, prodiamine, pyrimethanil, thiazopyr, ziram, zineb, not shown in Table 1) [13–16].
At the environmental level, wildlife is particularly vulnerable to the endocrine disrupting effects of pesticides. Effects linked to endocrine disruption have been largely noted in invertebrates [17–21], reptiles [22–27], fish [28,29], birds [30–34] and mammals [35–38] as reviewed by Mnif et al. [39]. Most of them are linked to exposure to organochlorine pesticides (OC) and affect the reproductive function. For example, a study on Daphnia magna has shown that endosulfan sulphate disrupts the ecdysteroidal system (regulating processes such as molting and embryonic development) and juvenile hormone activity (regulating the sex ratio) of crustaceans [40,41]. Another example is the influence of linuron on reproductive hormone production [42], testosterone production in rats being significantly reduced after in utero exposure to linuron, whereas progesterone production was not affected [42].
At the human level, endocrine disruptor pesticides have also been shown to disrupt reproductive and sexual development, and these effects seem to depend on several factors, including gender, age, diet, and occupation.
Age is a particularly sensitive factor. Human fetuses, infants and children show greater susceptibility than adults [43–45]. Much of the damage caused by EDC occurs during gametogenesis and the early development of the fetus [45–48]. However, the effects may not become apparent until adulthood. Moreover, fetuses and infants receive greater doses due to the mobilization of maternal fat reserves during pregnancy [47–50] and breastfeeding [49,51]. Infants are extremely vulnerable to pre and postnatal exposure to endocrine disruptor pesticides, resulting in a wide range of adverse health effects including possible long-term impacts on intellectual function [52,53] and delayed effects on the central nervous system functioning [54,55].
Likewise, residential proximity to agricultural activity is a factor often described to explain developmental abnormalities in epidemiological studies of low birth weight [56], fetal death [57], and childhood cancers [58]. Additionally, a higher prevalence of cryptorchidism and hypospadias [59,60] was found in areas with extensive farming and pesticide use and in sons of women working as gardeners [61]. Recently, a relation has been reported between cryptorchidism and persistent pesticide concentration in maternal breast milk [47,62,63]. The impact of endocrine disruptor pesticides on fertility has also been discussed [64].
Based on the epidemiological studies since 2000, the study concluded that pesticide exposure may affect spermatogenesis leading to poor semen quality and reduced male fertility. Furthermore, an increasing number of epidemiological studies tend to link environmental exposure to pesticides and hormone-dependent cancer risks. High levels of PCBs, DDE, and DDT have been found in fat samples from women with breast cancer [141]. The risk of breast cancer is said to be four times greater in women with increased blood levels of DDE 142]. One of the latest epidemiological studies performed in Spain between 1999 and 2009 shows that among a total of 2,661 cases of breast cancer reported in the female population, 2,173 (81%) were observed in areas of high pesticide contamination [143]. Moreover, it was also suggested that women with hormone responsive breast cancer have a higher DDE body burden than women with benign breast disease [144]. Similar studies have revealed correlations between damage to the immune system and increased amounts of organochlorine residues in certain cancerous tissues [145]. Numerous other studies support the hypothesis that pesticide exposure influences the risk of breast cancer [146], but few of them are really conclusive due to some inconsistent data across the study. Further research is required to explore long-term follow-up beginning in early life, with opportunities for exposure measurement at critical periods of vulnerability. Moreover, improvements are needed in the cohort sample size and standardization of exposure assessments methods. Finally, researchers also need to consider simultaneous co-exposures to these substances and other chemicals and whether they may act in an additive, synergistic, or antagonistic manner [147].
There may also be a connection between pesticide exposure and prostate cancer. Various studies have consistently demonstrated a higher risk in agricultural populations than in the general population [148–150]. For example, pesticides (in particular DDT) were associated with a statistically significant higher rate of prostate cancer among farmers (exposed to organochloride pesticides) in a multi-site case-control study carried out in five rural areas between 1990–92 in Italy [151]. Several studies in the USA and Sweden showed that farmers and commercial pesticide applicators have a slightly and/or significantly higher rate of prostate cancer than the general population [148,152,153].
Several meta-analyses, cohort studies and case-control studies on the risk of prostate cancer in populations exposed occupationally or professionally to pesticides have been conducted in recent years [154] (and reference therein). They all showed a significantly higher risk of prostate cancer estimated at between 10 and 40%, the higher values being for professional exposure. Quite recently, a study analyzed the relationship between exposure to chlordecone (organochloride pesticide extensively used for more than 30 years in the French West Indies to control the banana root borer) and the risk of prostate cancer [155]. It showed a significant increase in the risk of prostate cancer with increasing plasma chlordecone concentrations and supported the hypothesis that exposure to environmental estrogens may increase the risk of prostate cancer.
However, in spite of these outcomes, the hypothesis that such excess risk is related to the use of pesticides has not yet been formally demonstrated. Various other factors have been suggested to explain the increase in prostate cancer in agricultural or rural populations, such as dietary issues, contact with infectious agents via livestock, dust, tobacco and chemical products [154]. Rigorous studies with larger cases that accurately and objectively estimate pesticide exposures and consider gene-environment interactions are needed to determine a potential relationship between pesticides and prostate cancer.
3. Biomonitoring for Human Exposure Assessment
Exposure to pesticides can occur via numerous pathways, including household use of pesticide products, dietary exposure to pesticide residues, and exposure to agricultural drift. Biological monitoring studies indicate that pesticide exposures are widespread in the human population. Dietary exposure comes from residues in fruits, vegetables, and from contaminated meat, fish, rice and dairy products. The European Commission [156] estimated that, in 2005, consumer intake was always below the acceptable daily intakes (ADI) for long-term exposures. Several recent studies also show the difference between EDI and ADI [157–159]. However, according to the European report, the acute reference dose (a parameter for high short-term intakes, usually in one day or one meal) was exceeded for some pesticides in different vegetables and fruits and 26.7% of samples show residues of more than one pesticide, with a significant upward trend as compared to previous years. Food intake is not the only exposure pathway for the general population. Living near sites where pesticides are used, manufactured or disposed of may significantly increase environmental exposure through inhalation and contact with air, water and soil [160–163].
Human exposure to pesticides is assessed by measuring the levels of pesticides in human samples such as breast milk, maternal blood and serum, urine and sometime umbilical cord blood. Improvements in analytical techniques have made it possible to detect pesticides and their metabolites at trace levels (from milligrams per kilogram to femtograms per kilogram in some laboratories) in almost all human samples. Table 1 reports the detection of pesticides in human samples.
