Abstract
Seaweeds have been utilized for millennia in Asian countries, although they have only more recently become popular in Western society. They began to be used in ancient times because of their long-term properties and, nowadays, seaweeds are being targeted as a potential tool to combat climate change. There are not many laws governing seaweeds because they have just lately been utilized as food. However, guidelines are being developed to regulate their manufacture and use. Because of seaweed’s tendency to accumulate components, whether helpful or poisonous, limited doses of certain substances have been established to prevent consumer overdosage. Aside from chemical safety, microbiological safety is important for people, and preventing any pathogen from spreading and infecting seaweeds is critical. As a result, systems and ways to safeguard consumers must be developed. Because various seaweed species have varied compositions, certain seaweeds may be safer nutraceuticals than others. To ensure the safety of seaweed-based food items, the HACCP (Hazard Analysis Critical Control Point) system needs to be used. The majority of seaweeds consumed come from aquaculture; however, others come from wild harvesting. To ensure the success of the cultures, the waters must be tested for chemicals and biological risks, as well as for the pH, salinity, and temperature. Seaweeds have enormous promise in many industries, but in the food industry, they are beginning to play a major role, and seizing the chances to produce innovative, safe, and sustainable food sources is strongly advised. This critical review investigates the real potential of seaweed as a human food source and as a nutraceutical solution. This review also focuses on the usage of seaweed as a food product and the procedures required to prepare it. In addition, it compiles information on the applicable legislation and regulations, and it addresses the lengthy road that has to be traveled to increase human well-being by employing a new food source in a controlled manner while simultaneously reducing the human population’s health problems.
1. Introduction
The world’s population is expected to reach ten billion in the next three decades. Food production must be increased by 70% [1]. In addition, meat output will double in the same time period. As a result, the hunt for alternative food sources is critical [2]. Agriculture and intensive farming have led to arable land saturation and restricted availability of fresh water [3,4]. Thus, aquatic organisms can be key for food safety and security due to being cultivated in aquatic systems, with usage of terrestrial land, where agriculture, farms, and food industries near coastal areas can be used to transform and work the seaweeds into the food industry [5].
Seaweeds are one of the most promising sustainable food types. Their capacity to absorb CO2 from the water and atmosphere is an excellent way to combat climate change [6]. Furthermore, they have the ability for rapid development in the water, which simplifies manufacturing methods and decreases production costs [4]. Despite the fact that seaweeds have been used for millennia in nations such as China, Japan, and Korea, they were only recently introduced as a food source in Western civilizations [5]. After WWII, it was discovered that there was inadequate protein consumption due to exponential population expansion. To combat this nutrient deficiency during the war, seaweeds played an important role, as they are high in various macronutrients and micronutrients, including vitamins, minerals and proteins [7,8]. Also, nowadays, seaweed could play this important role again to mitigate malnutrition and food insufficiency. The nutritional potential of seaweeds is directly tied to their biochemical profiles and bioactive qualities, which are known to vary greatly between species [9]. Isolated polysaccharides (e.g., alginate and fucoidan), proteins (phycobiliproteins), polyphenols (e.g., phlorotannins), carotenoids (e.g., fucoxanthin), and n-3 long-chain polyunsaturated fatty acids (e.g., eicosapentaenoic acid) are among the highlights [10]. The biochemical composition and functional properties of algae feedstocks have been extensively studied over the last few decades, aided greatly by the advancement and development of new techniques that enable high-resolution profiling of proteins, lipids, polysaccharides, pigments, and others [11,12,13]. Overall, seaweeds are a sustainable supply of natural high-value bioactive chemicals with the potential to produce novel human nutrition products.
They have also been employed as a new food due to their polymers, such as carrageenan, agar, and alginate, as well as their gelling, emulsifying, and thickening capabilities [14,15].
The FAO and WHO published a report that evaluated the current food safety information regarding seaweed produced from both wild stocks and aquaculture and proposed more discussion as well as international advice [16]. The report noted that morbidities and deaths associated with eating seaweed are uncommon, but it warned that the limited evidence raises worries that some risks may be present in seaweed [17]. These risks include chemical hazards such as heavy metals (principally inorganic arsenic and cadmium), persistent organic pollutants (e.g., dioxins and polychlorinated biphenyls), radionuclides and pesticide residues; microbiological hazards (e.g., Salmonella spp., Bacillus spp., and norovirus); physical hazards (e.g., metal pieces, glass splinters, crustacean shells, micro- and nanoplastics); and allergens [17]. Thus, there is a need for technology and methods to ensure seaweed’s potential for human welfare and reduce the inherent risks to human health.
