Air Quality Modeling for the Urban Jackson, Mississippi Region Using a High Resolution WRF/Chem Model
Abstract
:1. Introduction
2. Experimental Section
2.1. WRF/Chem Model
2.2. Model Configuration and Initialization
3. Results and Discussion
4. Conclusions
- The WRF/Chem model shows the capability of simulating ozone production and its diurnal variations at a fine resolution of 1 km. This shows the potential of WRF/Chem model for urban pollution studies in contrast to the earlier usage of running separate models for urban pollution integrated with independently generated atmospheric model outputs.
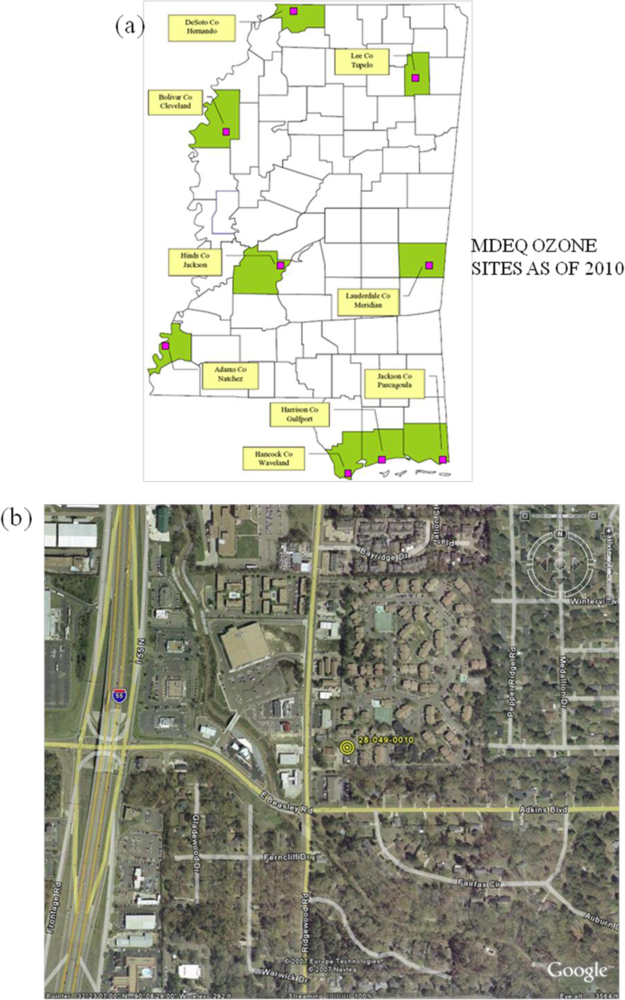
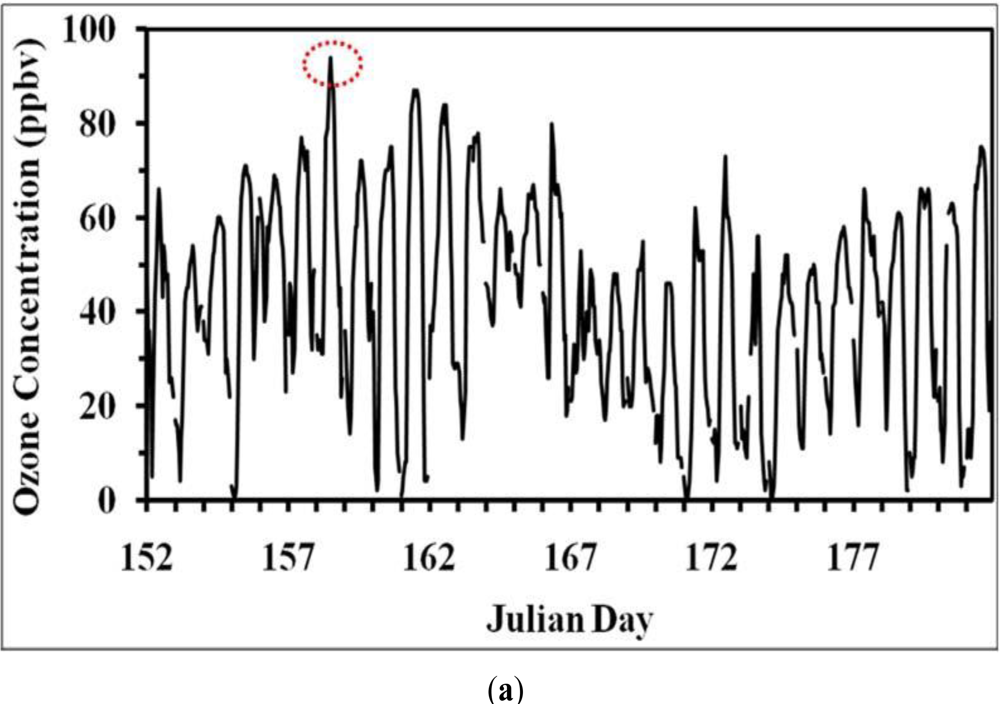
- The air pollution records of the last decade show the occurrence of a moderate air pollution episode of ozone formation on 7 June 2006 with a magnitude of 90 ppbv in the study region. Prior to and after the episode the surface ozone concentrations were low on 6 and 8 June (about 70–75 ppbv).
- The model simulation has a fairly good agreement with the observed diurnal variation of ozone concentration in the PBL. The model simulated maximum levels of ozone concentration (50 ppb) at afternoon due to photochemical production of ozone associated with the NOx emission. In early morning, NOx has high concentration due to high mobile traffic and low PBL, while ozone concentration has a minimum concentration (about 10 ppbv), indicating a low photochemical activity condition. Thus emission of NOx plays an important role on the ozone levels in the city.
