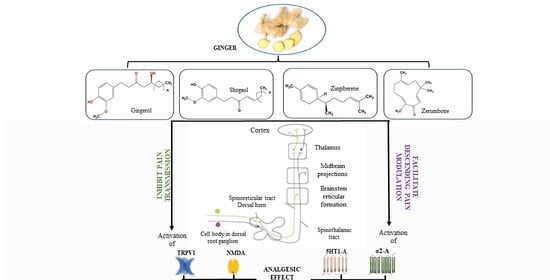
Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger
Abstract
:1. Introduction
2. Materials and Methods
3. Molecular Mechanisms Underlying the Analgesic Effect of Ginger
3.1. In Vitro Studies
3.2. In Vivo Studies
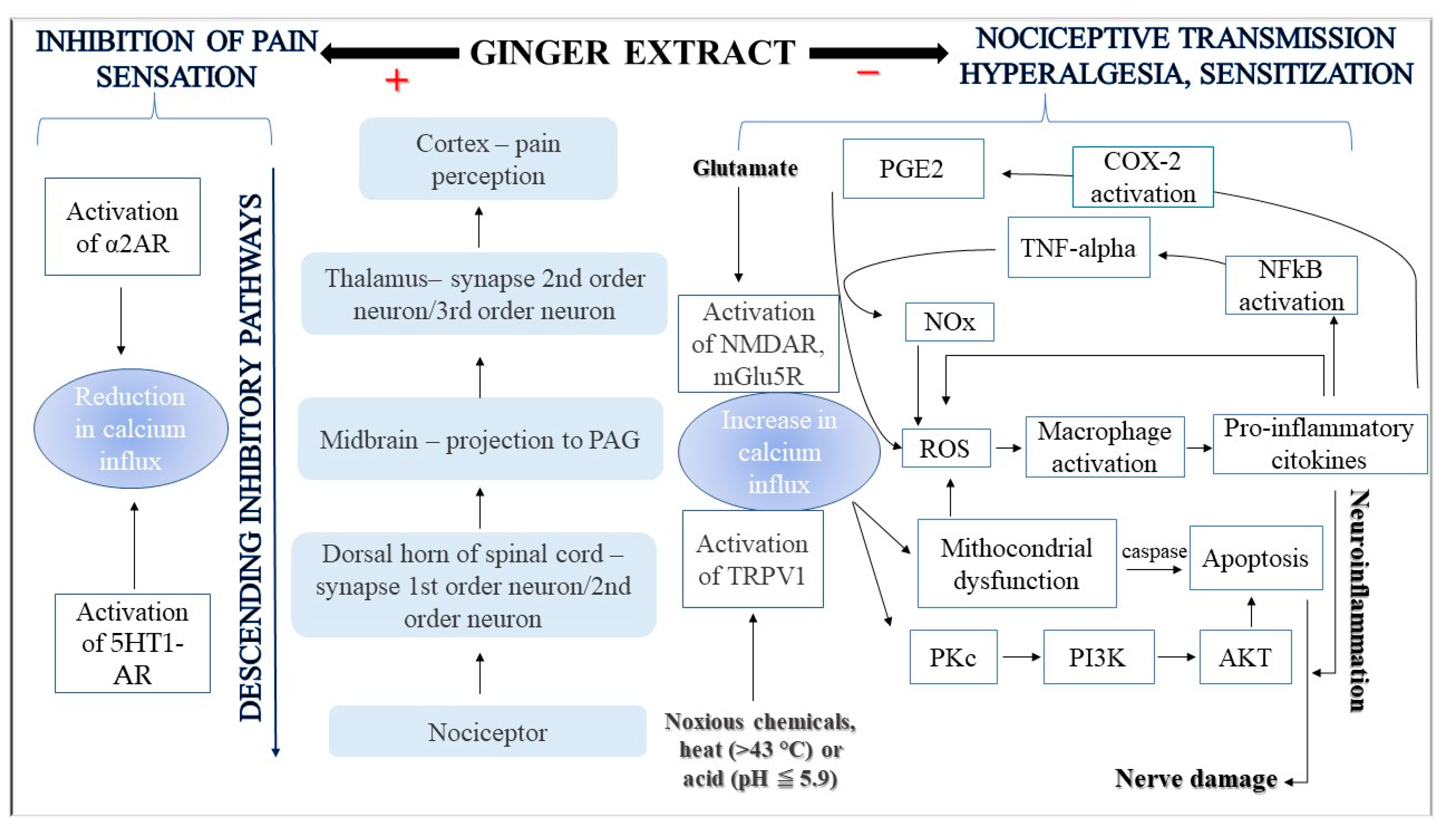
4. Discussion
- Modulation of pain-related neurotransmissions
- Inhibition of NF-κB signaling activation
- Inhibition of arachidonic acid metabolism
- Reduction in levels of proinflammatory cytokines
- Modulation of mitochondrial activity and reduction in oxidative stress
- Increase in zonulin and claudin-1 expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Begum, T.; Pandey, S.K.; Borah, A.; Paw, M.; Lal, M. Essential Oil Composition of Different Accessions of Ginger Collected from Northeast Region of India. J. Essent. Oil Bear. Plants 2018, 21, 1475–1486. [Google Scholar] [CrossRef]
- Menon, V.; Elgharib, M.; El-awady, R.; Saleh, E. Ginger: From serving table to salient therapy. Food Biosci. 2021, 41, 100934. [Google Scholar] [CrossRef]
- Nemati, Z.; Moradi, Z.; Alirezalu, K.; Besharati, M.; Raposo, A. Impact of ginger root powder dietary supplement on productive performance, egg quality, antioxidant status and blood parameters in laying japanese quails. Int. J. Environ. Res. Public Health 2021, 18, 2995. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Ni, Z.J.; Zhu, Y.Y.; Thakur, K.; Zhang, F.; Zhang, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. 2021, 12, 519–542. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytosci. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical composition and antimicrobial activity of fresh rhizome essential oil of zingiber officinale roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Munda, S.; Dutta, S.; Haldar, S.; Lal, M. Chemical Analysis and Therapeutic Uses of Ginger (Zingiber officinale Rosc.) Essential Oil: A Review. J. Essent. Oil Bear. Plants 2018, 21, 994–1002. [Google Scholar] [CrossRef]
- Wilson, R.; Haniadka, R.; Sandhya, P.; Palatty, P.L.; Baliga, M.S. Ginger (Zingiber officinale Roscoe) the dietary agent in skin care: A review. In Bioactive Dietary Factors and Plant Extracts in Dermatology; Humana Press: Totowa, NJ, USA, 2013; pp. 103–111. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Bradley, P. British Herbal Compendium Bournemouthe; British Herbal Midicine Association: Bournemouth, UK, 1992; Volume 1. [Google Scholar]
- Faraji, M.H.; Taherianfard, M. The Effect of Pretreatment with the Hydroalcoholic Extract of Ginger on the Modulation of Dopamine D2 Receptor Agonist and Antagonist Impacts on Pain Sensitivity in Male Rats. Herb. Med. J. 2022, 6, 1–5. [Google Scholar]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Li, P.; Chen, X.; Liu, F.; Hou, Q. Ginger relieves intestinal hypersensitivity of diarrhea predominant irritable bowel syndrome by inhibiting proinflammatory reaction. BMC Complement. Med. Ther. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Karampour, N.S.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Dang, M.; Kumar, M. Anti-inflammatory and renal protective effect of gingerol in high-fat diet/streptozotocin-induced diabetic rats via inflammatory mechanism. Inflammopharmacology 2019, 27, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, L.; Chen, X.; Li, Y.; Qin, Y.; Meng, X.; Zhang, Q. 6-Shogaol ameliorates diabetic nephropathy through anti-inflammatory, hyperlipidemic, anti-oxidative activity in db/db mice. Biomed. Pharmacother. 2018, 97, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediators Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Qiu, J.L.; Chai, Y.N.; Duan, F.Y.; Zhang, H.J.; Han, X.Y.; Chen, L.Y.; Duan, F. 6-Shogaol alleviates CCl4-induced liver fibrosis by attenuating inflammatory response in mice through the NF-κB pathway. Acta Biochim. Pol. 2022, 69, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, J.G.; Yang, W.; Xu, P.; Xiao, Y.L.; Zhang, H.T. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed. Pharmacother. 2018, 107, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wu, T.; Dai, Y.; Ji, K.; Zhong, Y.; Xue, Y. The effect of 6-gingerol on inflammatory response and Th17/Treg balance in DSS-induced ulcerative colitis mice. Ann. Transl. Med. 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Ohshita, M.; Tamaki, H.; Marutani, Y.; Nakayama, Y.; Akagi, M.; Miyata, M.; Maehara, S.; Hata, T.; Inoue, A. Shogaol but not gingerol has a neuroprotective effect on hemorrhagic brain injury: Contribution of the α β-unsaturated carbonyl to heme oxygenase-1 expression. Eur. J. Pharmacol. 