This critical review attempts to explore seaweed as food for humans. It focuses on the application of seaweed as a food product and the methods needed to make it safe to eat. In addition, it gathers information on the relevant legislation and regulations and discusses the long road that needs to be traveled to improve human well-being by using a new food source, in a controlled way, and also to decrease the health problems of the human population.
2. Seaweeds as a Possible Nutraceutical Food Source
Seaweeds are a basic ingredient in South Asiatic cuisine; however, there has been a considerable increase in seaweed consumption in European countries over the last decade, from traditional sushi to afternoon snacks and novel foods (processed or not) [18]. The current move in Western diets has shifted to more plant-based and sustainable food sources, and it is expected to amplify in the next years. Thus, it is expected that by 2050, 0.1% of our seas would be dedicated to growing seaweed as a food source, producing 15 times more seaweed than is currently produced to meet the rising world demand [19]. Despite the recent spectacular rise of seaweed as a food product, outside of Asia, there are presently no food standards governing the safety and quality of seaweed, posing a potential harm to consumer health [20].
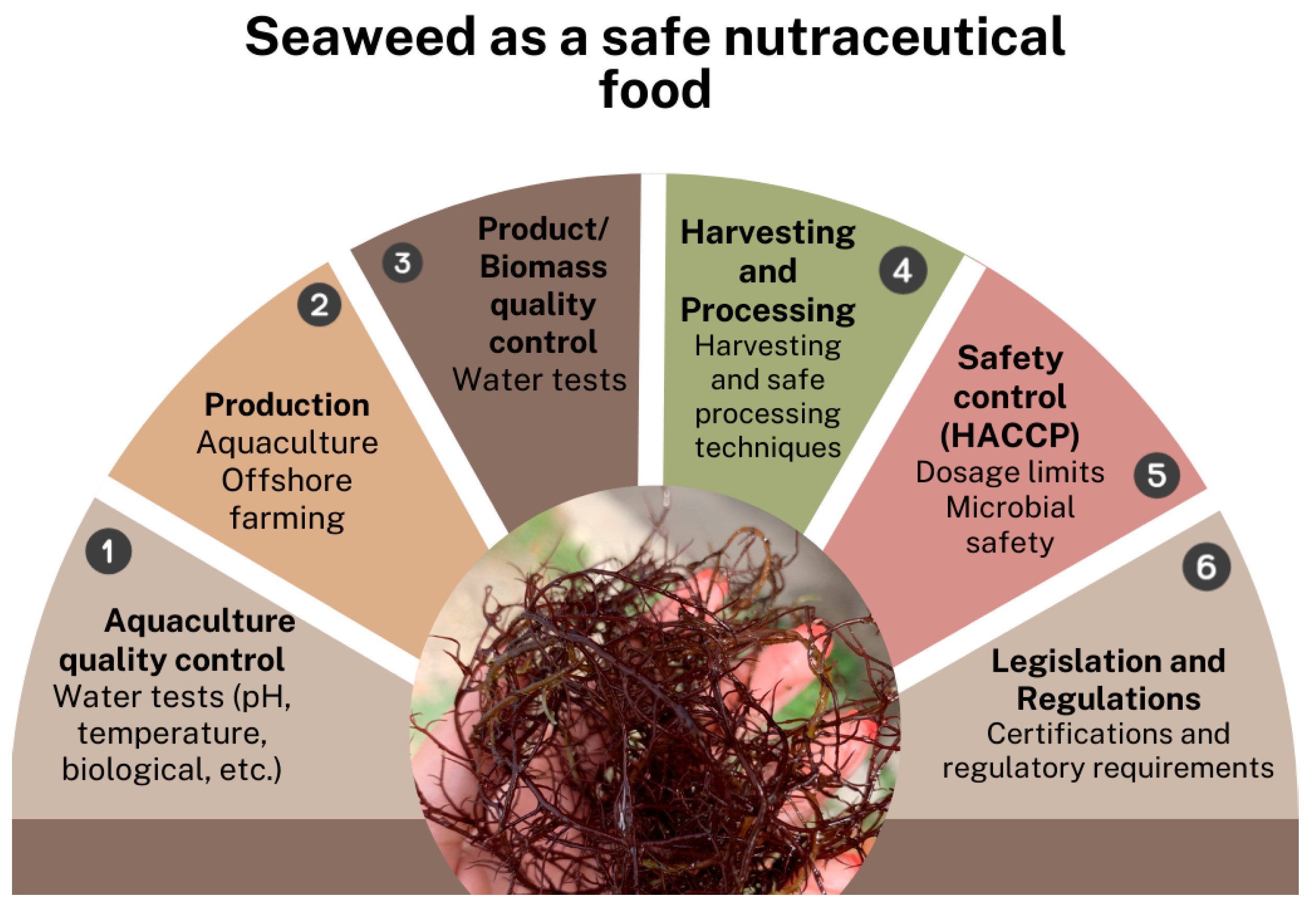
Seaweed is often regarded as a ‘superfood’ in the Western world, connected with healthy lifestyles and sustainability. Its significant nutritional value is derived from macro- and micronutrients such as vitamin B12, dietary fibers, and omega-3 fatty acids, among others, and it is a rich source of various bioactive substances that have health advantages (e.g., polyphenols, sulphated polysaccharides, pigments) [20]. However, for seaweed to be a potential safe nutraceutical food source, there is a need to overcome several steps from biomass production until the market (Figure 1), although there is need for a more robust system.