- The occurrence of NO maximum around 11 AM, 2-hours prior to the occurrence of ozone maximum, indicates the splitting of NO2 as NO and O and the formation of ozone through reaction of O and O2. Similarly a maximum of CO indicates the production of ozone in the troposphere by the photochemical oxidation of hydrocarbons in the presence of nitrogen oxides. The occurrence of maximum NO and HONO levels, 1–2 hours prior to the occurrence of the O3 maximum, indicate their direct contribution to O3 formation in the presence of UV radiation.
- These results show that the chemical reactions adapted in WRF/Chem model for the present study seem to be appropriate although there is an underestimation of magnitude of O3. The underestimation of ozone by 30% in the present study could be due to exclusion of biogenic emissions and use of ideal profiles.
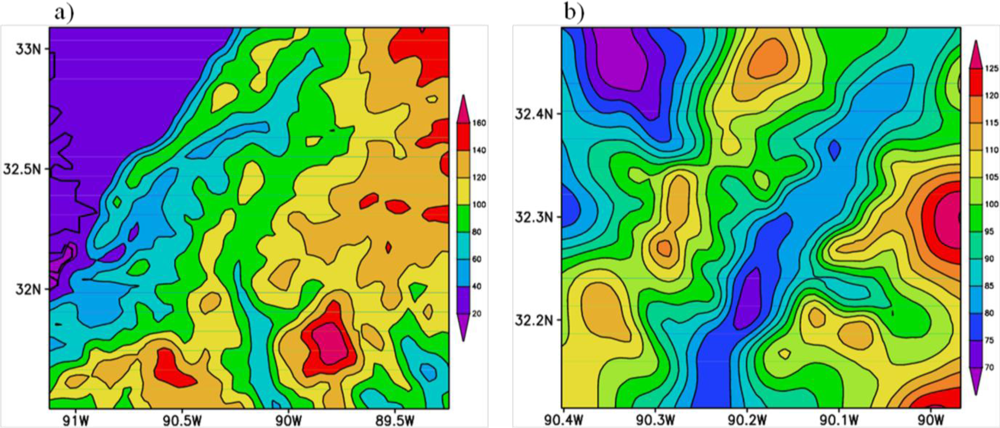
- The model simulated maximum levels of ozone and precursors NO, NO2, CO and HONO over Jackson city area and surrounding regions towards its northeast covering Ridgeland, Flowood, Pearl and parts of Madison and minimum over Clinton, Richland, Florence and Raymond indicating the regions that are vulnerable to stronger pollution.