Most of these studies show evidence of higher levels of pesticides in the exposed population (for example due to their occupation or geographical location) than in non-exposed control people. For example, a relation was established between employment in agriculture of Spanish women during pregnancy and serum levels of organochlorine endocrine disruptor pesticides, including DDT and isomers (despite their being banned in Spain since 1977) [164]. Also, higher levels of DDTs and HCHs were found in maternal milk and blood samples in Chinese provinces than in developed or industrialized countries [110]. Finally, higher concentrations of several pesticides were also found in urine and plasma of pregnant Israeli women compared to other populations of pregnant women in the United States and the Netherlands.
Pesticide metabolites are also monitored in human samples because they can be representative of a global contamination. This is particularly true for organophosphorus compounds. Alkyl phosphates have been reported in human samples (urine, hair) as representative of exposure to organophosphate pesticides [165–168], and some authors have observed a significant difference in the levels of total dialkyl phosphates among exposed and no exposed groups.
4. Discussion and Perspectives
Endocrine disruptor pesticides are widely used for agricultural, municipal, home and medical purposes worldwide. Humans are exposed to these compounds, and due to their toxic properties, the consequences of this exposure on human hormoel-dependent pathologies are being established. Most risk assessment studies and some epidemiologic studies have looked at the exposure and toxicology of a single compound. However, two other considerations must be included: the presence of pesticide by-products and the cumulative exposure to pesticides multiresidue.
It is undisputed that in some cases, pesticide by-products can exhibit greater harmful effects than their parent compounds. For example, one study showed that, at the organism level, the only sublethal effect seen was an increase in heart rate at low concentration and a decrease at higher concentration with the use of aldicarb-sulfoxide but not with aldicarb [169]. Another study reported that the oxons of methyl-parathion, chlorpyrifos and diazinon were 15 to 10 times more toxic (to sperm DNA) than their corresponding parent compounds [170]. As another example, in vitro studies confirmed that 2,4-dichlorophenoxyacetic acid (2,4-D), a commonly used organophosphate herbicide promoting the proliferation of androgen-sensitive cells [171], is a known estrogen receptor ligand [172]. Vinclozolin degrades to several metabolites in the soil, in the plants and in animal organisms [173]. Two hydrolytic degradation products, 2-[[(3,5- dichlorophenyl)-carbamoyl]oxy]-2-methyl-3-butenoic acid and 3′,5″-dichloro-2-hydroxy-methylbut-3-enalide, have been identified as anti-androgenic compounds that mediate the adverse effects of vinclozolin [173].
Furthermore, the combined actions of pesticides also need also to be addressed in the risk assessment process because mixtures of these substances may cause higher toxic effects than those expected from the single compounds [174]. For example an equimolar mixture of three pesticides (deltamethrin, methiocarb, and prochloraz) suppressed androgen receptor (AR) activation in vitro [175]. Also, under the additional presence of simazin and tribenuronmethyl, weight changes of the adrenal gland and alterations in gene expression of AR-associated genes were observed in vivo in castrated testosterone-treated rats. The question of the combined effect of mixtures of contaminants has attracted the attention of the scientific community. Predictive approaches are generally based on the mathematical concepts of Concentration Addition (CA) and Independent Action (IA), both predicting the toxicity of a mixture based on the individual toxicities of the mixture components (e.g., [176] and references therein). Recently, a review showed that some other models could be useful as tools to assess combined tissue doses and to help predict potential interactions including thresholds for such effects [177].
Finally, the impact of synthetic pesticides, due in particular to an excessive use (including environmental pollution and implications to human health) have led to modifications in agricultural practices and various national and international regulations limiting their use. Further limitations and/or bans should be sought, along with alternative solutions that are safer and non-toxic to the environment and humans. One such alternative is so called “natural pesticides” that are not synthetically produced, but are derived from nature such as botanicals pesticides (pyrethrum, limonene, and many others), microbial/biological agents (microbes, parasites) and inorganic minerals (boric acid, limestone, diatomaceous earth). These solutions are generally assumed to be less toxic for human health than synthetic pesticides and could represent an interesting alternative. But their usefulness is actually questionable because some such pesticides are not potent enough to control pests but at the same time do exhibit adverse effects for human health (i.e. “natural pyrethroids”). Further studies are needed on the occurrence, fate and impact of such pesticides on the ecosystem and public health.
Abbreviations:
| ADI: | Acceptable Daily Intake; |
| AhR: | ArylHydrocarbon Receptor; |
| AR: | Androgen Receptor; |
| CA: | Concentration Addition; |
| CAR: | Constitutive Androstane Receptor; |
| EDC: | Endocrine Disruptor Chemical; |
| ER: | Estrogen Receptor; |
| ERR: | Estrogen Related Receptor; |
| HCH: | Hexachlorocyclohexane; |
| IA: | Independent Action; |
| LOD: | Limit of Detection; |
| PCB: | PolyChloroBiphenyl; |
| PXR: | Pregnane X Receptor; |
| WHO: | World Health Organisation |
References
- Mellanby, K. The DDT Story; British Crop Protection Council: Hampshire, UK, 1992. [Google Scholar]
- Briggs, J. Green revolution. Int Encycl Hum Geogr 2009, 634–638. [Google Scholar]
