Impact of Growing Location on Kakadu Plum Fruit Composition and In Vitro Bioactivity as Determinants of Its Nutraceutical Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Mammalian Cell Lines
2.2. Plant Material Collection and Extract Preparation
2.3. Total Phenolic Content (TPC), Total Ellagic Acid (TEA), and Vitamin C
2.4. Nitric Oxide Production in RAW 264.7 Cells Using the Diaminonaphthalene Assay
2.4.1. RAW 264.7 Cell Culture
2.4.2. Nitric Oxide Production by RAW264.7 Cells
2.5. Hep G2 Cell Viability Assay
2.5.1. Cell Culture
2.5.2. Cell Viability Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. TPC, TEA, and Vitamin C
3.2. Effect of KP Fruit Extracts on Inflammation Induced by LPS In Vitro
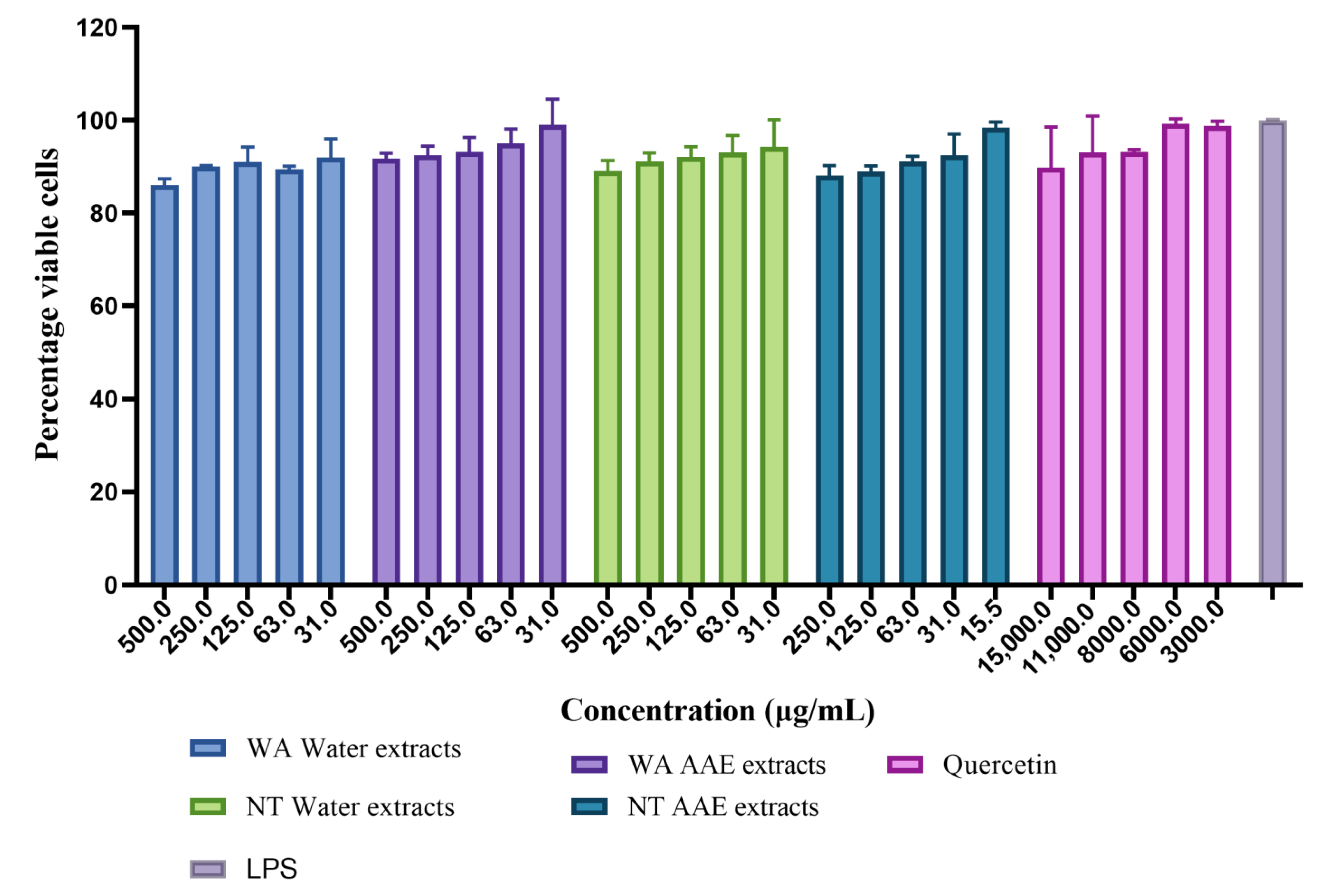
3.2.1. Cell Viability
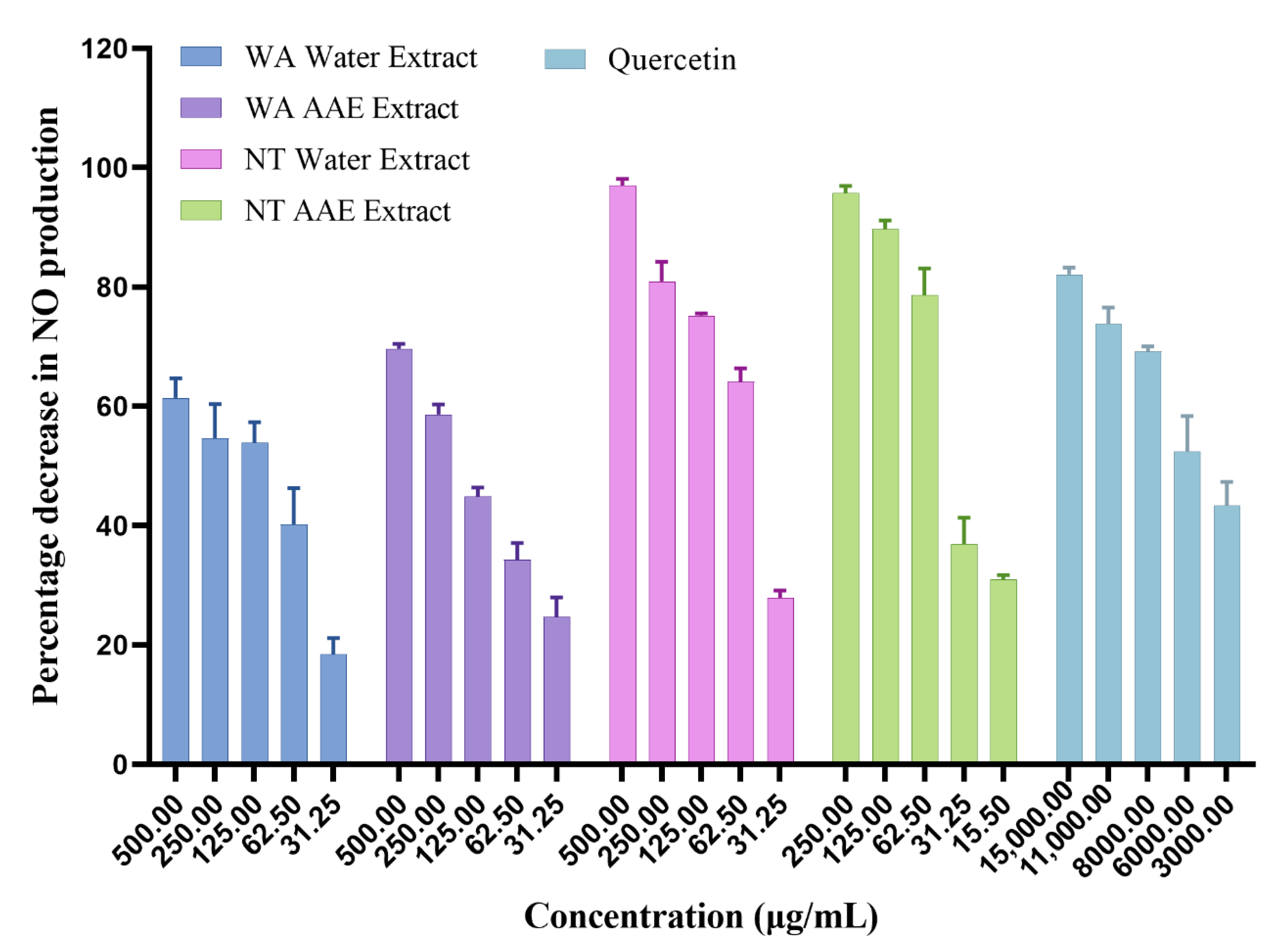
3.2.2. Inhibition of NO Production
3.3. Hep G2 Cell Viability Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215s–217s. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014, 151, 248–256. [Google Scholar] [CrossRef]
- Bobasa, E.M.; Phan, A.D.T.; Netzel, M.E.; Cozzolino, D.; Sultanbawa, Y. Hydrolysable tannins in Terminalia ferdinandiana Exell fruit powder and comparison of their functional properties from different solvent extracts. Food Chem. 2021, 358, 129833. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Akter, S.; Netzel, M.E.; Tinggi, U.; Osborne, S.A.; Fletcher, M.T.; Sultanbawa, Y. Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods 2019, 8, 281. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.G.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.J.; Munch, G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts—Identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015, 6, 910–919. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Modelling and prediction of global non-communicable diseases. BMC Public Health 2020, 20, 822. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Ohno-Machado, L.; Sandborn, W.J. Population Health Management for Inflammatory Bowel Disease. Gastroenterology 2018, 154, 37–45. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Addepalli, R.; Masci, P.; Gobe, G.; Osborne, S.A. In vitro anti-inflammatory activities of blacklip abalone (Haliotis rubra) in RAW 264.7 macrophages. Food Agric. Immunol. 