Supplemental Psyllium Fiber Increases Antimicrobial Proteins via the Tuft Cell-ILC2 Circuit and Type II Immune Response in the Mouse Small Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mice
2.3. Experimental Design
2.4. Whole Transcriptome Analysis of Mouse Small Intestine via RNA Sequencing (Expt. 1)
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis (Expt. 1–5)
2.6. Immunoblot Analysis (Expt. 1–5)
2.7. Immunofluorescence Analysis (Expt. 1, 2, 4, and 5)
2.8. Metagenomic Analysis of 16S rRNA Genes in Intestinal Microbiota (Expt. 1)
2.9. Statistical Analyses
3. Results
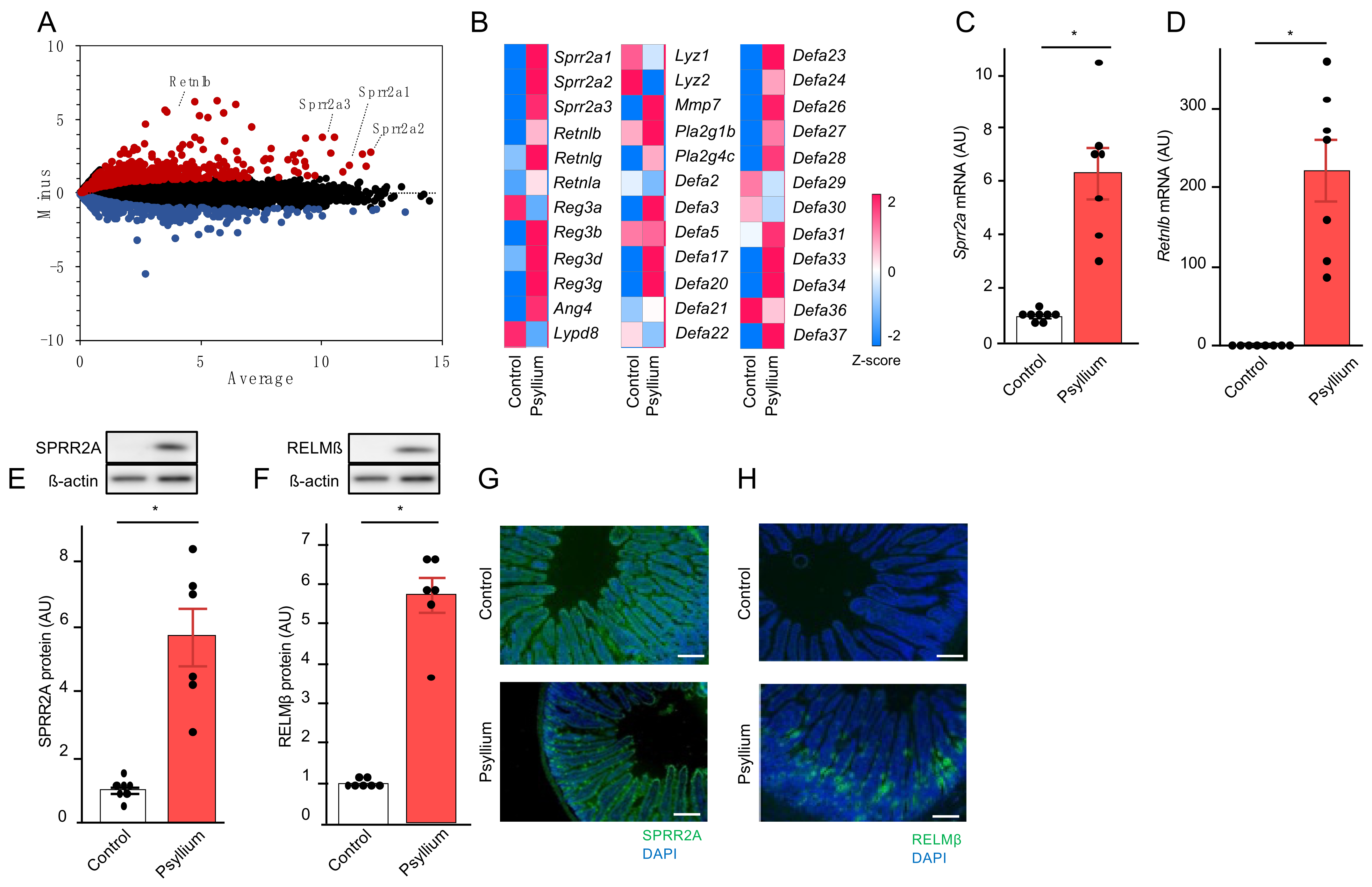
3.1. Psyllium Supplementation Upregulates the Production of Antimicrobial Proteins in the Mouse Small Intestine (Expt. 1)
3.2. Psyllium Supplementation Alters Cecal Microbiota Composition (Expt. 1)
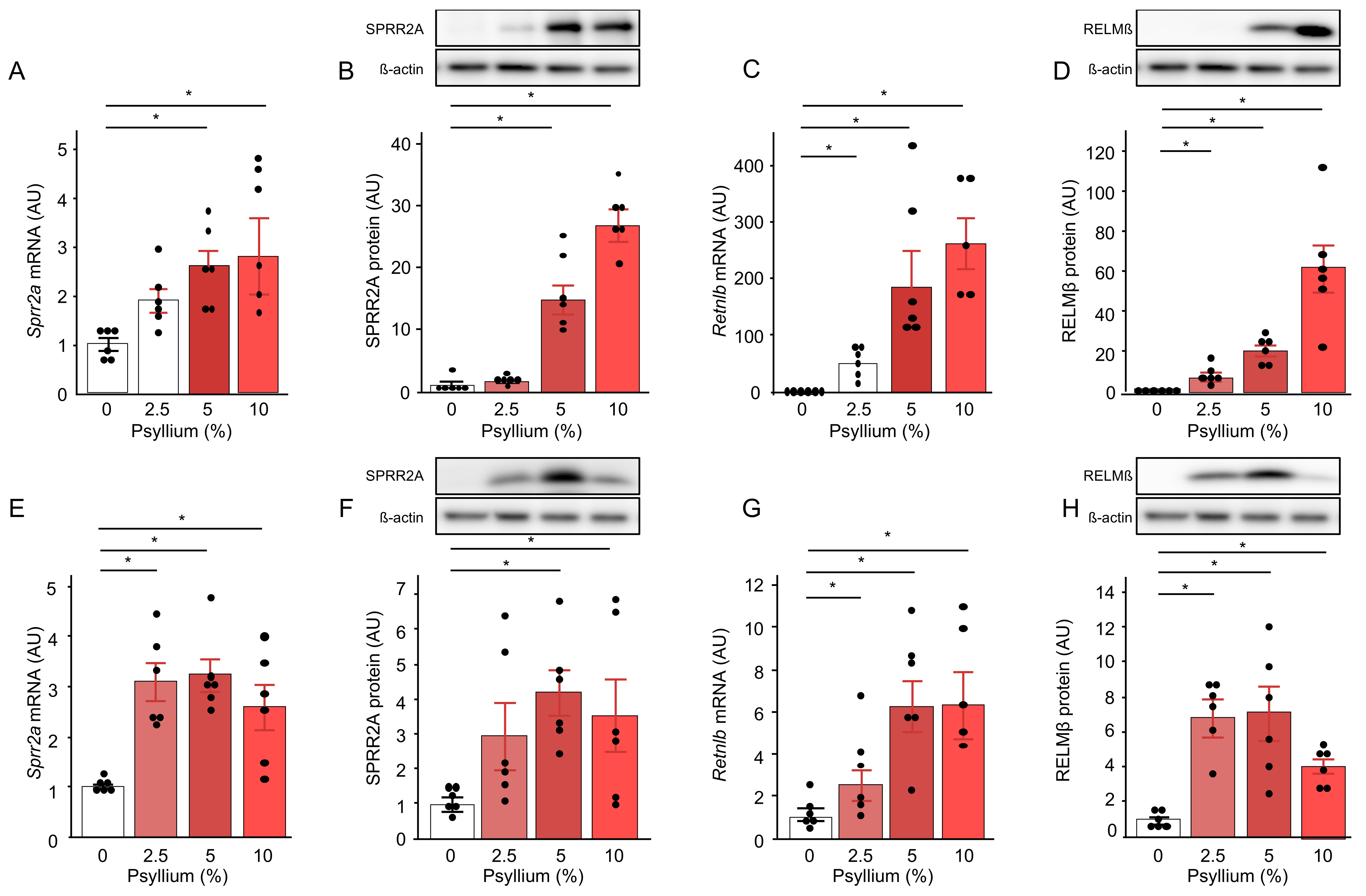
3.3. Psyllium Supplementation Upregulates Antimicrobial Proteins, SPRR2A, and RELMβ in a Dose-Dependent Manner (Expt. 2)
3.4. Psyllium Fiber Sustains Upregulation of Antimicrobial Proteins for 15 d (Expt. 3)
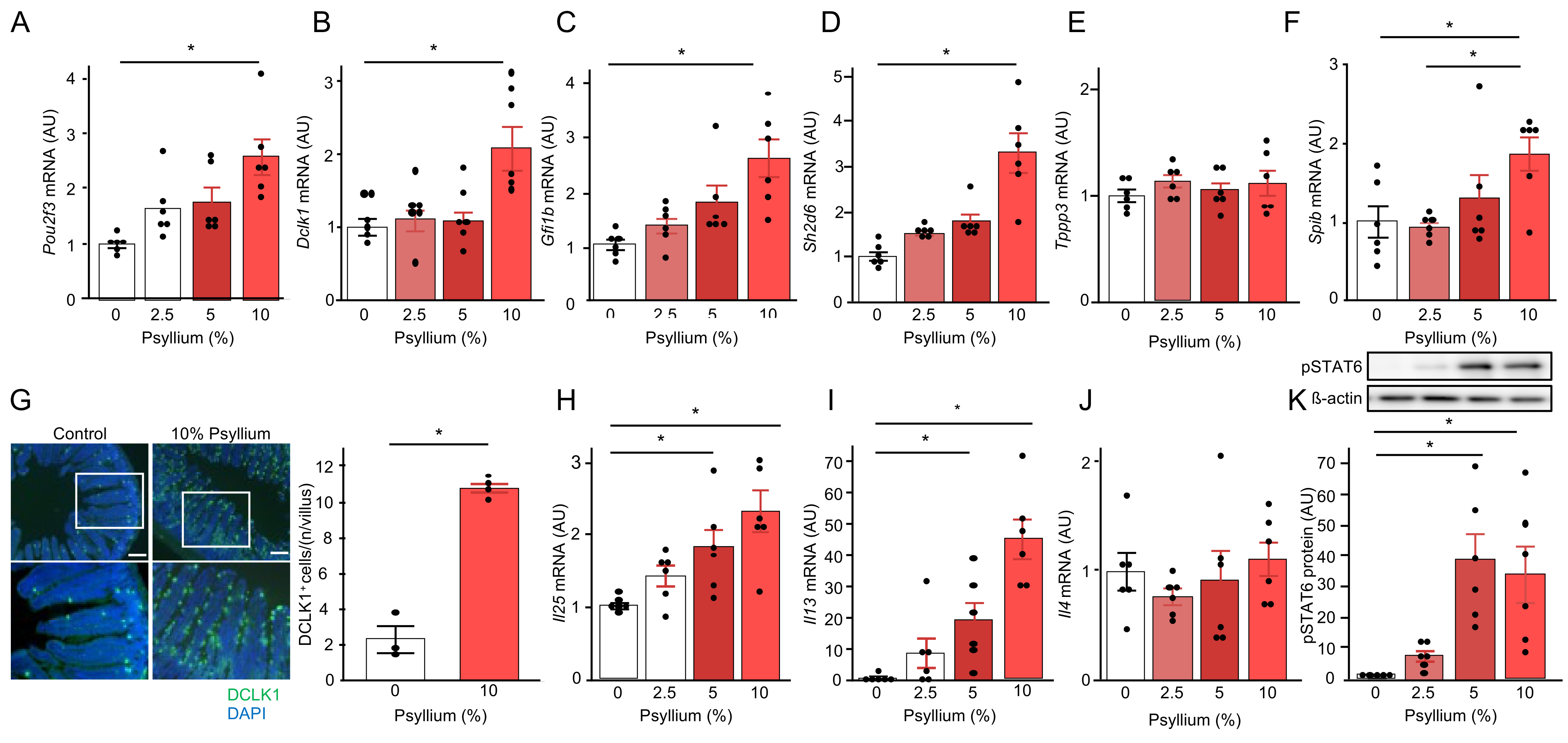
3.5. Psyllium Supplementation Increases Tuft Cell Proliferation via IL-13 Signaling (Expt. 2)
3.6. ILC2 Is Involved in the Psyllium-Induced Antimicrobial Protein Production (Expt. 4)
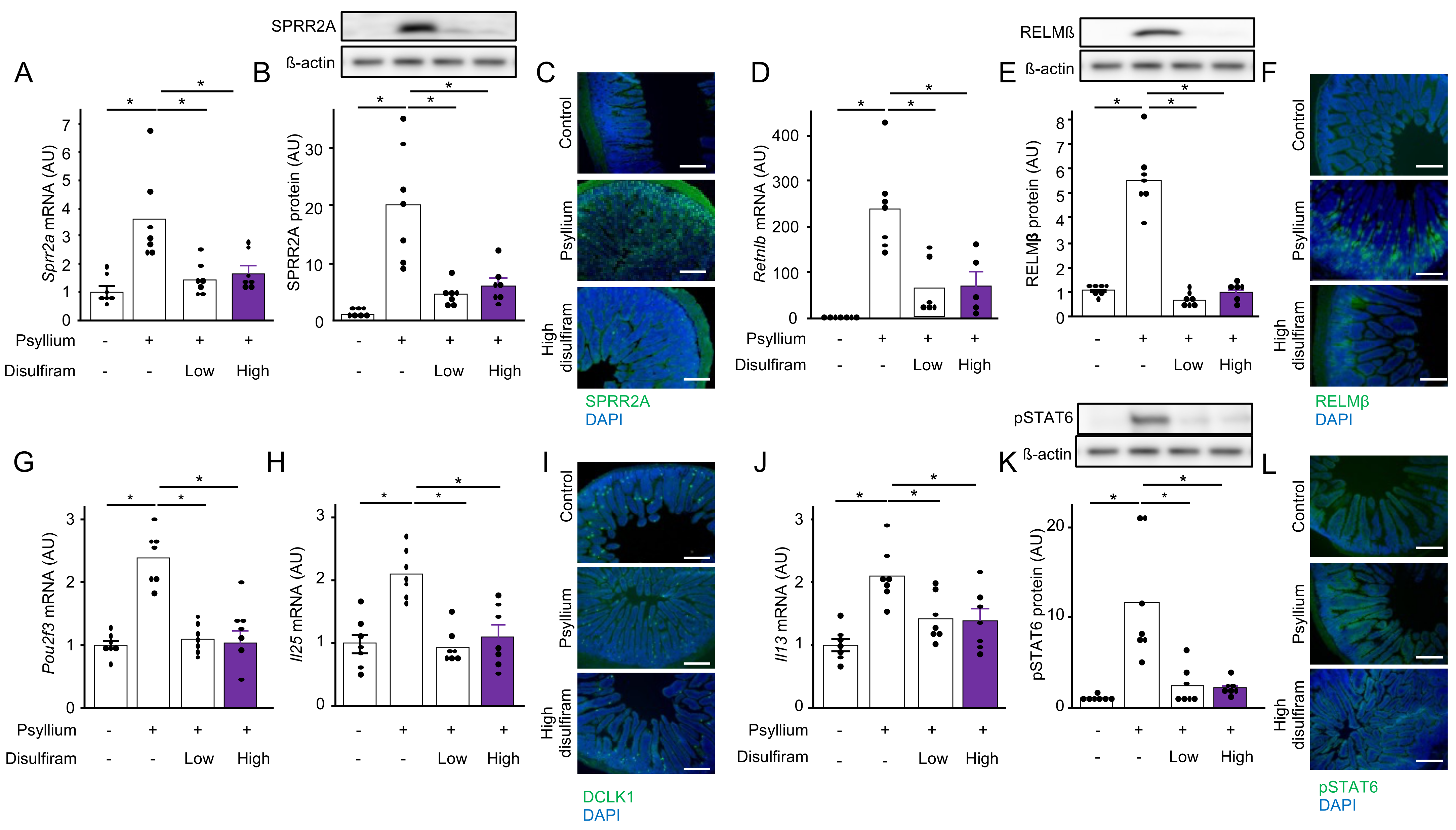
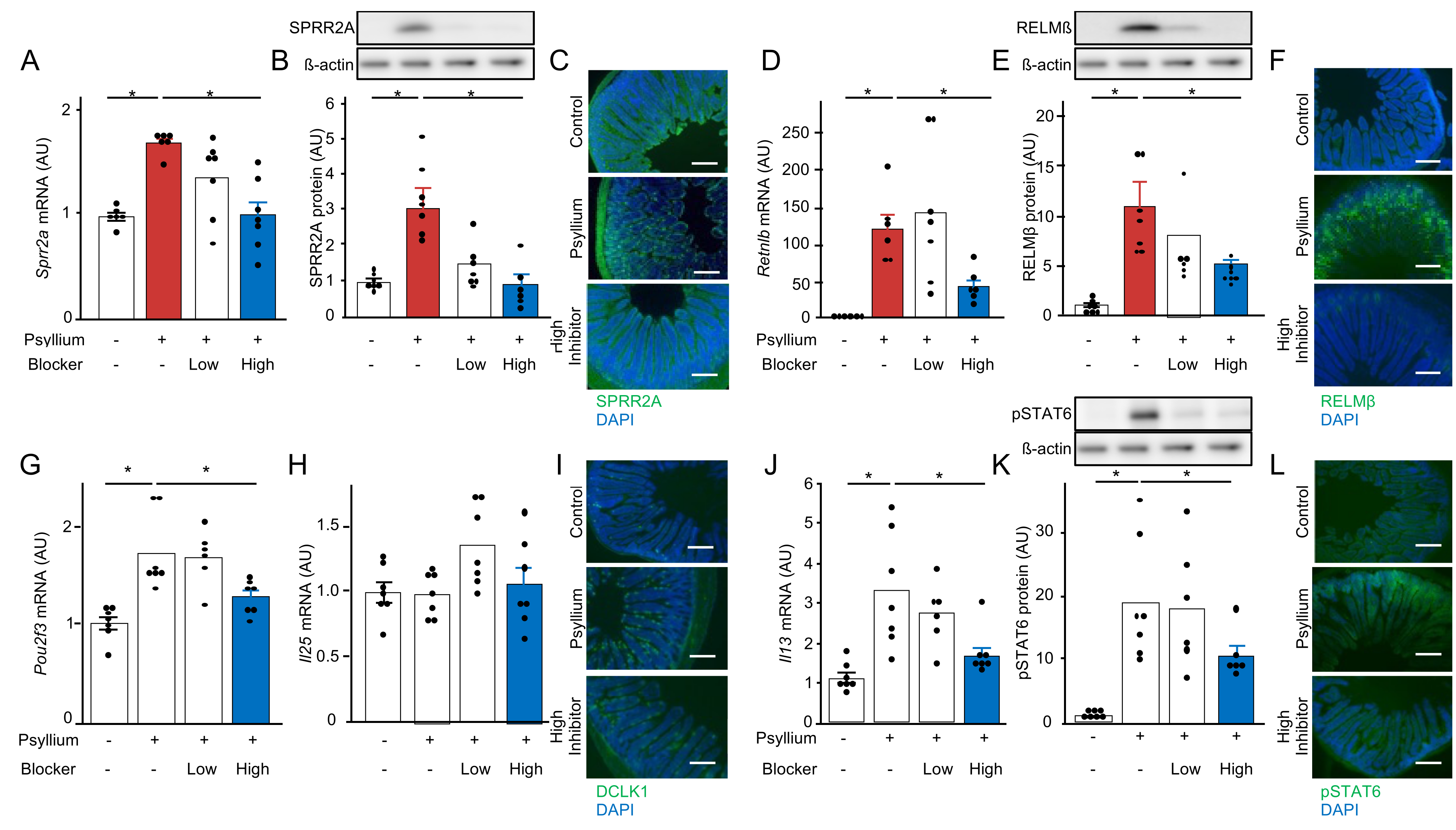