- Cooper, J; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot 2007, 26, 1337–1348. [Google Scholar]
- Carson, R. Silent Spring; Houghton Mifflin: Boston, MA, USA, 1962. [Google Scholar]
- Kolpin, DW; Thurman, EM; Linhart, SM. Finding minimal herbicide concentrations in ground water? Try looking for their degradates. Sci. Total Environ 2000, 248, 115–122. [Google Scholar]
- WHO. Our Planet, Our Health; Report of the WHO Commission on Health and Environment; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Vinggaard, AM; Hnida, C; Breinholt, V; Larsen, JC. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. In Vitro 2000, 14, 227–234. [Google Scholar]
- Andersen, HR; Cook, SJ; Waldbillig, D. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol. Appl. Pharmacol 2002, 179, 1–12. [Google Scholar]
- Kojima, H; Katsura, E; Takeuchi, S; Niiyama, K; Kobayashi, K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect 2004, 112, 524–531. [Google Scholar]
- Lemaire, G; Mnif, W; Mauvais, P; Balaguer, P; Rahmani, R. Activation of alpha- and beta- estrogen receptors by persistent pesticides in reporter cell lines. Life Sci 2006, 79, 1160–1169. [Google Scholar]
- Lemaire, G; Mnif, W; Pascussi, JM; Pillon, A; Rabenoelina, F; Fenet, H; Gomez, E; Casellas, C; Nicolas, JC; Cavailles, V; et al. Identification of new human PXR ligands among pesticides using a stable reporter cell system. Toxicol. Sci 2006, 91, 501–509. [Google Scholar]
- Tabb, MM; Blumberg, B. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol 2006, 20, 475–482. [Google Scholar]
- Cocco, P. On the rumors about the silent spring. Review of the scientific evidence linking occupational and environmental pesticide exposure to endocrine disruption health effects. Cad. Saúde Pública 2002, 18, 379–402. [Google Scholar]
- Akhtar, N; Kayani, SA; Ahmad, MM; Shahab, M. Insecticide-induced changes in secretory activity of the thyroid gland in rats. J. Appl. Toxicol 1996, 16, 397–400. [Google Scholar]
- Leghait, J; Gayrard, V; Picard-Hagen, N; Camp, M; Perdu, E; Toutain, PL; Viguié, C. Fipronil-induced disruption of thyroid function in rats is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxicology 2009, 255, 38–44. [Google Scholar]
- Sugiyama, S; Shimada, N; Miyoshi, H; Yamauchi, K. Detection of thyroid systemdisrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol. Sci 2005, 88, 367–374. [Google Scholar]
- Bryan, GW; Gibbs, PE; Hummerstone, LG; Burt, GR. The decline of the gastropod Nucella lapillus around south-west England: Evidence for the effect of tributyltin from antifouling paints. J. Mar. Biol. Assoc. UK 1986, 66, 611–640. [Google Scholar]
- Short, JW; Rice, SD; Brodersen, CC; Stickle, WB. Occurrence of tri-N-butyltin caused imposex in the north pacific marine snail Nucella limaz in Auke Bay; Alaska. Mar. Biol 1989, 102, 291–297. [Google Scholar]
- Ellis, DV; Pattisina, LA. Widerspread neogastropod imposex; a biological indicator of TBT contamination. Mar. Pollut. Bull 1990, 21, 248–253. [Google Scholar]
- Heidrich, DD; Steckelbroeck, S; Klingmuller, D. Inhibition of human cytochrome P450 aromatase activity by butyltins. Steroids 2001, 66, 763–769. [Google Scholar]
- Gooding, MP; Wilson, VS; Folmar, LC; Marcovich, DT; LeBlanc, GA. The biocide tributyltin reduces the accumulation of testosterone as fatty acid esters in the mud snail (Ilyanassa obsoleta). Environ. Health Perspect 2003, 111, 426–230. [Google Scholar]
- Bishop, CA; Brooks, RJ; Carey, JH; Norstrom, PNRJ; Lean, DRS. The case for a cause-effect linkage between environmental contamination and development in egg of the common snapping turtle (chelydra serpentina) from Ontario. Canada. J. Toxicol. Environ. Health 1991, 33, 521–547. [Google Scholar]
- Bishop, CA; Lean, DRS; Brooks, RJ; Carey, JH; Norstrom, PNRJ. Chlorinated hydrocarbons in early life stages of the common snappling turtle (chelydra serpentina serpentina) from a coastal wetland on lake Ontario. Canada. Environ. Toxicol. Chem 1995, 14, 421–426. [Google Scholar]
- Guillette, LJ; Gross, TS; Masson, GR; Matter, JM; Percival, HF; Woodward, AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect 1995, 102, 680–688. [Google Scholar]
- Guillette, LJ; Pickford, DB; Crain, DA; Rooney, AA; Percival, HF. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environnement. Gen. Comp. Endocrinol 1996, 101, 32–42. [Google Scholar]
- Guillette, LJ; Brock, JW; Rooney, AA; Woodward, AR. Serum concentration of various environmental contaminants and their relationship to sex steroid concentration and phallus size in juvenile American alligators. Arch. Environ. Contam. Toxicol 1999, 36, 447–455. [Google Scholar]
- Crain, DA; Guillette, LJ; Pickford, DB; Rooney, A. Alterations in steroidogenesis in alligators (alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect 1997, 105, 528–533. [Google Scholar]
- Munkittrick, KR; Port, CB; Van Der Kraak, GJ; Smith, IR; Rokosh, DA. Impact of bleach kraft mill effluent on population characteristics; liver MFO activity; and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni). Can. J. Fish Aquat. Sci 1991, 48, 1371–1380. [Google Scholar]
- Purdom, CE; Hardiman, PA; Bye, VJ; Eno, NC; Tyler, CR; Sumpter, JP. Estrogenic effects of effluents from sewage treatment works. Chem. Ecol 1994, 8, 275–285. [Google Scholar]
- Fry, DM; Toone, CK. DDT-induced feminization of gull embryos. Science 1981, 213, 922–924. [Google Scholar]
- Fry, DM; Toone, CK; Speich, SM; Peard, RJ. Sex ratio skew and breeding patterns of gulls, demographic and toxicological considerations. Stud. Avian Biol 1987, 10, 26–43. [Google Scholar]
- Crisp, TM; Clegg, ED; Cooper, RL; Wood, WP; Anderson, DG; Baetcke, KP; Hoffmann, JL; Morrow, MS; Rodier, DJ; Schaeffer, JE; et al. Environmental endocrine disruption: An effects assessment and analysis [Review]. Environ. Health Perspect 1998, 106, 11–56. [Google Scholar]
- Tyler, CR; Jobling, S; Sumpter, JP. Endocrine disruption in wildlife, a critical review of the evidence. Crit. Rev. Toxicol 1998, 28, 319–361. [Google Scholar]