2017, 28, 711–724. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Folkes, L.K.; O’Neill, P. DNA damage induced by nitric oxide during ionizing radiation is enhanced at replication. Nitric Oxide 2013, 34, 47–55. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Freeman, B.A. Interactions Between Nitric Oxide and Lipid Oxidation Pathways. Circ. Res. 2001, 88, 12–21. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Zabaras, D.; Sze, D.M. Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of Kakadu plum and Illawarra plum polyphenolic fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef]
- Tu, D.G.; Chyau, C.C.; Chen, S.Y.; Chu, H.L.; Wang, S.C.; Duh, P.D. Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds. Foods 2020, 9, 1514. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Mayer, F.; Honecker, F.; Schittenhelm, M.; Bokemeyer, C. Efficacy of cytotoxic agents used in the treatment of testicular germ cell tumours under normoxic and hypoxic conditions in vitro. Br. J. Cancer 2003, 89, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Breger, J.C.; Lyle, D.B.; Shallcross, J.C.; Langone, J.J.; Wang, N.S. Defining critical inflammatory parameters for endotoxin impurity in manufactured alginate microcapsules. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 755–765. [Google Scholar] [CrossRef]
- Qian, Z.J.; Kim, S.A.; Lee, J.S.; Kim, H.J.; Choi, I.L.W.; Jung, W.K. The antioxidant and anti-inflammatory effects of abalone intestine digest, Haliotis discus hannai in RAW 264.7 macrophages. Biotechnol. Bioprocess Eng. 2012, 17, 475–484. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Lyle, D.B.; Shallcross, J.C.; Durfor, C.N.; Hitchins, V.M.; Breger, J.C.; Langone, J.J. Screening biomaterials for stimulation of nitric oxide-mediated inflammation. J. Biomed. Mater. Res. Part A 2009, 90A, 82–93. [Google Scholar] [CrossRef]
- Florento, L.; Matias, R.; Tuaño, E.; Santiago, K.; Dela Cruz, F.; Tuazon, A. Comparison of Cytotoxic Activity of Anticancer Drugs against Various Human Tumor Cell Lines Using In Vitro Cell-Based Approach. Int. J. Biomed. Sci. 2012, 8, 76–80. [Google Scholar]