3.7. Bitter Taste Receptors of Tuft Cells Are Involved in Psyllium-Induced Antimicrobial Protein Production (Expt. 5)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blyth, G.A.D.; Connors, L.; Fodor, C.; Cobo, E.R. The Network of Colonic Host Defense Peptides as an Innate Immune Defense Against Enteropathogenic Bacteria. Front. Immunol. 2020, 11, 538574. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein Kinase Cσ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef]
- Van Hung, T.; Suzuki, T. Guar Gum Fiber Increases Suppressor of Cytokine Signaling-1 Expression via Toll-like Receptor 2 and Dectin-1 Pathways, Regulating Inflammatory Response in Small Intestinal Epithelial Cells. Mol. Nutr. Food Res. 2017, 61, 1700048. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The Effect of Fiber Supplementation on Chronic Constipation in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2022, 116, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Van Hung, T.; Tari, H.; Arakawa, T.; Suzuki, T. Dietary Psyllium Fiber Increases Intestinal Heat Shock Protein 25 Expression in Mice. Nutr. Res. 2017, 39, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bretin, A.; Yeoh, B.S.; Ngo, V.L.; Reddivari, L.; Pellizzon, M.; Vijay-Kumar, M.; Gewirtz, A.T. Psyllium Fiber Protects Mice against Western Diet-Induced Metabolic Syndrome via the Gut Microbiota-Dependent Mechanism. Gut Microbes 2023, 15, 2221095. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small Intestinal Goblet Cell Proliferation Induced by Ingestion of Soluble and Insoluble Dietary Fiber Is Characterized by an Increase in Sialylated Mucins in Rats. J. Nutr. 2012, 142, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Bretin, A.; Zou, J.; San Yeoh, B.; Ngo, V.L.; Winer, S.; Winer, D.A.; Reddivari, L.; Pellizzon, M.; Walters, W.A.; Patterson, A.D.; et al. Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- France, M.M.; Turner, J.R. The Mucosal Barrier at a Glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Pernitzsch, S.R.; Bäumler, A.J. Healthy Hosts Rule within: Ecological Forces Shaping the Gut Microbiota. Mucosal Immunol. 2018, 11, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Fraser, L.M.; Barrón, G.M.; Gologorsky, M.B.; Atkinson, S.N.; Gerrick, E.R.; Hayward, M.; Ziegelbauer, J.; Li, J.A.; Nico, K.F.; et al. Tuft Cells Mediate Commensal Remodeling of the Small Intestinal Antimicrobial Landscape. Proc. Natl. Acad. Sci. USA 2023, 120, e2216908120. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, C.; Sifuentes-Dominguez, L.; Zarek, C.M.; Propheter, D.C.; Kuang, Z.; Wang, Y.; Pendse, M.; Ruhn, K.A.; Hassell, B.; et al. Small Proline-Rich Protein 2A Is a Gut Bactericidal Protein Deployed during Helminth Infection. Science 2021, 374, abe6723. [Google Scholar] [CrossRef] [PubMed]