2019, 842, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Park, S.J.; Choi, J.W. Neuroprotective effects of 6-shogaol and its metabolite, 6-paradol, in a mouse model of multiple sclerosis. Biomol. Ther. 2019, 27, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014, 449, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H.; Liu, T.; Yang, W.; Lv, W.; He, D.; Guo, P.; Li, L. 6-Gingerol induces cell-cycle G1-phase arrest through AKT–GSK 3β–cyclin D1 pathway in renal-cell carcinoma. Cancer Chemother. Pharmacol. 2020, 85, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuzer, A.M.; Martin, A.C.B.M.; Becceneri, A.B.; da Silva, J.A.; Vieira, P.C.; Cominetti, M.R. [10]-Gingerol Affects Multiple Metastatic Processes and Induces Apoptosis in MDAMB- 231 Breast Tumor Cells. Anticancer. Agents Med. Chem. 2018, 19, 645–654. [Google Scholar] [CrossRef]
- Hu, S.M.; Yao, X.H.; Hao, Y.H.; Pan, A.H.; Zhou, X.W. 8-Gingerol regulates colorectal cancer cell proliferation and migration through the EGFR/STAT/ERK pathway. Int. J. Oncol. 2020, 56, 390–397. [Google Scholar] [CrossRef]
- Pei, X.D.; He, Z.L.; Yao, H.L.; Xiao, J.S.; Li, L.; Gu, J.Z.; Shi, P.Z.; Wang, J.H.; Jiang, L.H. 6-Shogaol from ginger shows anti-tumor effect in cervical carcinoma via PI3K/Akt/mTOR pathway. Eur. J. Nutr. 2021, 60, 2781–2793. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.H.; Ni, Z.J.; Zhang, F.; Zhang, Y.Y.; Liu, M.M.; Thakur, K.; Zhang, J.G.; Wang, S.; Wei, Z.J. 6-Shogaol mediated ROS production and apoptosis via endoplasmic reticulum and mitochondrial pathways in human endometrial carcinoma Ishikawa cells. J. Funct. Foods 2020, 74, 104178. [Google Scholar] [CrossRef]
- Baptista Moreno Martin, A.C.; Tomasin, R.; Luna-Dulcey, L.; Graminha, A.E.; Araújo Naves, M.; Teles, R.H.G.; da Silva, V.D.; da Silva, J.A.; Vieira, P.C.; Annabi, B.; et al. [10]-Gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models. Cell. Oncol. 2020, 43, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Park, S.Y. Attenuation of autophagy flux by 6-shogaol sensitizes human liver cancer cells to TRAIL-induced apoptosis via p53 and ROS. Int. J. Mol. Med. 2019, 43, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Jo, M.; Hong, J.E.; Park, C.O.; Lee, C.G.; Rhee, K.J. Protective effects of zerumbone on colonic tumorigenesis in enterotoxigenic bacteroides fragilis (ETBF)-colonized AOM/DSS BALB/c mice. Int. J. Mol. Sci. 2020, 21, 857. [Google Scholar] [CrossRef]
- Sung, B.; Murakami, A.; Oyajobi, B.O.; Aggarwal, B.B. Zerumbone abolishes RaNKL-induced NF-κB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009, 69, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A.; Tanaka, T.; Lee, J.Y.; Surh, Y.J.; Kim, H.W.; Kawabata, K.; Nakamura, Y.; Jiwajinda, S.; Ohigashi, H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int. J. Cancer 2004, 110, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Liu, T.; Jing, L.; Wu, J. Zingiberene inhibits in vitro and in vivo human colon cancer cell growth via autophagy induction, suppression of PI3K/ AKT/mTOR Pathway and caspase 2 deactivation. J. BUON 2019, 24, 1470–1475. [Google Scholar]
- Ma, S.Q.; Guo, Z.; Liu, F.Y.; Hasan, S.G.; Yang, D.; Tang, N.; An, P.; Wang, M.Y.; Wu, H.M.; Yang, Z.; et al. 6-Gingerol protects against cardiac remodeling by inhibiting the p38 mitogen-activated protein kinase pathway. Acta Pharmacol. Sin. 2021, 42, 1575–1586. [Google Scholar] [CrossRef]
- Li, J.; Thangaiyan, R.; Govindasamy, K.; Wei, J. Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum. Exp. Toxicol. 2021, 40, 915–927. [Google Scholar] [CrossRef]
- Wang, S.; Tian, M.; Yang, R.; Jing, Y.; Chen, W.; Wang, J.; Zheng, X.; Wang, F. 6-Gingerol Ameliorates Behavioral Changes and Atherosclerotic Lesions in ApoE −/− Mice Exposed to Chronic Mild Stress. Cardiovasc. Toxicol. 2018, 18, 420–430. [Google Scholar] [CrossRef]
- Jain, M.; Singh, A.; Singh, V.; Maurya, P.; Barthwal, M.K. Gingerol Inhibits Serum-Induced Vascular Smooth Muscle Cell Proliferation and Injury-Induced Neointimal Hyperplasia by Suppressing p38 MAPK Activation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Heiss, E.H.; Sider, N.; Schinkovitz, A.; Gröblacher, B.; Guo, D.; Bucar, F.; Bauer, R.; Dirsch, V.M.; Atanasov, A.G. Identification and characterization of [6]-shogaol from ginger as inhibitor of vascular smooth muscle cell proliferation. Mol. Nutr. Food Res. 2015, 59, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Gratal, P.; Mediero, A.; Lamuedra, A.; Matamoros-Recio, A.; Herencia, C.; Herrero-Beaumont, G.; Martín-Santamaría, S.; Largo, R. 6-Shogaol (enexasogoal) treatment improves experimental knee osteoarthritis exerting a pleiotropic effect over immune innate signalling responses in chondrocytes. Br. J. Pharmacol. 2022, 179, 5089–5108. [Google Scholar] [CrossRef]