- The model simulated spatial pattern of O3, NO, NO2, CO and HONO and the back trajectories indicate that the mobile sources in Jackson, Ridgeland and Madison are contributing significantly to their formation.
- The results of this study distinctly indicated the regions of higher and lesser ozone concentrations. Although the direct effects of surface ozone were not the focus of this study the health effects of surface ozone are well known. The study indirectly indicates the vulnerable urban regions within the City of Jackson area for ozone pollution.
- The present study demonstrates the applicability of WRF/Chem model to generate quantitative information at high spatial and temporal resolution for the development of decision support systems for air quality regulatory agencies and health administrators. Further studies are under progress to make sensitivity experiments with respect to photochemical processes, surface physics, radiation and vertical resolution and fine tune WRF/Chem model towards improvement of ozone simulation over urban regions.
Acknowledgments
References
- Paul, RA; Biller, WF; McCurdy, T. National Estimates of Population Exposure to Ozone. Presented at the Air Pollution Control Association 80th Annual Meeting and Exhibition, New York, NY, USA, 21–26 June 1987.
- Seaman, NL. Meteorological modeling for air-quality assessments. Atmos. Environ 2000, 34, 2231–2259. [Google Scholar]
- Carvalho, AC; Carvalho, A; Gelpi, I; Barreiro, M; Borrego, C; Miranda, AI; Perez-Munuzuri, V. Influence of topography and land use on pollutants dispersion in the Atlantic coast of Iberian Peninsula. Atmos. Environ 2006, 40, 3969–3982. [Google Scholar]
- Ferguson, SA. Air Quality Climate in the Columbia River Basin; General Technical Report PNW-GTR-434; Quigley, TM, Ed.; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1998. [Google Scholar]
- Bei, N; Lei, W; Zavala, M; Molina, LT. Ozone predictabilities due to meteorological uncertainties in Mexico City basin using ensemble forecasts. Atmos. Chem. Phys. Discuss 2010, 10, 3229–3263. [Google Scholar]
- Thielmann, A; Prévôt, ASH; Staehelin, J. Sensitivity of ozone production derived from field measurements in the Italian Po basin. J Geophys Res 2002, 107, 8194. [Google Scholar] [CrossRef]
- Altshuller, AP; Lefohn, AS. Background ozone in the planetary boundary layer over the United States. J. Air Waste Manage. Assoc 1996, 46, 134–141. [Google Scholar]
- Zaveri, RA; Berkowitz, CM; Kleinman, LI; Springston, SR; Doskey, PV; Lonneman, WA; Spicer, CW. Ozone production efficiency and NOx depletion in an urban plume: Interpretation of field observations and implications for evaluating O3−NOx-VOC sensitivity. J Geophys Res 2003, 108, 4436. [Google Scholar] [CrossRef]
- Kleinman, LI; Daum, PH; Imre, DG; Lee, JH; Lee, YN; Nunnermacker, LJ; Springston, SR; Weinstein-Lloyd, J; Newman, L. Ozone production in the New York City urban plume. J Geophys Res 2000, 105, 14495–14512. [Google Scholar] [CrossRef]
- Kleinman, LI; Daum, PH; Lee, YN; Nunnermacker, LJ; Springston, SR; Weinstein-Lloyd, J; Hyde, P; Doskey, P; Rudolph, J; Fast, J; Berkowitz, C. Photochemical age determinations in the Phoenix metropolitan area. J Geophys Res 2003, 108, 4096. [Google Scholar] [CrossRef]
- Liu, Y; Low-Nam, S; Sheu, R; Carson, L; Davis, C; Warner, T; Bowers, J; Xu, M; Hsu, H; Rife, D. Performance and Enhancements of the NCAR/ATEC Mesoscale FDDA and Forecasting System. Proceedings of the 15th Conference on Numerical Weather Prediction, San Antonio, TX, USA, 12–16 August, 2002; pp. 399–402.