- Propheter, D.C.; Chara, A.L.; Harris, T.A.; Ruhn, K.A.; Hooper, L.V. Resistin-like Molecule β Is a Bactericidal Protein That Promotes Spatial Segregation of the Microbiota and the Colonic Epithelium. Proc. Natl. Acad. Sci. USA 2017, 114, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.B.; Morampudi, V.; Chan, J.M.; Bhinder, G.; Lau, J.; Yang, H.; Ma, C.; Huang, T.; Ryz, N.; Sham, H.P.; et al. Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS Pathog. 2015, 11, 1005108. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of Immune Responses by Tuft Cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft Cells, Taste-Chemosensory Cells, Orchestrate Parasite Type 2 Immunity in the Gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-Cell-Derived IL-25 Regulates an Intestinal ILC2-Epithelial Response Circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [PubMed]
- McGinty, J.W.; Ting, H.A.; Billipp, T.E.; Nadjsombati, M.S.; Khan, D.M.; Barrett, N.A.; Liang, H.E.; Matsumoto, I.; von Moltke, J. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-Helminth Immunity in the Small Intestine but Are Dispensable for Anti-Protist Immunity. Immunity 2020, 52, 528–541.e7. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.C.; Chen, Z.H.; Xue, J.B.; Zhao, D.X.; Lu, C.; Li, Y.H.; Li, S.M.; Du, Y.W.; Liu, Q.; Wang, P.; et al. Infection by the Parasitic Helminth Trichinella Spiralis Activates a Tas2r-Mediated Signaling Pathway in Intestinal Tuft Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 5564–5569. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Yamazaki, T.; Tanamoto, K. Analysis of Thickening Polysaccharides by the Improved Diethyldithioacetal Derivatization Method. Food Hyg. Saf. Sci. 2010, 52, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Mucke, H.A.M. Drug Repurposing Patent Applications January–March 2020. Assay Drug Dev. Technol. 2020, 18, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Allyl Isothiocyanate Ameliorates Angiogenesis and Inflammation in Dextran Sulfate Sodium-Induced Acute Colitis. PLoS ONE 2014, 9, e102975. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, F.; Dey, B.; Yoshida, Y.; Nishimura, S.; Tabata, S. Bitter Taste Receptor Antagonists Inhibit the Bitter Taste of Canola Meal Extract in Chickens. J. Poult. Sci. 2020, 57, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Prandi, S.; Bromke, M.; Hübner, S.; Voigt, A.; Boehm, U.; Meyerhof, W.; Behrens, M. A Subset of Mouse Colonic Goblet Cells Expresses the Bitter Taste Receptor Tas2r131. PLoS ONE 2013, 8, e82820. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; Gouka, R.J.; Gruppen, H.; Driesse, M.; Van Buren, L.; Smit, G.; Vincken, J.P. 6-Methoxyflavanones as Bitter Taste Receptor Blockers for HTAS2R39. PLoS ONE 2014, 9, e94451. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus Murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Li, L.; Ma, J. Bacterial Community Structure and Potential Microbial Coexistence Mechanism Associated with Three Halophytes Adapting to the Extremely Hypersaline Environment. Microorganisms 2022, 10, 1124. [Google Scholar] [CrossRef]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelde, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, S.; Albers, T.; Duerr, C.U.; Ménard, S.; Pütsep, K.; Andersson, M.; Hornef, M.W. Interleukin-13-Mediated Paneth Cell Degranulation and Antimicrobial Peptide Release. J. Innate Immun. 2014, 6, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhu, X.; Geng, J.; Xu, Y.; Wu, R.; Li, C.; Fan, D.; Qin, X.; Du, Y.; Tian, Y.; et al. Intestinal Tuft-2 Cells Exert Antimicrobial Immunity via Sensing Bacterial Metabolite N-Undecanoylglycine. Immunity 2022, 55, 686–700.e7. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Legraverend, C.; Jay, P. The Intestinal Epithelium Tuft Cells: Specification and Function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Moerings, B.G.J.; van Bergenhenegouwen, J.; Furber, M.; Abbring, S.; Schols, H.A.; Witkamp, R.F.; Govers, C.; Mes, J.J. Dectin-1b Activation by Arabinoxylans Induces Trained Immunity in Human Monocyte-Derived Macrophages. Int. J. Biol. Macromol. 2022, 209, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Atagozli, T.; Elliott, D.E.; Ince, M.N. Helminth Lessons in Inflammatory Bowel Diseases (IBD). Biomedicines 2023, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174, 