- Ruslay, S.; Abas, F.; Shaari, K.; Zainal, Z.; Maulidiani; Sirat, H.; Israf, D.A.; Lajis, N.H. Characterization of the components present in the active fractions of health gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS. Food Chem. 2007, 104, 1183–1191. [Google Scholar] [CrossRef]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Riva, A.; Petrangolini, G.; Iannello, G.; Nichetti, M.; et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phyther. Res. 2020, 34, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Malek Mahdavi, A.; Javadivala, Z.; Mahluji, S.; Zununi Vahed, S.; Ostadrahimi, A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phyther. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef]
- Mustafa, I.; Chin, N.L.; Fakurazi, S.; Palanisamy, A. Comparison of phytochemicals, antioxidant and anti-inflammatory properties of sun-, oven- and freeze-dried ginger extracts. Foods 2019, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, C.Y.; Cheng, H.M.; Lo, H.Y.; Li, C.C.; Chou, P.C.; Lee, Y.C.; Ho, T.Y. Ginger and Zingerone Ameliorate Lipopolysaccharide-Induced Acute Systemic Inflammation in Mice, Assessed by Nuclear Factor-κB Bioluminescent Imaging. J. Agric. Food Chem. 2015, 63, 6051–6058. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chang, K.S.; Lin, C.C. Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chem. 2013, 141, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1β in Cultured Chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Chia, J.S.M.; Izham, N.A.M.; Farouk, A.A.O.; Sulaiman, M.R.; Mustafa, S.; Hutchinson, M.R.; Perimal, E.K. Zerumbone Modulates α2A-Adrenergic, TRPV1, and NMDA NR2B Receptors Plasticity in CCI-Induced Neuropathic Pain In Vivo and LPS-Induced SH-SY5Y Neuroblastoma In Vitro Models. Front. Pharmacol. 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Luettig, J.; Rosenthal, R.; Lee, I.F.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Villalvilla, A.; da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol inhibits chondrocytes’ innate immune responses and cathepsin-K activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef]
- Villalvilla, A.; Almada, J.; Vieira, P.C.; Largo, R.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol inhibits cathepsin-K activity and has anticatabolic and anti-inflammatory properties in stimulated chondrocytes. Ann. Rheum. Dis. 2012, 71 (Suppl. S1), A1–A93. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-L.; Wang, R.; Yakhnitsa, V.; Santos, J.; Watson, C.; Kiritoshi, T.; Ji, G.; Kim, N.; Lovett, J.; Hamood, A.; et al. Ginger Root Extract Mitigates Neuropathic Pain via Suppressing Neuroinflammation: Gut-Brain Connection. Curr. Dev. Nutr. 2022, 6, 808. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.M.; Farouk, A.A.O.; Mohamad, T.A.S.T.; Sulaiman, M.R.; Zakaria, H.; Hassan, N.I.; Perimal, E.K. Zerumbone ameliorates neuropathic pain symptoms via cannabinoid and ppar receptors using in vivo and in silico models. Molecules 2021, 26, 3849. [Google Scholar] [CrossRef] [PubMed]
- Fajrin, F.A.; Hidayanti, E.D.; Khoiroh, N.L.; Sulistyaningrum, G.; Imandasari, N.; Afifah, A.; Hartono, S. Red ginger oil affects COX-2 and NMDAR expression during inflammatory- or neuropathy-induced chronic pain in mice. Jundishapur J. Nat. Pharm. Prod. 2021, 16, 112353. [Google Scholar] [CrossRef]
- Gopalsamy, B.; Chia, J.S.M.; Farouk, A.A.O.; Sulaiman, M.R.; Perimal, E.K. Zerumbone-Induced Analgesia Modulated via Potassium Channels and Opioid Receptors in Chronic Constriction Injury-Induced Neuropathic Pain. Molecules 2020, 25, 3880. [Google Scholar] [CrossRef] [PubMed]
- Mata-Bermudez, A.; Izquierdo, T.; de los Monteros-Zuñiga, E.; Coen, A.; Godínez-Chaparro, B. Antiallodynic effect induced by [6]-gingerol in neuropathic rats is mediated by activation of the serotoninergic system and the nitric oxide–cyclic guanosine monophosphate–adenosine triphosphate-sensitive K+ channel pathway. Phyther. Res. 2018, 32, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Gopalsamy, B.; Farouk, A.A.O.; Tengku Mohamad, T.A.S.; Sulaiman, M.R.; Perimal, E.K. Antiallodynic and antihyperalgesic activities of zerumbone via the suppression of IL-1β, IL-6, and TNF-α in a mouse model of neuropathic pain. J. Pain Res. 2017, 10, 2605–2619. [Google Scholar] [CrossRef] [Green Version]
- Chia, J.S.M.; Omar Farouk, A.A.; Mohamad, A.S.; Sulaiman, M.R.; Perimal, E.K. Zerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomed. Pharmacother. 2016, 83, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J. Ethnopharmacol. 2020, 249, 112396. [Google Scholar] [CrossRef]