- Aslantürk, Z.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Addepalli, R.; Netzel, E.M.; Tinggi, U.; Fletcher, T.M.; Sultanbawa, Y.; Osborne, A.S. Antioxidant-Rich Extracts of Terminalia ferdinandiana Interfere with Estimation of Cell Viability. Antioxidants 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Prudnikova, T.Y.; Mostovich, L.A.; Domanitskaya, N.V.; Pavlova, T.V.; Kashuba, V.I.; Zabarovsky, E.R.; Grigorieva, E.V. Antiproliferative effect of D-glucuronyl C5-epimerase in human breast cancer cells. Cancer Cell Int. 2010, 10, 27. [Google Scholar] [CrossRef]
- Zhang, L.; He, M.; Zhang, Y.; Nilubol, N.; Shen, M.; Kebebew, E. Quantitative high-throughput drug screening identifies novel classes of drugs with anticancer activity in thyroid cancer cells: Opportunities for repurposing. J. Clin. Endocrinol. Metab. 2012, 97, E319–E328. [Google Scholar] [CrossRef]
- Pandey, S.; Walpole, C.; Shaw, P.N.; Cabot, P.J.; Hewavitharana, A.K.; Batra, J. Bio-Guided Fractionation of Papaya Leaf Juice for Delineating the Components Responsible for the Selective Anti-proliferative Effects on Prostate Cancer Cells. Front. Pharmacol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Akter, S.; Addepalli, R.; Netzel, M.; Fletcher, M.; Sultanbawa, Y.; Osborne, S. Impact of polyphenol-rich extracts of Terminalia ferdinandiana fruits and seeds on viability of human intestinal and liver cells in vitro. Food Chem. Mol. Sci. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Chaliha, M.; Sultanbawa, Y. Measuring the three forms of ellagic acid: Suitability of extraction solvents. Chem. Pap. 2016, 70, 144–152. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crop. Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Mridusmita, C.; David, W.; David, E.; Sharon, P.; Heather, S.; Yasmina, S. Bioactive rich extracts from Terminalia ferdinandiana by enzyme-assisted extraction: A simple food safe extraction method. J. Med. Plants Res. 2017, 11, 96–106. [Google Scholar] [CrossRef]
- Phillips, K.M.; Tarrago-Trani, M.T.; McGinty, R.C.; Rasor, A.S.; Haytowitz, D.B.; Pehrsson, P.R. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J. Sci. Food Agric. 2018, 98, 4191–4204. [Google Scholar] [CrossRef]
- Akhtar, M.; Raju, R.; Beattie, K.; Bodkin, F.; Münch, G. Medicinal Plants of the Australian Aboriginal Dharawal People Exhibiting Anti-Inflammatory Activity. Evid.-Based Complement. Altern. Med. 2016, 2016, 2935403. [Google Scholar] [CrossRef]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-inflammatory Activity of Herbal Medicines: Inhibition of Nitric Oxide Production and Tumor Necrosis Factor-α Secretion in an Activated Macrophage-like Cell Line. Am. J. Chin. Med. 2005, 33, 415–424. [Google Scholar] [CrossRef]