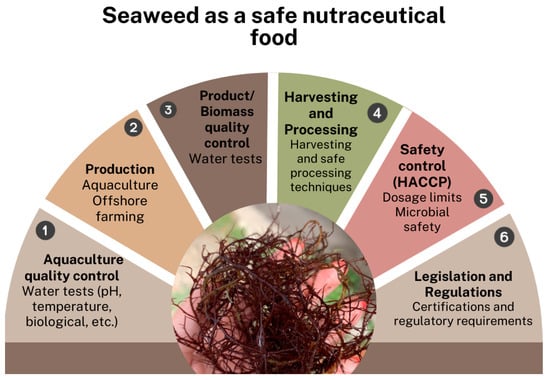
Figure 1.
The real and normal steps of seaweed biomass as a human food source.
In general, compared to green and brown algae, red algae contains a high amount of proteins, reaching 47% (Pyropia tenera) of the dry matter [21]. On the other hand, the lipids in these seaweeds present relatively lower contents. Also, there are not significant differences between red and brown algae, as they both revealed low fat and high fiber content. Red algae contains soluble fibers such as sulphated galactans (agars and carrageenans), xylans and floridean starch [22,23,24]. Brown seaweeds demonstrate lower protein content when compared with the other seaweeds but have more mineral and phenolic compound content [23].
2.1. A Good Source of Nutrients?
Seaweeds are presented as a sustainable source of protein and dietary fibers from the sea, decreasing the pressure on wild-capture fisheries and being an eco-friendly alternative to meat protein [25]. Seaweed mariculture seems like a perfectly fitting solution to the secure food and feed demand, all the while avoid placing additional pressure on arable/available land and freshwater resources (leaving