271–284.e14. [Google Scholar] [CrossRef] [PubMed]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, Y.; Matsunaga, T.; Yasui, T.; Rini, D.M.; Inoue, R.; Yamamoto, Y.; Suzuki, T. Supplemental Psyllium Fiber Increases Antimicrobial Proteins via the Tuft Cell-ILC2 Circuit and Type II Immune Response in the Mouse Small Intestine. Nutraceuticals 2024, 4, 307-322. https://doi.org/10.3390/nutraceuticals4020019
Ishii Y, Matsunaga T, Yasui T, Rini DM, Inoue R, Yamamoto Y, Suzuki T. Supplemental Psyllium Fiber Increases Antimicrobial Proteins via the Tuft Cell-ILC2 Circuit and Type II Immune Response in the Mouse Small Intestine. Nutraceuticals. 2024; 4(2):307-322. https://doi.org/10.3390/nutraceuticals4020019
Chicago/Turabian StyleIshii, Yoshiki, Taiyo Matsunaga, Tomoki Yasui, Dina Mustika Rini, Ryo Inoue, Yoshinari Yamamoto, and Takuya Suzuki. 2024. "Supplemental Psyllium Fiber Increases Antimicrobial Proteins via the Tuft Cell-ILC2 Circuit and Type II Immune Response in the Mouse Small Intestine" Nutraceuticals 4, no. 2: 307-322. https://doi.org/10.3390/nutraceuticals4020019
APA StyleIshii, Y., Matsunaga, T., Yasui, T., Rini, D. M., Inoue, R., Yamamoto, Y., & Suzuki, T. (2024). Supplemental Psyllium Fiber Increases Antimicrobial Proteins via the Tuft Cell-ILC2 Circuit and Type II Immune Response in the Mouse Small Intestine. Nutraceuticals, 4(2), 307-322. https://doi.org/10.3390/nutraceuticals4020019