- Vos, JG; Dybing, E; Greim, HA; Ladefoged, O; Lambré, C; Tarazona, JV; Brandt, I; Vethaak, AD. Health effects of endocrine-disrupting chemicals on wildlife; with special reference to the European sitation. Crit. Rev. Toxicol 2000, 30, 71–133. [Google Scholar]
- Reijnders, PJ. Reproductive failure in common seals feeding on fish from polluted coastal waters. Nature 1986, 324, 456–457. [Google Scholar]
- Norstrom, RJ; Muir, DC. Chlorinated hydrocarbon contaminants in arctic marine mammals. Sci. Total Environ 1994, 154, 107–128. [Google Scholar]
- Facemire, CF; Gross, TS; Guillette, LJ. Reproductive impairment in the Florida panther: Nature or nuture? Environ. Health Perspect 1995, 103, 79–86. [Google Scholar]
- Oskam, IC; Ropstad, E; Dahl, E; Lie, E; Derocher, AE; Wiig, O; Larsen, S; Wiger, R; Skaare, JU. Organochlorines affect the major androgenic hormone; testosterone; in male polar bears (Ursus maritimus) at Svalbard. J. Toxicol. Environ. Health A 2003, 66, 2119–2139. [Google Scholar]
- Mnif, W; Pillon, A; Balaguer, P; Bartegi, A. Endocrine xenoestrogenics disrupters, molecular mechanisms and detection methods. Therapie 2007, 62, 369–386. [Google Scholar]
- Palma, P; Palma, VL; Matos, C; Fernandes, RM; Bohn, A; Soares, AMVM; Barbosa, IR. Effects of atrazine and endosulfan sulphate on the ecdysteroid system of Daphnia magna. Chemosphere 2009, 74, 676–681. [Google Scholar]
- Palma, P; Palma, VL; Matos, C; Fernandes, RM; Bohn, A; Soares, AMVM; Barbosa, IR. Assessment of the pesticides atrazine; endosulfan sulphate and chlorpyrifos for juvenoid-related endocrine activity using Daphnia magna. Chemosphere 2009, 76, 335–340. [Google Scholar]
- Wilson, VS; Lambright, CR; Furr, JR; Howdeshell, KL; Gray, LE, Jr. The Herbicide Linuron Reduces Testosterone Production from the Fetal Rat Testis During Both In Utero and In Vitro Exposures. Toxicol. Lett 2009, 186, 73–77. [Google Scholar]
- Birnbaum, LS; Fenton, SE. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect 2003, 111, 389–394. [Google Scholar]
- Goldman, L; Falk, H; Landrigan, PJ; Balk, SJ; Reigart, R; Etzel, RA. Environmental pediatrics and its impact on government health policy. Pediatrics 2004, 113, 1146–1157. [Google Scholar]
- Sharpe, RRM. Pathways of endocrine disruption during male sexual differentiation and masculinisation. Best. Pract. Res. Clin. Endocrinol. Metab 2006, 20, 91–110. [Google Scholar]
- Sultan, C; Balaguer, P; Terouanne, B; Georget, V; Paris, F; Jeandel, C; Lumbroso, S; Nicolas, J. Environmental xenoestrogens; antiandrogens and disorders of male sexual differentiation. Mol. Cell. Endocrinol 2001, 178, 99–105. [Google Scholar]
- Skakkebaek, NN. Endocrine disrupters and testicular dysgenesis syndrome. Horm. Res 2002, 57, 43. [Google Scholar]
- Hardell, L; Bavel, B; Lindström, G; Eriksson, M; Carlberg, M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int. J. Androl 2006, 29, 228–234. [Google Scholar]
- Anderson, H; Wolff, MS. Environmental contaminants in human milk. J. Expo. Anal. Environ. Epidemiol 2000, 10, 755–760. [Google Scholar]
- Waliszewski, S; Aguirre, AA; Infanzón, RM; Siliceo, J. Carry-over of persistent organochlorine pesticides through placenta to fetus. Salud. Publica Mex 2000, 42, 384–390. [Google Scholar]
- Przyrembel, H; Heinrich-Hirsch, B; Vieth, B. Exposition to and health effects of residues in human milk. Adv. Exp. Med. Biol 2000, 478, 307–325. [Google Scholar]
- Jacobson, JL; Jacobson, SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med 1996, 335, 783–789. [Google Scholar]
- Eskenazi, B; Marks, AR; Bradman, A; Fenster, L; Johnson, C; Barr, DB. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics 2006, 118, 233–241. [Google Scholar]
- Ribas-Fito, N; Cardo, E; Sala, M; Eulalia de Muga, M; Mazon, C; Verdu, A; Kogevinas, M; Grimalt, JO; Sunyer, J. Breastfeeding exposure to organochlorine compounds and neurodevelopment in infants. Pediatrics 2003, 111, 580–585. [Google Scholar]
- Beard, J. Australian rural health research collaboration. DDT and human health. Sci. Total Environ 2006, 355, 78–89. [Google Scholar]
- Xiang, H; Nuckols, JR; Stallones, L. A geographic information assessment of birth weight and crop production patterns around mother’s residence. Environ. Res. A 2000, 82, 160–167. [Google Scholar]
- Bell, EM; Hertz-Picciotto, I; Beaumont, JJ. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology 2001, 12, 148–156. [Google Scholar]
- Reynolds, P; Von Behren, J; Gunier, RB; Goldberg, DE; Hertz, A; Harnly, ME. Childhood cancer and agricultural pesticide use, an ecologic study in California. Environ. Health Perspect 2002, 110, 319–324. [Google Scholar]
- Kristensen, P; Irgens, LM; Andersen, A; Bye, AS; Sundheim, L. Birth defects among offspring of Norwegian farmers; 1967–1991. Epidemiology 1997, 8, 537–544. [Google Scholar]
- Carbone, P; Giordano, F; Nori, F; Mantovani, A; Taruscio, D; Lauria, L; Figà-Talamanca, I. Cryptorchidism and hypospadias in the Sicilian district of Ragusa and the use of pesticides. Reprod. Toxicol 2006, 22, 8–12. [Google Scholar]
- Weidner, IS; Moller, H; Jensen, TK; Skakkebæk, NE. Cryptorchidism and hypospadias in sons of gardeners and farmers. Environ. Health Perspect 1998, 106, 793–796. [Google Scholar]
- Skakkebæk, NE; Rajpert-De Meyts, E; Main, KM. Testicular dysgenesis syndrome, an increasingly common developmental disorder with environmental aspects. Hum. Reprod 2001, 16, 972–978. [Google Scholar]
- Damgaard, IN; Skakkebaek, NE; Toppari, J; Virtanen, HE; Shen, H; Schramm, KW; Petersen, JH; Jensen, TK; Main, KM. Persistent pesticides in human breast milk and cryptorchidism. Environ. Health Perspect 2006, 114, 1133–1138. [Google Scholar]
- Roeleveld, N; Bretveld, R. The impact of pesticides on male fertility. Curr. Opin. Obstet. Gynecol 2008, 20, 229–233. [Google Scholar]
- McKinlay, R; Plant, JA; Bell, JNB; Voulvoulis, N. Endocrine disrupting pesticides, Implications for risk assessment. Environ. Int 2008, 34, 168–183. [Google Scholar]
- PPDB: Pesticide Properties DataBase. University of Hertfordshire. Available online: http://sitem.herts.ac.uk/aeru/footprint/fr/index.htm (accessed on 12 March 2011).