aside possible land-based cultivation of certain seaweed species) [26]. Studies generally agree that seaweeds contain high-quality proteins with essential amino acids (lysine, methionine, to name a couple, depending on the strain) and are a rich source of other bioactives, including taurine, lipids, carotenoids, and pigments [27,28,29].
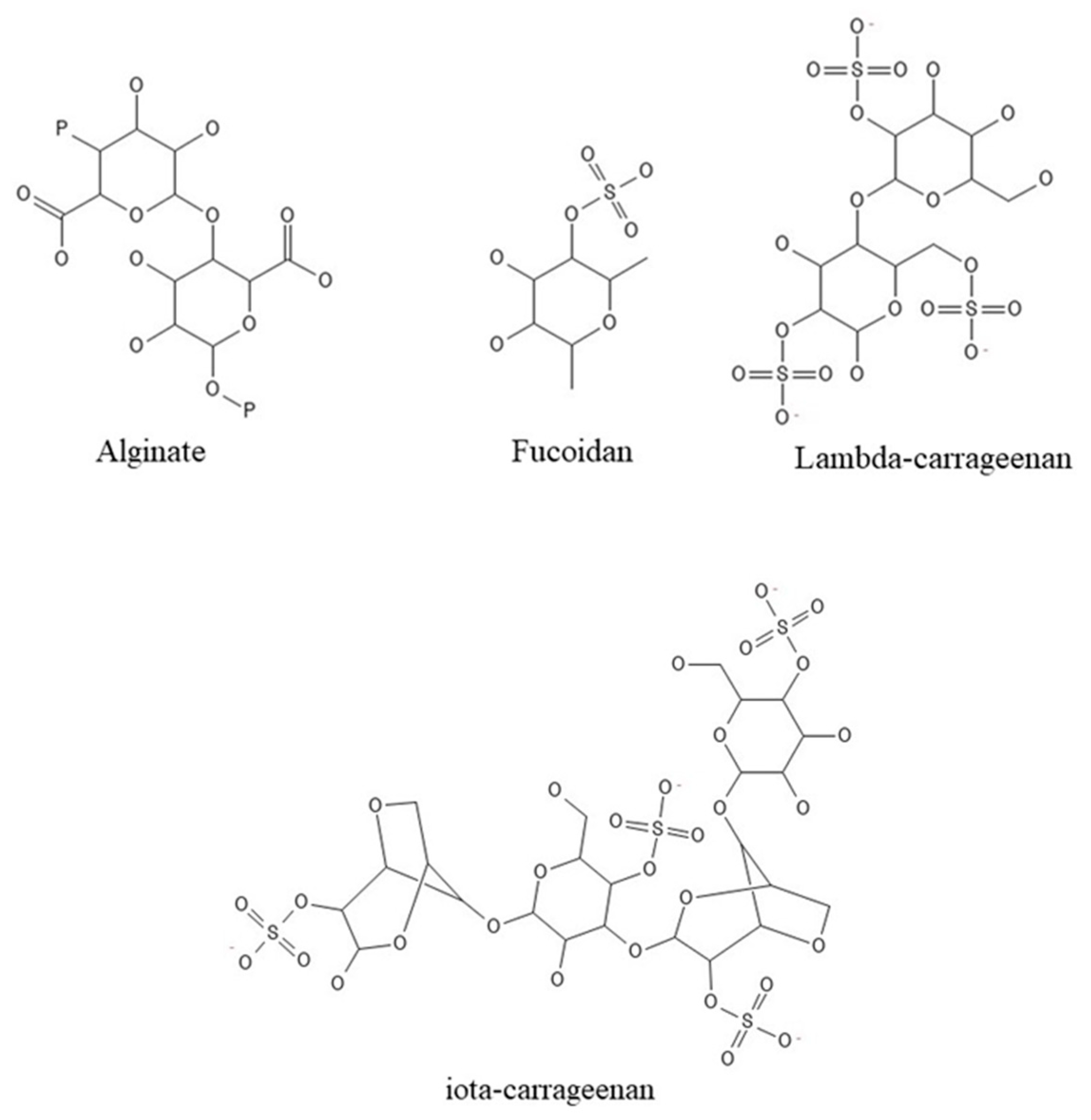
In reality, seaweeds are a low-calorie meal, making them appealing for inclusion in the human daily diet (Table 1). In addition, the incorporation of vitamins, fibers, and proteins provides a high nutritional value as well as a variety of health advantages [18]. Polysaccharides from seaweeds (Figure 2), for example, have a positive effect on the digestive system but are calorie-free, unlike fibers. Because of their positive properties, these biological molecules might be employed to create innovative and functional meals, as well as implemented in pharmacological and medical applications [30,31,32,33].

Table 1.
Nutrient composition of some edible seaweed (% dry weight) (adapted from Pereira [32,33]; Guiry and Guiry [24]).
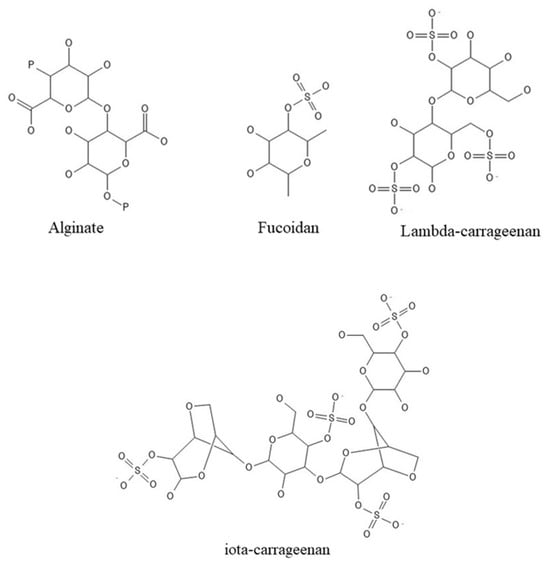
Figure 2.
Polysaccharides’ structures.