- Jo, W.S.; Choi, Y.J.; Kim, H.J.; Lee, J.Y.; Nam, B.H.; Lee, J.D.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. The Anti-inflammatory Effects of Water Extract from Cordyceps militaris in Murine Macrophage. Mycobiology 2010, 38, 46–51. [Google Scholar] [CrossRef]
- Altundag, E.M.; Gençalp, D.; Özbilenler, C.; Toprak, K.; Kerküklü, N. In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi. Turk. J. Biochem. 2020, 45, 365–372. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I.R.N.A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, S.L.; Tao, J.Y.; Pang, R.; Jin, F.; Guo, Y.J.; Dong, J.H.; Ye, P.; Zhao, H.Y.; Zheng, G.H. Preliminary exploration on anti-inflammatory mechanism of Corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose) in vitro. Int. Immunopharmacol. 2008, 8, 1059–1064. [Google Scholar] [CrossRef]
- Yang, M.H.; Ali, Z.; Khan, I.A.; Khan, S.I. Anti-inflammatory Activity of Constituents Isolated from Terminalia chebula. Nat. Prod. Commun. 2014, 9, 965–968. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Meena, D.K.; Sahoo, A.K.; Srivastava, P.P.; Sahu, N.P.; Jadhav, M.; Gandhi, M.; Swain, H.S.; Borah, S.; Das, B.K. On valorization of solvent extracts of Terminalia arjuna (arjuna) upon DNA scission and free radical scavenging improves coupling responses and cognitive functions under in vitro conditions. Sci. Rep. 2021, 11, 10656. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Byun, H.S.; Seok, J.H.; Park, K.A.; Won, M.; Seo, W.; Lee, S.R.; Kang, K.; Sohn, K.C.; Lee, I.Y.; et al. Terminalia chebula provides protection against dual modes of necroptotic and apoptotic cell death upon death receptor ligation. Sci. Rep. 2016, 6, 25094. [Google Scholar] [CrossRef]
- Sheng, Z.; Yan, X.; Zhang, R.; Ni, H.; Cui, Y.; Ge, J.; Shan, A. Assessment of the antidiarrhoeal properties of the aqueous extract and its soluble fractions of Chebulae Fructus (Terminalia chebula fruits). Pharm. Biol. 2016, 54, 1847–1856. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Suresh Kumar, B.; Esakkidurai, T.; Pitchumani, K. Green Synthesis of Silver Nanoparticles Using a Characterized Polyphenol Rich Fraction from Terminalia bellirica and the Evaluation of its Cytotoxicity in Normal and Cancer Cells. J. Biol. Act. Prod. Nat. 2018, 8, 352–363. [Google Scholar] [CrossRef]