- Kim, DO; Lee, SK; Jeon, TW; Jin, CH; Hyun, SH; Kim, EJ; Moon, GI; Kim, JA; Lee, ES; Lee, BM; et al. Role of metabolism in parathion-induced hepatotoxicity and immunotoxicity. J. Toxicol. Environ. Health A 2005, 68, 2187–2205. [Google Scholar]
- Panuwet, P; Prapamontol, T; Chantara, S; Thavornyuthikarn, P; Montesano, MA; Whitehead, R, Jr; Barr, DB. Concentrations of urinary pesticide metabolites in small-scale farmers in Chiang Mai Province; Thailand. Sci. Total Environ 2008, 407, 655–668. [Google Scholar]
- Swan, SH; Kruse, RL; Liu, F; Barr, DB; Drobnis, EZ; Redmon, JB; Wang, C; Brazil, C; Overstreet, JW; Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect 2003, 111, 1478–1484. [Google Scholar]
- Singh, AK. Acute effects of acephate and methamidophos and interleukin-1 on corticotropin-releasing factor (CRF) synthesis in and release from the hypothalamus in vitro. Comp. Biochem. Physiol. C Toxicol. Pharmacol 2002, 132, 9–24. [Google Scholar]
- Inoue, S; Saito, T; Mase, H; Suzuki, Y; Takazawa, K; Yamamoto, I; Inokuchi, S. Rapid simultaneous determination for organophosphorus pesticides in human serum by LC–MS. J. Pharm. Biomed. Anal 2007, 44, 258–264. [Google Scholar]
- Rollerová, EE. Interaction of acetochlor with estrogen receptor in the rat uterus. Acetochlor—possible endocrinemodulator? Gen. Physiol. Biophys 2000, 19, 73–84. [Google Scholar]
- Crump, D; Werry, K; Veldhoen, N; Van Aggelen, G; Helbing, CC. Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression and metamorphosis in Xenopus laevis. Environ Health Perspect 2002, 110, 1199–205. [Google Scholar]
- Mikamo, E; Harada, S; Nishikawa, J; Nishihara, T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol Appl. Pharmacol 2003, 193, 66–72. [Google Scholar]
- Klotz, D; Arnold, SF; McLachlan, JA. Inhibition of 17 beta-estradiol and progesterone activity in human breast and endometrial cancer cells by carbamate insecticides. Life Sci 1997, 60, 1467–1475. [Google Scholar]
- Lemaire, G; Terouanne, B; Mauvais, P; Michel, S; Rahmani, R. Effect of organochlorine pesticides on human androgen receptor activation in vitro. Toxicol. Appl. Pharmacol 2004, 196, 235–246. [Google Scholar]
- Cruz, S; Lino, C; Silveira, MI. Evaluation of organochlorine pesticide residues in human serum from an urban and two rural populations in Portugal. Sci. Total Environ 2003, 317, 23–35. [Google Scholar]
- Botella, B; Crespo, J; Rivas, A; Cerrillo, I; Olea-Serrano, MF; Olea, N. Exposure of women to organochlorine pesticides in Southern Spain. Environ. Res 2004, 96, 34–40. [Google Scholar]
- Nasir, K; Bilto, YY; Al-Shuraiki, Y. Residues of chlorinated hydrocarbon insecticides in human milk of Jordanian women. Environ. Pollut 1998, 99, 141–148. [Google Scholar]
- Cooper, R; Stoker, TE; Tyrey, L; Goldman, JM; McElroy, WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci 2000, 53, 297–307. [Google Scholar]
- Sanderson, JT; Seinen, W; Giesy, JP; van den Berg, M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells, a novel mechanism for estrogenicity? Toxicol. Sci 2000, 54, 121–127. [Google Scholar]
- Hayes, T; Tsui, M; Hoang, A; Haeffele, C; Vonk, A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens); laboratory and field evidence. Environ. Health Perspect 2003, 111, 568–575. [Google Scholar]
- Thibaut, R; Porte, C. Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J. Steroid Biochem. Mol. Biol 2004, 92, 485–494. [Google Scholar]
- Curwin, BD; Hein, MJ; Sanderson, WT; Striley, C; Heederik, D; Kromhout, H; Reynolds, SJ; Alavanja, MC. Urinary Pesticide Concentrations Among Children; Mothers and Fathers Living in Farm and Non-Farm Households in Iowa. Ann. Occup. Hyg 2007, 51, 53–65. [Google Scholar]
- Barr, DB; Barr, JR; Maggio, VL; Whitehead, RD, Jr; Sadowski, MA; Whyatt, RM; Needham, LL. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2002, 778, 99–111. [Google Scholar]
- Morinaga, H; Yanase, T; Nomura, M; Okabe, T; Goto, K; Harada, N; Nawata, H. A benzimidazole fungicide; benomyl; and its metabolite; carbendazim; induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology 2004, 145, 1860–1869. [Google Scholar]
- Kim, IY; Shin, JH; Kim, HS; Lee, SJ; Kang, IH; Kim, TS; Moon, HJ; Choi, KS; Moon, A; Han, SY. Assessing estrogenic activity of pyrethroid insecticides using in vitro combination assays. J. Reprod. Dev 2004, 50, 245–255. [Google Scholar]
- Ostrea, EM, Jr; Bielawski, DM; Posecion, NC, Jr; Corrion, M; Villanueva-Uy, E; Bernardo, RC; Jin, Y; Janisse, JJ; Ager, JW. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair; cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ. Res 2009, 10, 116–122. [Google Scholar]
- Trosken, EE; Scholz, K; Lutz, RW; Volkel, W; Zarn, JA; Lutz, WK. Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr. Res 2004, 30, 387–394. [Google Scholar]
- Okubo, T; Yokoyama, Y; Kano, K; Soya, Y; Kano, I. Estimation of estrogenic and antiestrogenic activities of selected pesticides by MCF-7 cell proliferation assay. Arch. Environ. Contam. Toxicol 2004, 46, 445–453. [Google Scholar]
- Goad, R; Goad, J; Atieh, B; Gupta, R. Carbofuran-Induced endocrine disruption in adult male rats. Toxicol. Mech. Methods 2004, 14, 233–239. [Google Scholar]
- Barr, DB; Ananth, CV; Yan, X; Lashley, S; Smulian, JC; Ledoux, TA; Hore, P; Robson, MG. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Sci. Total Environ 2010, 408, 790–795. [Google Scholar]
- Tessier, D; Matsumura, F. Increased ErbB-2 tyrosine kinase activity; MAPK phosphorylation; and cell proliferation in the prostate cancer cell line LNCaP following treatment by select pesticides. Toxicol. Sci 2001, 60, 38–43. [Google Scholar]
- Röllin, HB; Sandanger, TM; Hansen, L; Channa, K; Odland, JØ. Concentration of selected persistent organic pollutants in blood from delivering women in South Africa. Sci. Total Environ 2009, 408, 146–152. [Google Scholar]