- Umit, A; Sema, T; Selahattin, I. An application of a photochemical model for urban airshed in Istanbul. Air Pollut Model Its Appl 2004, XV(Part 2), 167–175. [Google Scholar] [CrossRef]
- De Foy, B; Lei, W; Zavala, M; Volkamer, R; Samuelsson, J; Mellqvist, J; Galle, B; Martinez, AP; Grutter, M; Retama, A; Molina, LT. Modeling constraints on the emission inventory and on vertical diffusion for CO and SO2 in the Mexico City Metropolitan Area using Solar FTIR and Zenith Sky UV Spectroscopy. Atmos. Chem. Phys 2007, 7, 781–801. [Google Scholar]
- Lei, W; de Foy, B; Zavala, M; Volkamer, R; Molina, LT. Characterizing ozone production in the Mexico City Metropolitan Area: A case study using a chemical transport model. Atmos. Chem. Phys 2007, 7, 1347–1366. [Google Scholar]
- Tie, X; Madronich, S; Li, GH; Ying, ZM; Zhang, R; Garcia, A; Lee-Taylor, J; Liu, Y. Characterizations of chemical oxidants in Mexico City: A regional chemical/dynamical model (WRF-Chem) study. Atmos. Environ 2007, 41, 1989–2008. [Google Scholar]
- Lei, W; Zavala, M; de Foy, B; Volkamer, R; Molina, LT. Characterizing ozone production and response under different meteorological conditions in Mexico City. Atmos. Chem. Phys 2008, 8, 7571–7581. [Google Scholar]
- Zhang, Y; Dubey, MK; Olsen, SC. Comparisons of WRF/Chem simulations in Mexico 20 City with ground-based RAMA measurements during the MILAGRO-2006 period. Atmos. Chem. Phys. Discuss 2009, 9, 1329–1376. [Google Scholar]
- Grell, GA; Peckham, SE; Schmitz, R; McKeen, SA; Frost, G; Skamarock, WC; Eder, B. Fully coupled “online” chemistry within the WRF model. Atmos. Environ 2005, 39, 6957–6975. [Google Scholar]
- Fast, JD; Gustafson, WI, Jr; Easter, RC; Zaveri, RA; Barnard, JC; Chapman, EG; Grell, G; Peckham, SE. Evolution of Ozone, particulates, and aerosol direct radiative forcing in the vicinity of Houston using a fully coupled meteorology-chemistry-aerosol model. J Geophys Res 2006, 111, D21305. [Google Scholar] [CrossRef]
- Zhang, Y; Vijayaraghavan, K; Seigneur, C. Evaluation of three probing techniques in a three-dimensional air quality model. J Geophys Res 2005, 110, D02305. [Google Scholar] [CrossRef]
- Misenis, C; Hu, XM; Krishnan, S; Zhang, Y; Fast, J. Sensitivity of WRF/Chem predictions to meteorological schemes. Presented at the 86th Annual Conference/14th Joint Conference on the Applications of Air Pollution Meteorology with the A&WMA, Atlanta, GA, USA, 27 January–3 February, 2006.