- Qu, T.; Duan, X.; Pang, X.; Huang, J.; Gao, L.; Wang, J.; Zhu, Y.; Liang, X.; Chen, L.; Zhang, K.; et al. Commonalities and characteristics of aqueous extracts from three Uighur medicines were analyzed by using three-stage infrared spectroscopy combined with ultra-performance liquid chromatography-time of flight-mass spectra. J. Tradit. Chin. Med. 2019, 39, 118–126. [Google Scholar]
- Rubab, I.; Ali, S. Dried fruit extract of Terminalia chebula modulates the immune response in mice. Food Agric. Immunol. 2016, 27, 1–22. [Google Scholar] [CrossRef]
- Diop, E.A.; Jacquat, J.; Drouin, N.; Queiroz, E.F.; Wolfender, J.-L.; Diop, T.; Schappler, J.; Rudaz, S. Quantitative CE analysis of punicalagin in Combretum aculeatum extracts traditionally used in Senegal for the treatment of tuberculosis. Electrophoresis 2019, 40, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Markom, M.; Hasan, M.; Daud, W.R.W. Pressurized Water Extraction of Hydrolysable Tannins from Phyllanthus niruri Linn. Sep. Sci. Technol. 2010, 45, 548–553. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of l-ascorbic acid, sugar, pectin and freeze–thaw treatment on polyphenol content of frozen strawberries. LWT-Food Sci. Technol. 2009, 42, 581–586. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhao, M.; Yang, B.; Ren, J.; Shen, G.; Rao, G. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011, 126, 277–282. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Nagao, T.; Tanaka, T.; Yang, C.R.; Okabe, H.; Kouno, I. Antiproliferative Activity of the Main Constituents from Phyllanthus emblica. Biol. Pharm. Bull. 2004, 27, 251–255. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and Antiproliferative Activities of Anthocyanin/Ellagitannin-Enriched Extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef]
- Pellati, F.; Bruni, R.; Righi, D.; Grandini, A.; Tognolini, M.; Pio Prencipe, F.; Poli, F.; Benvenuti, S.; Del Rio, D.; Rossi, D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 2013, 147, 277–285. [Google Scholar] [CrossRef] [PubMed]