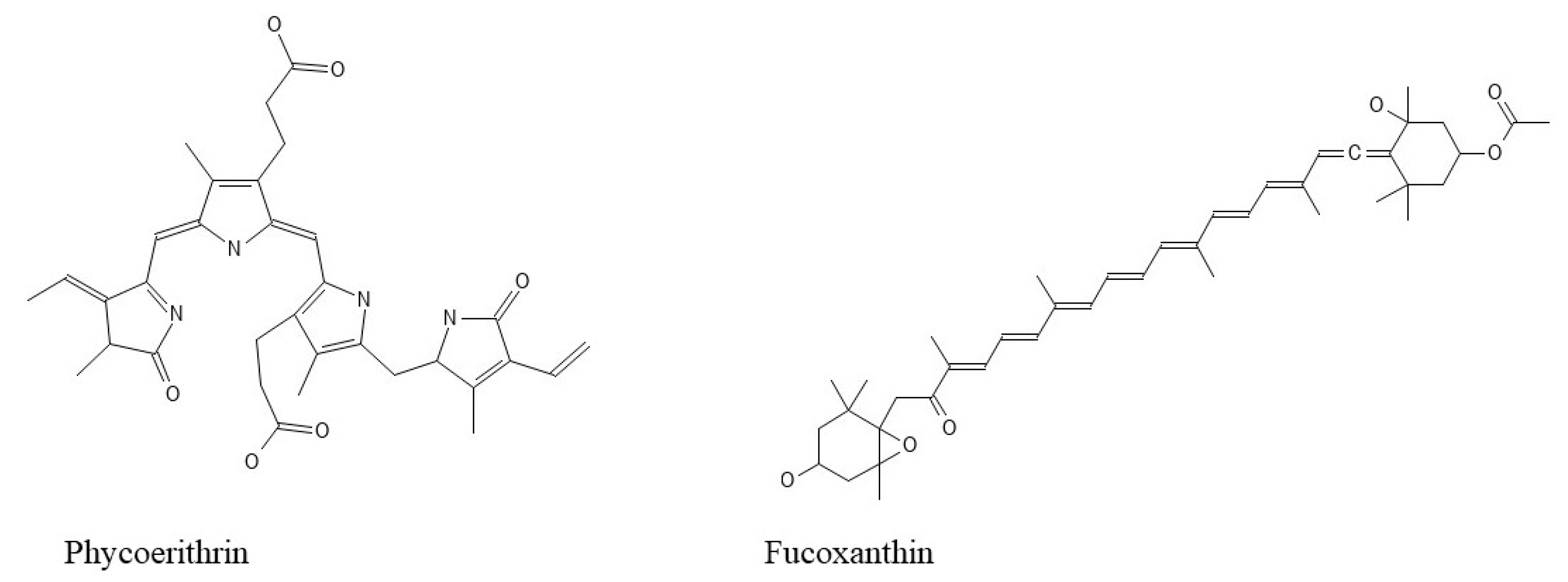
Seaweed bioactive substances have fueled nutraceutical interest in these functional meals. Polysaccharides (for instance, alginate, fucoidan, ulvan, agar, and carrageenan), proteins (for example, amino acids, phycobiliproteins (for example, phycoerythrin) (Figure 3), carotenoids (beta-carotene and fucoxanthin (Figure 3)), phenolic compounds (such as phlorotannins), vitamins (particularly vitamins A, B, C, D, E, and K), essential minerals (such as calcium, iron, iodine, magnesium, and potassium) and polyunsaturated fatty acids (namely ω-3 fatty acids) constitute the interesting group of compounds [34].
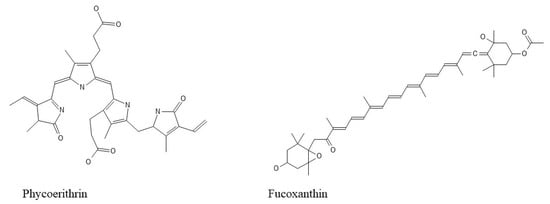
Figure 3.
Pigments’ structures.
These seaweed-derived compounds have been studied in the treatment of human diseases and pathologies such as hyperglycemia, diabetes, metabolic disorders, cancer, pathogenic diseases, aging, obesity, bone-related diseases, and neurodegenerative and cardiovascular diseases [4,8,10,35,36,37,38].
There are about 600 edible seaweed species recognized globally, with Porphyra/Pyropia sp. (Rhodophyta), Undaria pinnatifida (Figure 4), and Saccharina/Laminaria sp. (Phaeophyceae) being the main three used in Asian meals, known as the food product names “nori”, “wakame”, and “kombu” [39]. Although their contents vary greatly according to the species, location, growing/production circumstances, and harvest season, they are all capable of delivering essential macro- and micronutrients. Macronutrients are proteins, lipids, and carbohydrates that must be consumed in bigger amounts on a regular basis to provide energy. Micronutrients are vitamins, minerals, and trace elements that, although required in minute amounts, are essential for maintaining key processes [4].

Figure 4.
Undaria pinnatifida young specimen.
Seaweeds are complete protein sources because they include all nine essential amino acids (EAAs) required for protein synthesis, tissue repair, and nutrition absorption: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine [40,41]. We are unable to synthesize EAAs, so we must consume them through our food to ensure optimal organ function. Seaweeds have very similar patterns in terms of the non-essential amino acids (NEAAs), with aspartic and glutamic acid accounting for a significant portion (20–32%) of the total amino acids [42,43]. In fact, significant amounts of these two amino acids are responsible for the seaweeds’ characteristic taste and “umami” flavor [39,43].