- Jiang, X; Li, Q; Liang, M; Shia, RL; Chahine, MT; Olsen, ET; Chen, LL; Yung, YL. Simulation of upper troposphere CO2 from chemistry and transport models. Global Biogeochem Cycles 2008, 22, 1–11. [Google Scholar] [CrossRef]
- de Foy, B; Fast, JD; Paech, SJ; Phillips, D; Walters, JT; Coulter, RL; Martin, TJ; Pekour, MS; Shaw, WJ; Kastendeuch, PP; Marley, NA; Retama, A; Molina, LT. Basin-scale wind transport during the MILAGRO field campaign and comparison to 25 climatology using cluster analysis. Atmos. Chem. Phys 2008, 8, 1209–1224. [Google Scholar]
- Tie, X; Geng, FH; Peng, L; Gao, W; Zhao, CS. Measurement and modeling of O3 variability in Shanghai, China; Application of the WRF-Chem model. Atmos. Environ 2009, 43, 4289–4302. [Google Scholar]
- Srinivas, CV; Jayakumar, I; Baham, JM; Hughes, R; Patrick, C; Young, JH; Rabbarison, M; Swanier, S; Hardy, MG; Anjaneyulu, YA. Simulation Study of meso-scale coastal circulations in Mississippi Gulf coast for atmospheric dispersion. J. Atmos. Res 2009, 91, 9–25. [Google Scholar]
- Anjaneyulu, Y; Srinivas, CV; Dasari, HP; White, LD; Baham, JM; Young, JH; Hughes, R; Patrick, C; Hardy, MG; Swanier, SJ. Simulation of atmospheric dispersion of air-borne effluent releases from point sources in Mississippi Gulf coast with different meteorological data. Int. J. Environ. Res. Public Health 2009, 6, 1055–1074. [Google Scholar]
- Srinivas, CV; Jayakumar, I; Baham, JM; Hughes, R; Patrick, C; Young, J; Rabbarison, M; Swanier, S; Hardy, MG; Anjaneyulu, Y. Sensitivity of Atmospheric dispersion simulations by HYSPLIT to the meteorological predictions from a mesoscale model. Environ. Fluid Mechanics 2008, 8, 367–387. [Google Scholar]
- Anjaneyulu, Y; Srinivas, CV; Jayakumar, I; Hariprasad, D; Baham, J; Patrick, C; Young, J; Hughes, L; White, D; Hardy, MG; Swanier, S. Some observational and modeling studies of the coastal atmospheric boundary layer at Mississippi Gulf coast for Air Pollution Dispersion assessment. Int. J. Environ. Res. Public Health 2008, 5, 484–497. [Google Scholar]
- Anjaneyulu, Y; Srinivas, CV; Bhaskar, DV; Hariprasad, D; Young, JH; Chuck, P; Baham, JM; Hughes, RL; Hardy, MG; Swanier, SJ. Simulation of surface ozone pollution in the central gulf coast region using WRF/Chem Model: Sensitivity to PBL and Land Surface Physics. Adv Meteorol 2010, 2010, 1–24. [Google Scholar] [CrossRef]
- Skamarock, WC; Klemp, JB; Dudhia, J; Gill, DO; Barker, DM; Wang, W; Powers, JG. A description of the Advanced Research WRF version 2; NCAR Technical Note: NCAR/TN-468+STR 2005; NCAR: Boulder, CO, USA, 2005. [Google Scholar]
- Guenther, A; Zimmerman, PR; Harley, PC; Monson, RK; Fall, R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analysis. J. Geophys. Res 1993, 98D, 12609–12617. [Google Scholar]
- Guenther, A; Zimmerman, P; Wildermuth, M. Natural volatile organic compound emission rate estimates for US woodland landscapes. Atmos. Environ 1994, 28, 1197–1210. [Google Scholar]
- Lin, YL; Farley, RD; Orville, HD. Bulk parameterization of the snow field in a cloud model. J. Clim. Appl. Meteor 1983, 22, 1065–1092. [Google Scholar]
- Chou, MD; Suarezm, MJ. An efficient thermal infrared radiation parameterization for use in general circulation models. NASA Tech Memo 1994, 104606, 85. [Google Scholar]
- Mlawer, EJ; Taubman, SJ; Brown, PD; Iacono, MJ; Clough, SA. Radiative transfer for inhomogeneous atmosphere: RRTM, a validated correlated-k model for the longwave. J. Geophys. Res 1997, 102, 16663–16682. [Google Scholar]