- Kang, JH; Park, H; Chang, YS; Choi, JW. Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human serum from urban areas in Korea. Chemosphere 2008, 73, 1625–1631. [Google Scholar]
- Schinas, V; Leotsinidis, M; Alexopoulos, A; Tsapanos, V; Kondakis, XG. Organochlorine pesticide residues in human breast milk from southwest Greece, associations with weekly food consumption patterns of mothers. Arch. Environ. Health 2000, 55, 411–417. [Google Scholar]
- Tanabe, S; Kunisue, T. Persistent organic pollutants in human breast milk from Asian countries. Environ. Pollut 2007, 146, 400–413. [Google Scholar]
- Polder, A; Thomsen, C; Lindström, G; Løken, KB; Skaare, JU. Levels and temporal trends of chlorinated pesticides; polychlorinated biphenyls and brominated flame retardants in individual human breast milk samples from Northern and Southern Norway. Chemosphere 2008, 73, 14–23. [Google Scholar]
- Devanathan, G; Subramanian, A; Someya, M; Sudaryanto, A; Isobe, T; Takahashi, S; Chakraborty, P; Tanabe, S. Persistent organochlorines in human breast milk from major metropolitan cities in India. Environ. Pollut 2009, 157, 148–154. [Google Scholar]
- Jarrell, JF; Villeneuve, D; Franklin, C; Bartlett, S; Wrixon, W; Kohut, J; Zouves, CG. Contamination of human ovariam follicular fluid and serum by chlorinated organic compounds in three Canadian cities. Can. Med. Assoc. J 1993, 148, 1321–1327. [Google Scholar]
- Tapiero, HT; Nguyen, BG; Tew, KD. Estrogens and environmental estrogens. Biomed. Pharmacother 2002, 56, 36–44. [Google Scholar]
- Fang, H; Tong, W; Branham, WS; Moland, CL; Dial, SL; Hong, H; Xie, Q; Perkins, R; Owens, W; Sheehan, DM. Study of 202 natural; synthetic; and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol 2003, 16, 1338–1358. [Google Scholar]
- Vinggaard, A; Breinholt, V; Larsen, JC. Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit. Contam 1999, 16, 533–542. [Google Scholar]
- Kang, HG; Jeong, SH; Cho, JH; Kim, DG; Park, JM; Cho, MH. Chlropyrifos-methyl shows anti-androgenic activity without estrogenic activity in rats. Toxicology 2004, 199, 219–230. [Google Scholar]
- Chen, H; Xiao, J; Hu, G; Zhou, J; Xiao, H; Wang, X. Estrogenicity of organophosphorus and pyrethroid pesticides. J. Toxicol. Environ. Health A 2002, 65, 1419–1435. [Google Scholar]
- McCarthy, AR; Thomson, BM; Shaw, IC; Abella, AD. Estrogenicity of pyrethroid insecticide metabolites. J. Environ. Monit 2006, 8, 197–202. [Google Scholar]
- Hardt, J; Angerer, J. Biological monitoring of workers after the application of insecticidal pyrethroids. Int. Arch. Occup. Environ. Health 2003, 76, 492–498. [Google Scholar]
- Tapiero, HT; Nguyen, BG; Tew, KD. Estrogens and environmental estrogens. Biomed. Pharmacother 2002, 56, 36–44. [Google Scholar]
- Bulayeva, NN; Watson, CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ. Health Perspect 2004, 112, 1481–1487. [Google Scholar]
- Qu, W; Suri, RPS; Bi, X; Sheng, G; Fu, J. Exposure of young mothers and newborns to organochlorine pesticides (OCPs) in Guangzhou; China. Sci. Total Environ 2010, 408, 3133–3138. [Google Scholar]
- Fujii, Y; Haraguchi, K; Harada, KH; Hitomi, T; Inoue, K; Itoh, Y; Watanabe, T; Takenaka, K; Uehara, S; Yang, HR; et al. Detection of dicofol and related pesticides in human breast milk from China; Korea and Japan. Chemosphere 2011, 82, 25–31. [Google Scholar]
- Foster, W; Chan, S; Platt, L; Hughes, C. Detection of endocrine disrupting chemicals in samples of second trimester human amniotic fluid. J. Clin. Endocrinol. Metab 2000, 85, 2954–2957. [Google Scholar]
- Tsatsakis, AM; Tzatzarakis, MN; Tutudaki, M. Pesticide levels in head hair samples of Cretan population as an indicator of present and past exposure. Forensic Sci. Int 2008, 176, 67–71. [Google Scholar]
- Pathak, R; Ahmed, RS; Tripathi, AK; Guleria, K; Sharma, CS; Makhijani, SD; Banerjee, BD. Maternal and cord blood levels of organochlorine pesticides, Association with preterm labor. Clin. Biochem 2009, 42, 746–749. [Google Scholar]
- Manabe, M; Kanda, S; Fukunaga, K; Tsubura, A; Nishiyama, T. Evaluation of the estrogenic activities of some pesticides and their combinations using MtT/Se cell proliferation assay. Int. J. Hyg. Environ. Health 2006, 209, 413–421. [Google Scholar]
- Soto, AM; Chung, KL; Sonnenschein, C. The pesticides endosulfan; toxaphene; and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ. Health Perspect 1994, 102, 380–383. [Google Scholar]
- Rawlings, NC; Cook, SJ; Waldbillig, D. Effects of the pesticides carbofuran; chlorpyrifos; dimethoate; lindane; triallate; trifluralin; 2;4-D; and pentachlorophenol on the metabolic endocrine and reproductive endocrine system in ewes. J. Toxicol. Environ. Health A 1998, 54, 21–36. [Google Scholar]
- Mahjoubi-Samet, A; Hamadi, F; Soussia, L; Fadhel, G; Zeghal, N. Dimethoate effects on thyroid function in suckling rats. Ann. Endocrinol 2005, 66, 96–104. [Google Scholar]
- Hurst, MRR; Sheahan, DA. The potential for estrogenic effects of pesticides in headwater streams in the UK. Sci. Total Environ 2003, 301, 87–96. [Google Scholar]
- Grunfeld, HT; Bonefeld-Jorgensen, EC. Effect of in vitro estrogenic pesticides on human estrogen receptor alpha and beta mRNA levels. Toxicol. Lett 2004, 151, 467–480. [Google Scholar]
- Tamura, H; Yoshikawa, H; Gaido, KW; Ross, SM; DeLisle, RK; Welsh, WJ; Richard, AM. Interaction of organophosphate pesticides and related compounds with the androgen receptor. Environ. Health Perspect 2003, 111, 545–552. [Google Scholar]
- Verslycke, T. Testosterone and energy metabolism in the estuarine mysid Neomysis integer (Crustacea, Mysidacea) following exposure to endocrine disruptors. Environ. Toxicol. Chem 2004, 23, 1289–1296. [Google Scholar]