- Fajrin, F.A.; Imandasari, N.; Barki, T.; Sulistyaningrum, G.; Afifah; Kristiningrum, N.; Puspitasari, E.; Holidah, D. The activity of red ginger oil in antioxidant study in vitro and antihyperalgesia effect in alloxan-induced painful diabetic neuropathy in mice. Thai J. Pharm. Sci. 2019, 43, 69–75. [Google Scholar]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale roscoe rhizomes attenuate oxaliplatin-induced neuropathic pain in mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Garcia-Gimenez, M.D.; Quilez, A.M.; De la Puerta, R.; Fernandez-Arche, A. Ginger rhizome enhances the anti-inflammatory and anti-nociceptive effects of paracetamol in an experimental mouse model of fibromyalgia. Inflammopharmacology 2018, 26, 1093–1101. [Google Scholar] [CrossRef]
- Haleem, D.J. Targeting Serotonin1A Receptors for Treating Chronic Pain and Depression. Curr. Neuropharmacol. 2019, 17, 1098–1108. [Google Scholar] [CrossRef]
- Smith, H.; Elliott, J. Alpha2 receptors and agonists in pain management. Curr. Opin. Anaesthesiol. 2001, 14, 513–518. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Zhang, M.; Xue, Y.; Zheng, B.; Zhang, Y.; Liu, Y.; Chu, X.; Sun, Z.; Han, X. Inhibition of human ether-à-go-go-related gene K+ currents expressed in HEK293 cells by three gingerol components from ginger. J. Pharm. Pharmacol. 2022, 74, 1133–1139. [Google Scholar] [CrossRef]
- Öz, B.; Orhan, C.; Tuzcu, M.; Şahin, N.; Özercan, İ.H.; Demirel Öner, P.; Koca, S.S.; Juturu, V.; Şahin, K. Ginger extract suppresses the activations of NF-κB and Wnt pathways and protects inflammatory arthritis. Anatol. J. Cardiol. 2021, 8, 196–201. [Google Scholar] [CrossRef]
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Gang, J.; Lee, J.H.; Yang, H.; Cheon, C.; Ko, S.G.; Bae, H.; Kim, W. [6]-Shogaol Attenuates Oxaliplatin-Induced Allodynia through Serotonergic Receptors and GABA in the Spinal Cord in Mice. Pharmaceuticals 2022, 15, 726. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Benarroch, E.E. CGRP: Sensory neuropeptide with multiple neurologic implications. Neurology 2011, 77, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, A.J.; Wu, S.W.; Peters, J.H. Isolation of TRPV1 independent mechanisms of spontaneous and asynchronous glutamate release at primary afferent to NTS synapses. Front. Neurosci. 2014, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Vangeel, L.; Voets, T. Transient receptor potential channels and calcium signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048. [Google Scholar] [CrossRef]
- Ma, W.; Li, L.; Xing, S. PGE2/EP4 receptor and TRPV1 channel are involved in repeated restraint stress-induced prolongation of sensitization pain evoked by subsequent PGE2 challenge. Brain Res. 2019, 1721, 146335. [Google Scholar] [CrossRef]
- Yin, Y.; Dong, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. Structural mechanisms underlying activation of TRPV1 channels by pungent compounds in gingers. Br. J. Pharmacol. 2019, 176, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, W.; Zhou, L.; Lu, Z.; Jie, P.; Chen, L.; Chen, L. Activation of transient receptor potential vanilloid 4 increases NMDA-activated current in hippocampal CA1 pyramidal neurons. Front. Cell. Neurosci. 2013, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Weyerbacher, A.R.; Xu, Q.; Tamasdan, C.; Shin, S.J.; Inturrisi, C.E. N-Methyl-d-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain 2010, 148, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihara, Y.; Egashira, N.; Sada, H.; Kawashiri, T.; Ushio, S.; Yano, T.; Ikesue, H.; Oishi, R. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawel, K.; Kukula-koch, W.; Banono, N.S.; Nieoczym, D.; Targowska-duda, K.M.; Czernicka, L.; Parada-turska, J.; Esguerra, C.V. 6-gingerol, a major constituent of zingiber officinale rhizoma, exerts anticonvulsant activity in the pentylenetetrazole- induced seizure model in larval zebrafish. Int. J. Mol. Sci. 2021, 22, 7745. [Google Scholar] [CrossRef]
- Goncalves dos Santos, G.; Li, R.; Ng, M.P.E.; Lemes, J.B.P.; Vieira, W.F.; Nagy, I.; Tambeli, C.H.; Parada, C.A. CB1 receptor-dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons. Br. J. Pharmacol. 2020, 177, 4615–4626. [Google Scholar] [CrossRef]
- Banister, S.D.; Krishna Kumar, K.; Kumar, V.; Kobilka, B.K.; Malhotra, S.V. Selective modulation of the cannabinoid type 1 (CB1) receptor as an emerging platform for the treatment of neuropathic pain. Medchemcomm 2019, 10, 647–659. [Google Scholar] [CrossRef]
- Baldini, A.; von Korff, M.; Lin, E.H.B. A review of potential adverse effects of long-term opioid therapy: A practitioner’s guide. Prim. Care Companion J. Clin. Psychiatry 2012, 14, 27252. [Google Scholar] [CrossRef] [Green Version]