- Hong, SY; Noh, Y; Dudhia, J. A new vertical diffusion package with an explicit treatment of entrainment processes. Mon. Wea. Rev 2006, 134, 2318–2341. [Google Scholar]
- Chen, F; Dudhia, J. Coupling an advanced land-surface/hydrology model with the Penn State/NCAR MM5 modeling system. Part I: Model description and implementation. Mon. Wea. Rev 2001, 129, 569–585. [Google Scholar]
- Stockwell, WR; Middleton, P; Chang, JS; Tang, X. The second-generation regional acid deposition model chemical mechanism for regional air quality modeling. J. Geophys. Res 1990, 95, 16343–16367. [Google Scholar]
- Chang, JS; Middleton, PB; Stockwell, WR; Binkowski, FS; Byun, D. The regional acid deposition model and engineering model, State-of-Science/Technology; Report 4; National Acid Precipitation Assessment Program: Washington, DC, USA, 1989. [Google Scholar]
- Madronich, S. Photodissociation in the Atmosphere, actinic flux and the effects of ground reflections and clouds. J. Geophys.Res 1987, 92, 9740–9752. [Google Scholar]
- Fishman, J; Solomon, S; Crutzen, PJ. Observational and theoretical evidence in support of a significant insitu photo-chemical source of tropospheric Ozone. Tellus 1979, 31, 432–446. [Google Scholar]










| Dynamics | Primitive equation, non-hydrostatic | |||
|---|---|---|---|---|
| Vertical resolution | 40 levels | |||
| Domains | Domain 1 | Domain 2 | Domain 3 | Domain 4 |
| Horizontal resolution | 36 km | 12 km | 4 km | 1 km |
| Domains of integration | 104.074° W–76.2928° W; 19.8601° N–43.2371° N | 95.0053° W–85.6463° W; 27.6283° N–35.5859° N | 91.1234° W–89.2472° W; 31.5017° N–33.0888° N | 90.4022° W–89.9676° W; 32.1146° N–32.4836° N |
| Radiation | Goddard scheme for shortwave RRTM scheme for long wave | |||
| Sea surface temperature | NCEP FNL analysis | |||
| Cumulus convection | New Grell scheme on the outer grids domain 1and domain 2 | |||
| Explicit moisture | Lin scheme | |||
| PBL turbulence | Hong scheme (Yonsei State University PBL) | |||
| Surface processes | Noah LSM | |||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yerramilli, A.; Dodla, V.B.; Desamsetti, S.; Challa, S.V.; Young, J.H.; Patrick, C.; Baham, J.M.; Hughes, R.L.; Yerramilli, S.; Tuluri, F.; et al. Air Quality Modeling for the Urban Jackson, Mississippi Region Using a High Resolution WRF/Chem Model. Int. J. Environ. Res. Public Health 2011, 8, 2470-2490. https://doi.org/10.3390/ijerph8062470
Yerramilli A, Dodla VB, Desamsetti S, Challa SV, Young JH, Patrick C, Baham JM, Hughes RL, Yerramilli S, Tuluri F, et al. Air Quality Modeling for the Urban Jackson, Mississippi Region Using a High Resolution WRF/Chem Model. International Journal of Environmental Research and Public Health. 2011; 8(6):2470-2490. https://doi.org/10.3390/ijerph8062470
Chicago/Turabian StyleYerramilli, Anjaneyulu, Venkata B. Dodla, Srinivas Desamsetti, Srinivas V. Challa, John H. Young, Chuck Patrick, Julius M. Baham, Robert L. Hughes, Sudha Yerramilli, Francis Tuluri, and et al. 2011. "Air Quality Modeling for the Urban Jackson, Mississippi Region Using a High Resolution WRF/Chem Model" International Journal of Environmental Research and Public Health 8, no. 6: 2470-2490. https://doi.org/10.3390/ijerph8062470
APA StyleYerramilli, A., Dodla, V. B., Desamsetti, S., Challa, S. V., Young, J. H., Patrick, C., Baham, J. M., Hughes, R. L., Yerramilli, S., Tuluri, F., Hardy, M. G., & Swanier, S. J. (2011). Air Quality Modeling for the Urban Jackson, Mississippi Region Using a High Resolution WRF/Chem Model. International Journal of Environmental Research and Public Health, 8(6), 2470-2490. https://doi.org/10.3390/ijerph8062470