Seaweeds are made up of hydrocarbons (e.g., squalene), sterols (e.g., cholesterol), and mostly fatty acids (FAs), the amount of which varies depending on the species, environmental circumstances, and life cycle phases [39,44,45]. Myristic acid (C14:0) and palmitic acid (C16:0), the respective monounsaturated versions, palmitoleic acid (C16:1) and oleic acid (C18:1), and linoleic acid (C18:3), the most prevalent PUFA, were the most abundant FAs discovered in the three groups of examined seaweeds [45,46,47]. Seaweed structural polysaccharides are similar to terrestrial plant polysaccharides and primarily consist of celluloses, hemicelluloses, xylans, and mannans, whereas storage polysaccharides, such as carrageenan, alginate, and agar, are more specific to seaweed species and are the most commercially exploited components of seaweeds [15,48]. These textural and stabilizing storage polysaccharides are commonly extracted by the hydrocolloid industry and employed in culinary applications [49]. Vitamins, like micronutrients, are required for optimal physiological function in minute quantities and their solubility determines their classification. Vitamins A, D, E, and K are examples of fat-soluble vitamins. These necessitate appropriate dietary fat intake because lipids are required for absorption, transport, and cellular uptake. Water-soluble vitamins include vitamin C, vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B6, vitamin B12, niacin, pantothenic acid, biotin, and folate [4].
The mineral profile of seaweeds varies according to the species, geographical location of harvest, wave exposure, seasonal, annual, environmental and physiological factors, type of processing, and method of mineralization. However, the mineral content, especially I, Fe, Mg, Ca, P, Na, and K, is abundant in seaweeds [8,50].
Due to their bioactive compounds and enrichment in other nutritional compounds, they are considered an excellent nutraceutical raw source for human welfare [51]. There is also interest in nutraceutical preparations for health benefits, and macroalgae offer promise as dietary supplements, with ingestion connected with positive health impacts due to the components they contain. Nutraceutical products are non-specific biological medications intended to improve general health, alleviate signs and symptoms, and prevent cancer. The term “nutraceutical” is a combination of the words “nutrient” (a nutritious dietary component) and “pharmaceutical” (a therapeutic drug) [52]. Seaweed bioactive compounds have the potential to play an important therapeutic role in illness prevention in humans. However, for this concept to be applied in the fulfilment of the concept, there is a need to certify that seaweeds promote health benefits with reduced secondary effects [52,53].
2.2. A Danger to Human Welfare?
Seaweeds absorb minerals and important trace elements from their immediate surroundings due to their unique cell wall structure with excellent biosorption characteristics. This great accumulation capability, however, may cause seaweed to accumulate potentially toxic materials found in its surroundings [54]. As a result, hazardous metals, including cadmium, lead, mercury, inorganic arsenic, and iodine, to mention a few, are frequently present at quantities many orders of magnitude greater than in water [55]. Unregulated distribution of seaweed species containing these components in high quantities may have negative health consequences for the unwary consumer. Certain seaweed species can have extraordinarily high quantities of iodine, resulting in excessive iodine levels when even modest amounts are ingested [56]. Although a necessary element of thyroid hormone production, excessive iodine intake can cause thyroid gland malfunction. In contrast, mercury, lead, cadmium, and inorganic arsenic have no biological purpose in the human body and can be toxic in even minute doses [57]. Furthermore, lead is categorized as potentially carcinogenic and neurotoxic, while inorganic arsenic is recognized as a “class I carcinogen”, and their presence in seaweed puts customers’ health at risk [20,58]. It is also important to note that various seaweed species have varying biosorption preferences for these hazardous substances [59]. Furthermore, extrinsic factors such as the geographic location, aquatic habitat, season, sample or processing methods can all influence the elements’ composition, even within the same seaweed species. Several food regulators are worried about consumer health and safety as a result of seaweed intake due to its possible toxicological profile [60].
3. Seaweed as Food: Regulations
The usage of seaweeds, as well as their production and marketing, have changed dramatically in recent decades, particularly in Europe, where their use is not always customary [61,62]. However, this relatively young European sector is beset by a lack of rules and norms governing the production and use of seaweeds [63]. This absence of controls is especially concerning since seaweeds collect and retain substances that, when consumed, can be toxic to people. Biosorption of heavy metals, such as mercury or arsenic, as well as the mineral iodine, in particular, need regulatory attention and the establishment of appropriate intake recommendations [63,64]. Only a few laws for seaweeds and seaweed products exist in Europe to ensure safe consumption. These regulations cover aspects of biology (including the authorization of seaweed species) as well as the composition of imported and locally produced products. The EU has also set out to conduct risk evaluations to define appropriate levels of ingestion and, lastly, to inform laws. Adaptations and extensions of the present legislation and guidelines are expected based on these risk assessments as well as the developing nature of the industry in Europe [63,65].