- Rocha-González, H.I.; Castañeda-Corral, G.; Argüelles, C.F.; Granados-Soto, V. Role of serotonin receptors in inflammatory pain. In Acute Pain: Causes, Effects and Treatment; Balint, G., Antala, B., Carty, C., Mabieme, J.-M.A., Amar, I.B., Kaplanova, A., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; pp. 1–42. ISBN 9781607412236. [Google Scholar]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef]
- Fuxe, K.; Gago, B.; Suarez-Boomgaard, D.; Narvaez, M.; Agnati, L.F.; Skieterska, K.; Van Craenenbroeck, K.; Rivera, A.; Borroto-Escuela, D.O. Heteroreceptor Complexes in the Central Nervous System. Focus on Their Role in Pain Modulation. J. Acupunct. Meridian Stud. 2015, 8, 334–335. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, T.; Knight, Y.E.; Goadsby, P.J. Activation of 5-HT1B/1D receptor in the periaqueductal gray inhibits nociception. Ann. Neurol. 2004, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gibson, A.W.; Levinstein, M.R.; Lesiak, A.J.; Ong, S.E.; Neumaier, J.F. 5-HT1B Receptor-Mediated Activation of ERK1/2 Requires Both Gαi/o and β-Arrestin Proteins. ACS Chem. Neurosci. 2019, 10, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Laranjeira-Silva, M.F.; Carvalho-Sousa, C.E.; Floeter-Winter, L.M.; Markus, R.P. The RelA/cRel nuclear factor-κB (NF-κB) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J. Pineal Res. 2016, 60, 394–404. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Niederberger, E.; Geisslinger, G. The IKK-NF-κB pathway: A source for novel molecular drug targets in pain therapy? FASEB J. 2008, 22, 3432–3442. [Google Scholar] [CrossRef]
- Raha, S.; Lee, H.J.; Yumnam, S.; Hong, G.E.; Venkatarame Gowda Saralamma, V.; Ha, Y.L.; Kim, J.O.; Kim, Y.S.; Heo, J.D.; Lee, S.J.; et al. Vitamin D2 suppresses amyloid-β 25–35 induced microglial activation in BV2 cells by blocking the NF-κB inflammatory signaling pathway. Life Sci. 2016, 161, 37–44. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular specificity of NF-κB function in the nervous system. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef] [Green Version]
- Lilienbaum, A.; Israël, A. From Calcium to NF-κB Signaling Pathways in Neurons. Mol. Cell. Biol. 2003, 23, 2680–2698. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, T.G. Role of Nuclear Factor Kappa B (NF-κB) Signalling in Neurodegenerative Diseases: An Mechanistic Approach. Curr. Neuropharmacol. 2020, 18, 918–935. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, E.; Schmidtko, A.; Gao, W.; Kühlein, H.; Ehnert, C.; Geisslinger, G. Impaired acute and inflammatory nociception in mice lacking the p50 subunit of NF-κB. Eur. J. Pharmacol. 2007, 559, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, G.; Shimaoka, M.; Fukuoka, T.; Hiroi, T.; Inoue, T.; Hashimoto, N.; Sakaguchi, T.; Sawa, Y.; Morishita, R.; Kiyono, H.; et al. NF-κB decoy suppresses cytokine expression and thermal hyperalgesia in a rat neuropathic pain model. Neuroreport 2001, 12, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by Targeting of NF-κB and Nrf2 signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Bowles, R.D.; Mata, B.A.; Bell, R.D.; Mwangi, T.K.; Huebner, J.L.; Kraus, V.B.; Setton, L.A. In vivo luminescence imaging of nf-kb activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol. 2014, 66, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Segelcke, D.; Pradier, B.; Pogatzki-Zahn, E. Advances in assessment of pain behaviors and mechanisms of post-operative pain models. Curr. Opin. Physiol. 2019, 11, 85–92. [Google Scholar] [CrossRef]
- Song, Z.P.; Xiong, B.R.; Guan, X.H.; Cao, F.; Manyande, A.; Zhou, Y.Q.; Zheng, H.; Tian, Y.K. Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta Pharmacol. Sin. 2016, 37, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.M.; Parhami, F.; Xi, X.P.; Berliner, J.A.; Hsueh, W.A.; Law, R.E.; Demer, L.L. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2094–2104. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Quirion, R. Does COX2-dependent PGE2 play a role in neuropathic pain? Neurosci. Lett. 2008, 437, 165–169. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, B.S.; Lee, H.L.; Son, S.J.; Hwang, S.H.; Kim, D.S.; Park, J.S.; Cho, H.J. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 2004, 19, 3375–3381. [Google Scholar] [CrossRef]
- Egger, J.; Bretscher, P.; Freigang, S.; Kopf, M.; Carreira, E.M. Synthesis of epoxyisoprostanes: Effects in reducing secretion of pro-inflammatory cytokines IL-6 and IL-12. Angew. Chem. Int. Ed. 2013, 52, 5382–5385. [Google Scholar] [CrossRef]