- Garey, J; Wolff, MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem. Biophys. Res. Commun 1998, 251, 855–859. [Google Scholar]
- Eil, CC; Nisula, BC. The binding properties of pyrethroids to human skin fibroblast androgen receptors and to sex hormone binding globulin. J. Steroid Biochem 1990, 35, 409–414. [Google Scholar]
- Chen, HY; Liu, R; He, J; Song, L; Bian, Q; Xu, L; Zhou, J; Xiao, H; Dai, G; Chang, HC; Wang, X. Effects of fenvalerate on progesterone production in cultured rat granulosa cells. Reprod. Toxicol 2005, 20, 195–202. [Google Scholar]
- Richard, S; Moslemi, S; Sipahutar, H; Benachour, N; Seralini, G-E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect 2005, 113, 716–720. [Google Scholar]
- Ralph, JJ; Orgebin-Crist, MC; Lareyre, JJ; Nelson, CC. Disruption of androgen regulation in the prostate by the environmental contaminant hexachlorobenzene. Environ. Health Perspect 2003, 111, 461–466. [Google Scholar]
- Verreault, J; Skaare, JU; Jenssen, BM; Gabrielsen, GW. Effects of organochlorine contaminants on thyroid hormone levels in Arctic breeding glaucous gulls; Larus hyperboreus. Environ. Health Perspect 2004, 112, 532–537. [Google Scholar]
- Beard, AB; Rawlings, NC. Thyroid function and effects on reproduction in ewes exposed to the organochlorine pesticides lindane or pentachlorophenol (PCP) from conception. J. Toxicol. Environ. Health A 1999, 58, 509–530. [Google Scholar]
- Oduma, JA; Wango, EO; Oduor-Okelo, D; Makawiti, DW; Odongo, H. In vivo and In vitro effects of graded doses of the pesticide heptachlor on female sex steroid production in rats. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol 1995, 111, 191–196. [Google Scholar]
- Fang, H; Tong, W; Branham, WS; Moland, CL; Dial, SL; Hong, H; Xie, Q; Perkins, R; Owens, W; Sheehan, DM. Study of 202 natural; synthetic; and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol 2003, 16, 1338–1358. [Google Scholar]
- Schmutzler, C; Gotthardt, I; Hofmann, PJ; Radovic, B; Kovacs, G; Stemmler, L; Nobis, I; Bacinski, A; Mentrup, B; Ambrugger, P; et al. Endocrine Disruptors and the Thyroid Gland—A Combined in Vitro and in Vivo Analysis of Potential New Biomarkers. Environ. Health Perspect 2007, 115, 77–83. [Google Scholar]
- Ishihara, A; Nishiyama, N; Sugiyama, S; Yamauchi, K. The effect of endocrine disrupting chemicals on thyroid hormone binding to Japanese quail transthyretin and thyroid hormone receptor. Gen. Comp. Endocrinol 2003, 134, 36–43. [Google Scholar]
- Porter, W; Green, SM; Debbink, NL; Carlson, I. Groundwater pesticides, interactive effects of low concentrations of carbamates aldicarb and methomyl and the triazine metribuzin on thyroxine and somatotropin levels in white rats. J. Toxicol. Environ. Health 1993, 40, 15–34. [Google Scholar]
- Bradman, A; Barr, DB; Henn, BGC; Drumheller, T; Curry, C; Eskenazi, B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ. Health Perspect 2003, 111, 1779–1782. [Google Scholar]
- Sonnenschein, C; Soto, AM. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. Mol. Biol 1998, 65, 143–150. [Google Scholar]
- Vinggaard, AM; Hass, U; Dalgaard, M; Andersen, HR; Bonefeld-Jørgensen, E; Christiansen, S; Laier, P; Poulsen, ME. Prochloraz, an imidazole fungicide with multiple mechanisms of action. Int. J. Androl 2006, 29, 186–192. [Google Scholar]
- Salazar, KD; Schafer, R; Barnett, JB; Miller, MR. Evidence for a novel endocrine disruptor, the pesticide propanil requires the ovaries and steroid synthesis to enhance humoral immunity. Toxicol. Sci 2006, 93, 62–74. [Google Scholar]
- Kim, SS; Kwack, SJ; Lee, RD; Lim, KJ; Rhee, GS; Seok, JH; Kim, BH; Won, YH; Lee, GS; Jeung, EB; et al. Assessment of estrogenic and androgenic activities of tetramethrin in vitro and in vivo assays. J. Toxicol. Environ. Health A 2005, 68, 2277–2289. [Google Scholar]
- Nicolau, GG. Circadian rhythms of RNA; DNA and protein in the rat thyroid; adrenal and testis in chronic pesticide exposure. III. Effects of the insecticides (dichlorvos and trichlorphon). Physiologie 1983, 20, 93–101. [Google Scholar]
- Falck, F; Ricci, A; Wolff, MS; Godbold, J; Deckers, P. Pesticides and polichlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch. Environ. Health 1992, 47, 143–146. [Google Scholar]
- Davis, DL; Bradlow, HL; Wolff, M; Woodruff, T; Hoel, DG; Anton-Culver, H. Hypothesis, xenoestrogens as preventable causes of breast cancer. Environ. Health Perspect 1993, 101, 372–377. [Google Scholar]
- Parron, T; Alarcon, R; Requena, MDM; Hernandez, A. Increased breast cancer risk in women with environmental exposure to pesticides. Toxicol. Lett 2010, 196, S180. [Google Scholar]
- Dewailly, E; Nantel, A; Weber, JP; Meyer, F. High levels of PCBs in breast of Inuit women from arctic Quebec. Bull. Environ. Contam. Toxicol 1989, 4, 641–646. [Google Scholar]
- Krieger, N; Wolff, MS; Hiatt, RA; Rivera, M; Vogelman, J; Orentreich, N. Breast cancer and serum organochlorines, a prospective study among white; black; and Asian women. J. Natl. Cancer Inst 1994, 86, 589–599. [Google Scholar]
- Cohn, BA. Developmental and environmental origins of breast cancer: DDT as a case study. Reprod. Toxicol 2011, 31, 302–311. [Google Scholar]
- Meeker, JD. Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 2010, 66, 236–241. [Google Scholar]
- Alavanja, MC; Samanic, C; Dosemeci, M; Lubin, J; Tarone, R; Lynch, CF; Knott, C; Thomas, K; Hoppin, JA; Barker, J; Coble, J; Sandler, DP; Blair, A. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol 2003, 157, 800–814. [Google Scholar]
- Prins, GS. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 2008, 15, 649–656. [Google Scholar]
- Diamanti-Kandarakis, E; Bourguignon, JP; Giudice, LC; Hauser, R; Prins, GS; Soto, AM; Zoeller, RT; Gore, AC. Endocrine-disrupting chemicals; An Endocrine Society scientific statement. Endocr. Rev 2009, 30, 293–342. [Google Scholar]