Outside of Europe, regulatory systems governing seaweeds and seaweed-based foods are in various stages of development. The market for edible seaweeds and seaweed products in the United States, like in Europe, is currently increasing [66]. In the United States, a lack of national legislation has recently resulted in the provision of an extensive set of guidelines and regulations for the safe consumption of seaweeds and seaweed-derived products by the Connecticut Sea Grant in collaboration with the Connecticut Department of Agriculture Bureau of Aquaculture [67]. By bridging the gap in national legislation and locally different state rules, these latest recommendations constitute an important step toward the industry’s efficient and safe expansion, which is required to satisfy the rising demand [66].
In Asia, where seaweeds have long been used and consumed, regulatory frameworks are more developed than in places of the world where the seaweed business is a relatively new sector. More information on the particular rules in China and Japan, as well as the other regions mentioned above, may be found in Campbell et al. [68].
The different types of seaweed do not impact the regulations, as they are considered similar between them, although seaweed compounds approved for the food industry (carrageenans, alginates and agar) are separated and very controlled in terms of the origin and extraction and purification methods. The major critical point is the mineral content of seaweeds, which includes all types of seaweeds; however, brown seaweeds are more prone to accumulate heavy metals [4].
How the Regulations Can Make the Seaweed Secure, Reducing the Risks to Human Health
The primary goal of law and regulation is to create and translate policies concerning the environment and/or health into rights and responsibilities, as well as to establish means to guarantee that such obligations are met [69].
Legislation is an important aspect of every business, especially if it impacts all the parties involved in the project, such as the primary producers and end-product makers. The legislation’s goal is to safeguard both consumers and producers while also controlling the market for each commodity [63].
The accumulation level of pollutants is considerable due to the peculiarities of seaweeds’ cell wall structure and their growth environment [70]. To ensure that humans are not endangered, legislation was enacted to establish the maximum amounts of harmful components in macroalgae, to regulate the amount of certain compounds ingested and to prevent food poisoning [63].
Since macroalgae has only recently gained popularity as a food product in the EU (European Union), the quantity of the laws on seaweeds in the EU is limited. The European Commission in 2006 established limit amounts of harmful chemicals for various meals in Commission Regulation (EC) No. 1881/2006 [65,71]. However, the EU has not set any limits for the amount of cadmium or inorganic arsenic found in various seaweed products [71].
As previously stated, macroalgae food items are not heavily regulated; nevertheless, macroalgae-derived additives such as carrageenan, agar, alginate, and others are controlled under (EC) No. 1333/2008 [65,72]. Because of their emulsifying, thickening, and gelling qualities, they have been employed. These items are designated by the numbers E401–E407a, and creating these categories will aid in the control of these substances as well as in their identification when needed [63].
Despite these negative and positive impacts of the human food source and food regulations, there are various aspects that can be checked to observe and make seaweed a future secure food source [26].
4. Seaweed as a Future Secure Food
Seaweeds are rich in nutrients and contains high concentrations of proteins, amino acids and minerals [73]. However, because of their tendency to retain substances such as heavy metals and other hazardous chemicals, their consumption must be continuously regulated [74]. Anthropogenic activities began to expand significantly around two decades ago, increasing the number of harmful substances released into the atmosphere and seas. All of these actions have an effect on algae, either directly or indirectly [73,75].
Biological, chemical, and physical dangers are the three types of hazards evaluated in relation to food safety [76]. As previously stated, seaweeds are excellent storage for toxic compounds [70], which are the most dangerous to seaweeds [63]. Chemical intoxication can cause a variety of health issues, and excessive use of certain substances can harm the neurological, circulatory, enzymatic, endocrine, and immunological systems [77,78]. Monitoring and limiting human consumption of seaweeds is critical to this.
4.1. Seaweed Aquaculture
Even though aquaculture produces most of the seaweed consumed globally (almost 97%), involving key instruments for seaweed food safety and nutritional, water composition analysis is uncommon in offshore and inshore aquaculture (Figure 5) [79].

Figure 5.
Ulva sp. inshore cultivation in Mondego river estuary, Figueira da Foz, Portugal.
Thus, the nutritional profile of seaweed can have a large range of values due to the intrinsic differences across species and specimen, but also to changes in the nutrient concentrations in water, water temperature, and salinity [80,81].