- Rothman, S.M.; Ma, L.H.; Whiteside, G.T.; Winkelstein, B.A. Inflammatory cytokine and chemokine expression is differentially modulated acutely in the dorsal root ganglion in response to different nerve root compressions. Spine 2011, 36, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, T.; Ahn, M.; Matsumoto, Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: Recent insights from macrophages. Anat. Cell Biol. 2012, 45, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uttra, A.M.; Alamgeer; Shahzad, M.; Shabbir, A.; Jahan, S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and upregulating IL-4 and IL-10. J. Ethnopharmacol. 2018, 224, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Berta, T.; Xu, Z.Z.; Liu, T.; Park, J.Y.; Ji, R.R. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011, 152, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Yin, Y.; Yin, X.; Cao, F.; Luo, D.; Zhang, T.; Li, Y.; Ni, L. Anti-nociceptive effects of Tanshinone IIA (TIIA) in a rat model of complete Freund’s adjuvant (CFA)-induced inflammatory pain. Brain Res. Bull. 2012, 88, 581–588. [Google Scholar] [CrossRef]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial Cytokines in Pain States. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Fernyhough, P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium 2008, 44, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.G.M.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Mei, Y.; Chen, Y.; Li, Y.; Zhu, D.; Cui, J.; Yu, L. Perisciatic Nerve Dexmedetomidine Alleviates Spinal Oxidative Stress and Improves Peripheral Mitochondrial Dynamic Equilibrium in a Neuropathic Pain Mouse Model in an AMPK-Dependent Manner. Dis. Markers 2022, 2022, 6889676. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; Lee, I.; Chung, K.; Chung, J.M. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 2008, 138, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, J.; Fang, L.; Willis, W.D. The effects of protein phosphatase inhibitors on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain 2003, 106, 443–451. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Lei, Y.; Fang, L.; Willis, W.D. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Mol. Brain Res. 2005, 138, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, Y.V.; Kim, M.S.; Usachev, Y.M. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J. Neurosci. 2008, 28, 5295–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Jeong, J.J.; Woo, K.H.; Han, M.J.; Kim, D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.K.; Schwabe, J.; Lux, T.J.; Salvador, E.; Rittner, H.L. Quantitative and Microstructural Changes of the Blood-Nerve Barrier in Peripheral Neuropathy. Front. Neurosci. 2018, 12, 936. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H.H. IFN-γ IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef] [PubMed]
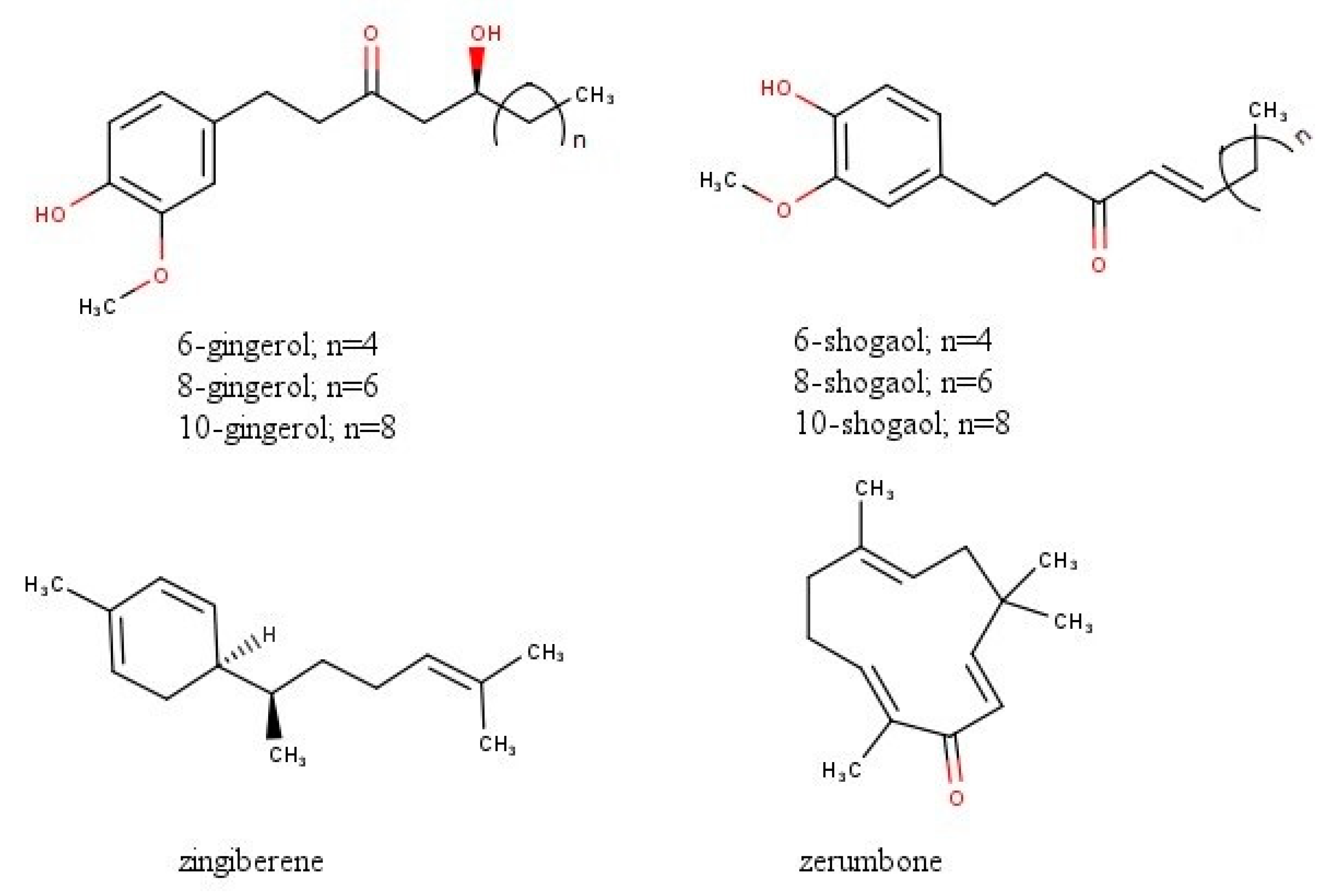

| First Author | Cell lines | Treatment | Results |
|---|---|---|---|
| Chia et al., 2020 [56] | LPS-induced neuronal sensitization in SH-SY5Y neuroblastoma cells | ZER 8 µg/mL for 24 h | increased the expression of α2A-adrenergic receptors downregulated TRPV1 and NMDAR2B receptors |
| Borgonetti et al., 2020 [48] | LPS-induced inflammation in murine microglial cells | GE 10 µg/mL 6-GEG 1 μg/mL SEG 0.17 μg/mL ZTE 3 μg/mL for 4 h | GE: decreased expression of pERK1, pERK2, HDAC1, TNF-α, IL-1β, IL-6, and NF-κB p65 signaling activation GEG: decreased protein levels of pERK1 and pERK2 increased HDAC1 and IKBα SEG: decreased protein levels of pERK1, pERK2, IL-1β, and IL-6 increased HDAC1 ZTE: decreased expression of HDAC1, IKBα, TNF-α, IL-1β, and IL-6 |
| Deng et al., 2019 [57] | HepG2 cell line | GE 1, 2.5, 5 mg/mL for 3 days | increased mitochondrial mass and mtDNA copy number (the effect was significant for 2.5, 5 mg/mL) determined the activities of mitochondrial respiratory chain complex I and IV increased levels of NDUFS1 (complex Ⅰ), SDHA (complex Ⅱ), UQCRC1 (complex Ⅲ), COX4 (complex Ⅳ), ATP5A1 (complex Ⅴ), p-AMPKɑ (Thr172), PGC1ɑ, NRF1, and TFAM |
| Mustafa et al., 2019 [49] | LPS-induced inflammation in murine macrophage cell line RAW 264.7 | GE 25, 50, 100 μg/mL | decreased NO production |
| Hosseinzadeh et al., 2017 [54] | IL-1β-induced oxidative stress in C28I2 human chondrocytes | GE 5, 25 μg/mL | increased the gene expression of catalase, superoxide dismutase-1, glutathione peroxidase-1, glutathione peroxidase-3, and glutathione peroxidase-4 reduced the IL-1β-induced elevation of ROS, lipid peroxidation, the Bax/Bcl-2 ratio, and caspase-3 activity |
| Luettig et al., 2016 [58] | HT-29/B6 and Caco-2 human intestinal epithelial cells | 6-SEG 100 μM for 1 h | inhibited the NF-κB and PI3K/Akt signaling activation |