| Samples | FEA (mg/100 g DW) | ETs & (mg EAE/100 g DW) | TEA (mg/100 g DW) | TPC (mg GAE/g DW) | Vitamin C (mg/g DW) | |||
|---|---|---|---|---|---|---|---|---|
| L-AA | DHAA | TVC | ||||||
| NT | NT AAE | 1228 ± 23.1 d | 1961.6 ± 24.4 d | 3189.6 ± 25.7 d | 196 ± 3.6 d | 171 ± 0.7 b | 9.6 ± 1.2 a | 180.5 ± 1.0 b |
| NT water | 148 ± 3.8 b | 373.1 ± 4.0 b | 521.1 ± 3.5 b | 88.8 ± 5.0 b | ||||
| WA | WA AAE | 1045 ± 9.4 c | 1449.7 ± 10.1 c | 2494.7 ± 11.1 c | 174 ± 5.0 c | 116.3 ± 1.3 a | 8.7 ± 1.0 a | 125 ± 3.0 a |
| WA water | 43 ± 1.3 a | 276 ± 5.2 a | 318.8 ± 9.1 a | 64.3 ± 5.0 a | ||||
| Extracts | Inhibition of NO Production (IC50 μg/mL) |
|---|---|
| WA Water | 166.3 ± 1.3 c |
| WA AAE | 157.0 ± 1.5 c |
| NT Water | 52.4 ± 2.1 b |
| NT AAE | 33.3 ± 1.3 a |
| Quercetin | 4269.3 ± 3.1 d |
| Extracts | Cytotoxicity (CC50 μg/mL) |
|---|---|
| WA Water | 5440 ± 1.0 c |
| WA AAE | 2456 ± 1.1 b |
| NT Water | 7337 ± 1.5 d |
| NT AAE | 1676 ± 1.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobasa, E.M.; Akter, S.; Phan, A.D.T.; Netzel, M.E.; Cozzolino, D.; Osborne, S.; Sultanbawa, Y. Impact of Growing Location on Kakadu Plum Fruit Composition and In Vitro Bioactivity as Determinants of Its Nutraceutical Potential. Nutraceuticals 2023, 3, 13-25. https://doi.org/10.3390/nutraceuticals3010002
Bobasa EM, Akter S, Phan ADT, Netzel ME, Cozzolino D, Osborne S, Sultanbawa Y. Impact of Growing Location on Kakadu Plum Fruit Composition and In Vitro Bioactivity as Determinants of Its Nutraceutical Potential. Nutraceuticals. 2023; 3(1):13-25. https://doi.org/10.3390/nutraceuticals3010002
Chicago/Turabian StyleBobasa, Eshetu M., Saleha Akter, Anh Dao Thi Phan, Michael E. Netzel, Daniel Cozzolino, Simone Osborne, and Yasmina Sultanbawa. 2023. "Impact of Growing Location on Kakadu Plum Fruit Composition and In Vitro Bioactivity as Determinants of Its Nutraceutical Potential" Nutraceuticals 3, no. 1: 13-25. https://doi.org/10.3390/nutraceuticals3010002
APA StyleBobasa, E. M., Akter, S., Phan, A. D. T., Netzel, M. E., Cozzolino, D., Osborne, S., & Sultanbawa, Y. (2023). Impact of Growing Location on Kakadu Plum Fruit Composition and In Vitro Bioactivity as Determinants of Its Nutraceutical Potential. Nutraceuticals, 3(1), 13-25. https://doi.org/10.3390/nutraceuticals3010002