- Settimi, L; Masina, A; Andrion, A; Axelson, O. Prostate cancer and exposure to pesticides in agricultural settings. Int. J. Cancer 2003, 104, 458–461. [Google Scholar]
- Alavanja, MC; Sandler, DP; Lynch, CF; Knott, C; Lubin, JH; Tarone, R; Thomas, K; Dosemeci, M; Barker, J; Hoppin, JA; et al. Cancer incidence in the agricultural health study. Scand. J. Work Environ. Health 2005, 31, 39–45. [Google Scholar]
- Dich, J; Wiklund, K. Prostate cancer in pesticide applicators in Swedish agriculture. Prostate 1998, 34, 100–112. [Google Scholar]
- Ndong, JR; Blanchet, P; Multigner, L. Pesticides et cancer de la prostate: données épidémiologiques. Bull. Cancer 2009, 96, 171–180. [Google Scholar]
- Multigner, L; Ndong, JR; Giusti, A; Romana, M; Delacroix-Maillard, H; Cordier, S; Jégou, B; Thome, JP; Blanchet, P. Chlordecone exposure and risk of prostate cancer. J. Clin. Oncol 2010, 28, 3457–3462. [Google Scholar]
- European Commission. Monitoring of Pesticide Residues in Products of Plant Origin in the European Union, Norway, Iceland and Liechtenstein; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Iñigo-Nuñez, S; Herreros, MA; Encinas, T; Gonzalez-Bulnes, A. Estimated daily intake of pesticides and xenoestrogenic exposure by fruit consumption in the female population from a Mediterranean country (Spain). Food Control 2010, 21, 471–477. [Google Scholar]
- Osman, KA; Al-Humaid, AI; Al-Rehiayani, SM; Al-Redhaiman, KN. Estimated daily intake of pesticide residues exposure by vegetables grown in greenhouses in al-qassim region; Saudi Arabia. Food Control 2010. [Google Scholar] [CrossRef]
- Fromberg, A; Granby, K; Højgård, A; Fagt, S; Larsen, JC. Estimation of dietary intake of PCB and organochlorine pesticides for children and adults. Food Chem 2011, 125, 1179–1187. [Google Scholar]
- Peakall, DB. Review of books on endocrine disruptors. Ecotoxicology 2000, 9, 137–144. [Google Scholar]
- Curl, CL; Fenske, RA; Kissel, JC; Shirai, JH; Moate, TF; Griffith, W; Coronado, G; Thompson, B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect 2002, 110, A787–A792. [Google Scholar]
- Whyatt, RM; Camann, D; Perera, FP; Rauh, VA; Tang, D; Kinney, PL; Garfinkel, R; Andrews, H; Hoepner, L; Barr, DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol 2005, 206, 246–254. [Google Scholar]
- Mantovani, A; Maranghi, F; La Rocca, C; Tiboni, GM; Clementi, M. The role of toxicology to characterize biomarkers for agrochemicals with potential endocrine activities. Reprod. Toxicol 2008, 26, 1–7. [Google Scholar]
- Carreno, J; Rivas, A; Granada, A; Lopez-Espinosa, MJ; Mariscal, M; Olea, N; Olea-Serrano, F. Exposure of young men to organochlorine pesticides in Southern Spain. Environ Res 2007, 103, 55–61. [Google Scholar]
- Berman, T; Hochner-Celnikier, D; Boyd Barr, D; Needham, LL; Amitai, Y; Wormser, U; Richter, E. Pesticide exposure among pregnant women in Jerusalem, Israel: Results of a pilot study. Environ. Int 2011, 37, 198–203. [Google Scholar]
- Duggan, A; Charnley, G; Chen, W; Chukwudebe, A; Hawk, R; Krieger, RI; Ross, J; Yarborough, C. Di-alkyl phosphate biomonitoring data; assessing cumulative exposure to organophosphate pesticides. Regul. Toxicol. Pharmacol 2003, 37, 382–395. [Google Scholar]
- Payne-Sturges, D; Cohen, J; Castorina, R; Axelrad, DA; Woodruff, TJ. Evaluating cumulative organophosphorus pesticide body burden of children, a national case study. Environ. Sci. Technol 2009, 43, 7924–7930. [Google Scholar]
- Tsatsakis, AM; Barbounis, MG; Kavalakis, M; Kokkinakis, M; Terzi, I; Tzatzarakis, MN. Determination of dialkyl phosphates in human hair for the biomonitoring of exposure to organophosphate pesticides. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2010, 878, 1246–1252. [Google Scholar]
- Kuster, E; Altenburger, R. Suborganismic and organismic effects of aldicarb and its metabolite aldicarb-sulfoxide to the zebrafish embryo (Danio rerio). Chemosphere 2007, 68, 751–760. [Google Scholar]
- Salazar-Arredondo, E; Solıs-Herediaa, M; Rojas-Garcıa, E; Hernandez-Ochoa, I; Quintanilla-Vega, B. Sperm chromatin alteration and DNA damage by methyl-parathion; chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reprod. Toxicol 2008, 25, 455–460. [Google Scholar]
- Kim, HJ; Park, YI; Dong, MS. Effects of 2,4-D and DCP on the DHT-induced androgenic action in human prostate cancer cells. Toxicol. Sci 2005, 88, 52–59. [Google Scholar]
- Blair, RM; Fang, H; Branham, WS; Hass, BS; Dial, SL; Moland, CL; Tong, W; Shi, L; Perkins, R; Sheehan, DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci 2000, 54, 138–153. [Google Scholar]
- Kelce, WR; Monosson, E; Gamcsik, MP; Law, SC; Gray, LE. Environmental hormone disruptors, evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol. Appl. Pharmacol 1994, 126, 276–285. [Google Scholar]
- Larsen, JC; Binderup, ML; Dalgaard, M; Dragsted, LO; Hossaini, A; Ladefoged, O; Lam, HR; Madsen, C; Meyer, O; Rasmussen, ES; et al. Combined Actions and Interactions of Chemicals in Mixtures. The Toxicological Effects of Exposure to Mixtures of Industrial and Environmental Chemicals; FødevareRapport 12; Larsen, JC, Ed.; Danish Veterinary and Food Administration: Søborg, Denmark, 2003. [Google Scholar]
- Birkhoj, M; Nellemann, C; Jarfelt, K; Jacobsen, H; Andersen, HR; Dalgaard, M; Vinggaard, AM. The combined antiandrogenic effects of five commonly used pesticides. Toxicol. Appl. Pharmacol 2004, 201, 10–20. [Google Scholar]
- Altenburger, R; Greco, WR. Extrapolation concepts for dealing with multiple contamination in environmental risk assessment. Integr. Environ. Assess. Manage 2009, 5, 62–68. [Google Scholar]
- Reffstrup, TK; Larsen, JC; Meyer, O. Risk assessment of mixtures of pesticides. Current approaches and future strategies. Regul. Toxicol. Pharmacol 2010, 56, 174–192. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).