| Hsiang et al., 2015 [50] | LPS-induced NF-κB activation in HepG2/NF-κB cells | Ginger 0.5; 1; 2.5; 5; 10; 50; 100 μg/mLZingerone 0.5; 1; 2.5; 5; 10; 50; 100 μg/mL | decreased NF-κB activity |
| Villalvilla et al., 2014 [59] | LPS- or IL-1β-challenged human chondrocytes/ ATDC5 murine chondrogenic cell lines | 6-shogaol 5 μM | completely inhibited the increase in NO production, IL-6, and MCP-1 expression, induced by LPS did not completely inhibit the increase in NO production, IL-6, and MCP-1 expression, induced by LPS |
| Ho et al., 2013 [51] | LPS-activated BV2 microglia cells | 6-GEG, -SEG 5, 10, 20 μM;8-GEG, -SEG 5, 10, 20 μM;10-GEG, -SEG 5, 10, 20 μM; Zingerone 5, 10, 20 μM; GE 0.125–0.5 mg/mL for 20 h | 8-GEG, 10-GEG, all SEG groups, and GE: decreased levels of TNF-α, IL-1α, IL-6, iNOS protein, mRNA expression, NO production, and NF-κB p65 activity |
| Ha et al., 2012 [52] | LPS-activated BV2 microglia cells | 6-GEG 1, 5, 10 μM; 6-SEG 1, 5, 10 μM for 24 h | 6-SEG 10 μM: decreased expression of iNOS 6-SEG 5 and 10 μM: decreased expression of iNOS and COX2 |
| LPS-activated primary microglia cells | All groups of 6-SEG: decreased NO production 6-SEG 10 μM: decreased expression of COX2 6-SEG 5 and 10 μM: decreased levels of IL-1β and TNF-α | ||
| Villalvilla et al., 2012 [60] | LPS- or IL-1β-challenged ATDC5 chondrocytes | 6-shogaol 5 μM 10-shogaol 5 μM | inhibited cathepsin-K activity, Only 6-shogaol: inhibited the LPS-induced increase in nitrite, NOS2, and MyD88 expressions. Inhibited ERK pathway activation and the activities of MMP-2 and MMP-9 did not significantly reduce IL-1β-induced nitrite accumulation |
| Dugasani et al., 2010 [55] | LPS-challenged RAW 264.7 cells | 6-shogaol 6 μM 8-shogaol 6 μM 10-shogaol 6 μM | reduced oxidative stress (direct scavenging effects against DPPH, superoxide, and hydroxyl radicals) inhibited the production of PGE2 and NO |
| Study | Animals | Model | Intervention | Results | Associated Molecular Mechanism |
|---|---|---|---|---|---|
| Kim et al., 2022 [78] | C57BL/6 mice | Oxaliplatin-induced neuropathic pain | [6]-shogaol 10 mg/kg, i.p., 1 dose | Significantly reduced cold and mechanical allodynia | activation of receptors 5-HT1A and 5-HT3 increase in GABA synthesis (increase in the levels of glutamic acid decarboxylase) |
| Shen et al., 2022 [61] | Sprague Dawley rats | Spinal nerve ligation-induced neuropathic pain | GEG 0.375% (w/w in diet) 0.75% (w/w in diet) 30 days | Significantly reduced mechanical hypersensitivity | reduction in the levels of gene expression of zonulin and claudin-1 in the amygdala and colon of animals with nerve ligation as well as NF-κB in the amygdala, colon, and ileum of animals with nerve ligation |
| Shen et al., 2022 [62] | Sprague Dawley rats | Spinal nerve ligation-induced neuropathic pain | GEG 0.75% (w/w in diet) 30 days | Reduced pain sensitivity after 10 days following operation. The effect persisted for up to 30 days. | reduction in mitochondrial oxidative stress, as reflected in the decreased plasma ccf-mtDNA levels the microbiome profile was strongly altered after SEG treatment |
| Chia et al., 2021 [63] | ICR mice | Spinal nerve ligation-induced neuropathic pain | ZER 10 mg/kg, i.p. Acute administration on day 14 postinjury | Reduced mechanical allodynia and thermal hyperalgesia | activation of CB1R and PPARα |
| Fajrin et al., 2021 [64] | Male BALB/c mice | (1) complete Freund’s adjuvant-induced inflammatory pain(2) partial sciatic nerve ligation-induced neuropathic pain | GE 100, 200, 400, or 600 mg/kg 14 days | Reduced thermal hyperalgesia | GE 600 mg/kg: reduction in COX-2 expression in the spinal cord and brain, and reduced NMDAR2B in the spinal cord increase in NMDAR2A expression in the spinal cord |
| Lee et al., 2021 [71] | C57BL/6 mice | Oxaliplatin-induced neuropathic pain | GE (100, 300, and 500 mg/kg)Acute administration | Significantly attenuated both cold and mechanical allodynia induced by oxaliplatin. | activation of 5-HT1A, but not 5-HT2A. The antiallodynic effect of GE against cold allodynia is also mediated by 5-HT3 activation. increase in mRNA expression of the spinal 5-HT1A receptor |
| Öz et al., 2021 [76] | Wistar rats | Complete Freund’s adjuvant-induced inflammatory pain | GE 50 mg/kg/daily 32 days | Reduction in arthritis symptoms | decrease in serum levels of TNF-α, IL-6, IL-17, and DKK-1 increased sclerostin serum level decreases in the tissue levels of IL-17, TNF-α, COX-2, and NF-κB |
| Gopalsamy et al., 2020 [65] | ICR mice | Spinal nerve ligation-induced neuropathic pain | ZER 10 mg/kg, i.p. Acute administration on day 14 postinjury | Reduced mechanical allodynia, and thermal hyperalgesia | activation of voltage-dependent K+ channel, ATP-sensitive K+ channel blocker, small-conductance Ca2+-activated K+ channel, large-conductance Ca2+-activated K+ channel activation of opioid receptors |
| Borgonetti et al., 2020 [48] | CD1 male mice/ BV2 cells | Spinal nerve ligation-induced neuropathic pain/LPS challenge | GE 200 mg kg−17 days | Reduced mechanical and thermal allodynia in the spared nerve injury mice model. | reduction in spinal neuroinflammation GE, 6-gingerol, and 6-shogaol reduced pERK levels GE and terpene fraction: reduced HDAC1 protein levels, inhibited NF-κB signaling activation, and decreased IL-1β, TNF-α, and IL-6 release |
| Chia et al., 2020 [56] | ICR mice | Spinal nerve ligation-induced neuropathic pain | ZER 10 mg/kg, i.p. Acute administration on day 14 postinjury | Decreased mechanical allodynia and thermal hyperalgesia | inhibition of TRPV1 increased the expression of α1, α2, β1, and β2 adrenoceptors downregulation of NMDA NR2B receptors |
| Fajrin et al., 2020 [69] | Balb/C mice | Streptozotocin-induced diabetic neuropathy | SEG (5, 10, 15 mg/kg/day, orally) GE (100, 200, 400 mg/kg/day, orally), Between days 28–49 after streptozotocin administration | SEG 15 mg/kg: Reduced mechanical allodynia and thermal hyperalgesia GE 200 and 400 mg/kg: Reduced thermal hyperalgesia, not mechanical allodynia | SEG 15 mg/kg: decreased mRNA expressions of TRPV1 and NMDAR2B in the spinal cord GE 400 mg/kg: decreased mRNA expressions of TRPV1 and NMDAR2B in the spinal cord |
| Gopalsamy et al., 2020 [65] | ICR mice | Spinal nerve ligation-induced neuropathic pain | ZER 10 mg/kg; i.p. 14 days | Reduced mechanical allodynia and thermal hyperalgesia | activation of voltage-dependent K+, ATP-sensitive K+ channel blocker, small-conductance Ca2+-activated K+ channel, or large-conductance Ca2+-activated K+ channel activation of opioid receptors |
| Fajrin et al., 2019 [70] | Balb/C mice | Alloxan-induced diabetic neuropathy | GE 100, 200, 400, 600 mg/kg orally Acute administration on day 14 postinjury | Decrease in thermal hyperalgesia | reduction in ROS and protection of cells in the spinal cord inhibition of ROS accumulation with a subsequent decrease in TRPV1 activation and deactivation of NMDAR2B in the dorsal horn of the spinal cord |
| Montserrat-de la Paz et al., 2018 [72] | C57BL/6 J mice/macrophages | Intermittent cold stress-induced fibromyalgia/LPS challenge | GER 0.5%, 1% in diet 56 days | Reduced mechanical and thermal allodynia and mechanical hyperalgesia and improved behavioral changes related to cognitive disturbances, anxiety, and depression. | reduction in the inflammatory response of proinflammatory mediators such as NO, PGE2, TXB2, and IL-1β in LPS-stimulated macrophages |
| Mata-Bermudez et al., 2018 [66] | Wistar rats | Spinal nerve ligation-induced neuropathic pain | GEG 10 µg, intrathecal one dose, on day | Reduced mechanical allodynia | activation of spinal 5-HT1A/1B/1D/5A receptors increase in nitric oxide-cyclic guanosine monophosphate and activated adenosine triphosphate-sensitive K+ channel pathway Naloxone (non-selective opioid receptor antagonist) did not prevent the [6]-gingerol-induced antiallodynic effect. |
| Gopalsamy et al., 2017 [67] | ICR mice | Chronic constriction injury | ZER (5, 10, 50 mg/kg/d, i.p.) 14 days | Decreased mechanical allodynia, mechanical hyperalgesia, thermal hyperalgesia, and cold allodynia | ZER 10, 50 mg/kg: reduced IL-1β, TNF-ɑ, and IL-6 levels in the plasma/spinal cord no change in IL-10 levels in plasma and spinal cord |
| Chia et al., 2016 [68] | ICR mice | Chronic constriction injury | ZER 10 mg/kg, i.p. Acute administration on day 14 postinjury. | Decreased mechanical allodynia and thermal hyperalgesia | activation of 5-HT receptors 1A, 1B, 2A, 3, 6, and 7 with subsequent enhancement of the descending serotoninergic transmission |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, C.; Zanfirescu, A.; Nițulescu, G.M.; Negreș, S. Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger. Nutraceuticals 2022, 2, 384-403. https://doi.org/10.3390/nutraceuticals2040029
Andrei C, Zanfirescu A, Nițulescu GM, Negreș S. Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger. Nutraceuticals. 2022; 2(4):384-403. https://doi.org/10.3390/nutraceuticals2040029
Chicago/Turabian StyleAndrei, Corina, Anca Zanfirescu, George Mihai Nițulescu, and Simona Negreș. 2022. "Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger" Nutraceuticals 2, no. 4: 384-403. https://doi.org/10.3390/nutraceuticals2040029
APA StyleAndrei, C., Zanfirescu, A., Nițulescu, G. M., & Negreș, S. (2022). Understanding the Molecular Mechanisms Underlying the Analgesic Effect of Ginger. Nutraceuticals, 2(4), 384-403. https://doi.org/10.3390/nutraceuticals2040029