Thus, these variations can make seaweed aquaculture a risky method for ensuring food safety (due to the possibility of heavy metal accumulation in seaweed); nowadays, to overcome this, chemical analysis and nutritional evaluation per cultivated seaweed batch are required [4], but it is expensive and one of the major issues in offshore aquaculture. Seaweed aquaculture may need novel techniques to regulate or manage the nutritional content of seaweeds, such as the use of data simulation to anticipate nutritional values and ensure the best nutritional values [38]. In comparison to wild specimens and offshore production, nutritional values in inshore aquaculture can be more precisely monitored and standardized since water and abiotic elements can be readily regulated [38,82].
Now, farming is being enhanced by incorporating technology that can be further explored to provide an overall insight and optimize the number and quality of seaweeds farmed, lowering the cultivation expenses, low seaweed quality, and nutrient pollution [83].
The next step in seaweed aquaculture involves the informatization of the cultivation system, allowing for more controlled cultivation systems and predictions of seaweeds’ nutritional value using a specific kinetic model to obtain similar values for different batches of seaweed cultivated in the same area, thereby improving seaweeds’ safety after cultivation by eliminating concerns such as water nutrient fluctuations and potential contaminations [81]. Sensors that provide real-time data regarding critical information in the offshore system, such as the nutrients, pH, temperature, and salinity, can also be incorporated. In this scenario, inshore culture is the optimal growing method for improving seaweed food safety; nevertheless, this safety comes at a cost to seaweed output [52,53,83]. Thus, in the future, cost-effective cultivation technologies (both offshore and onshore) that improve food safety while lowering costs will be required [83,84].
Green seaweeds (Chlorophyta), of which there are 2200 species, may grow to a maximum height of 1 m. They are mostly farmed in inshore systems or harvested wild. Red seaweeds (Rhodophyta), which number 6100 species, are effective photosynthesizers in deeper seas. They vary in length and are comparable to green seaweeds. They are grown for direct consumption and the polysaccharide extraction business. They are typically grown in nearshore systems (off-bottom line, floating raft, and basket methods). They are mostly farmed in longlines in offshore circumstances; after Porphyra sp., the giant brown seaweeds (Kelps–Phaeophyceae) are the most produced seaweeds in the world [23,83].
4.2. Seaweed Storage
Seaweed storage requirements are determined by variables such as the time required between harvest and processing, climatic conditions, the quality of the collected material, expenses, energy requirements, and environmental consequences. Temporary seaweed storage might occur at the seaweed farm, aboard ships, in the harbor, near the food processing business, or near stores. Pre-processing (for example, drying) is frequently necessary before seaweed may be preserved. There has been very little study on these crucial areas [85].
As seaweeds have a short shelf life, methods to reduce the possible biological dangers are critical. Dehydrating the seaweeds is one method. By drying the product, the water content decreases, which means there is less accessible water [86,87], reducing the possibility for infections to proliferate and reproduce and ensuring food safety [88]. Drying processes extend the shelf life of seaweeds but can have a detrimental influence on their chemical composition and their bioactive qualities [89].
4.3. Seaweed Commercial Products
For large-scale seaweed processing, drying technologies are unfeasible. Sun-drying needs wide areas and is weather-dependent; however, it is low-cost and already employed in feed production. Oven-drying (or convective air-drying) is an energy-intensive and costly industrial process. Other processes, such as lyophilization, are mostly utilized in businesses where the target components are small molecules rather than algae [31,90,91,92].
Another safe method to ingest seaweeds is in fermented foods. This is a food produced mostly of fermented and salted cabbages, but it has lately been tested with seaweeds. The acidity caused by the low pH and high salt concentration prevents undesired microbes from developing, therefore preserving the product [88]. When compared to terrestrial biomass, seaweeds have distinct carbohydrates, which provide a difficulty, particularly owing to the presence of mannitol and laminarin. Based on this understanding, current terrestrial biomass methods cannot be directly adapted to macroalgae biomass, and the selection of suitable microbes is critical for the success of seaweed fermentation [31].
Opportunities for marketing seaweeds as fresh vegetables are growing as a result of the increasing demand in foods that are minimally processed [4]. and call for more research on how the storage temperature affects the quality of fresh seaweeds and how to monitor the seaweed quality during refrigerated storage [85]. Because fresh seaweeds are highly perishable and begin to deteriorate quickly after harvest, rapid analysis techniques and more analysis are needed. Thus, the application of Modified Atmosphere Packaging has been demonstrated to be a viable strategy for conserving minimally processed seaweed, outperforming the efficacy of vacuum packing [93]. It should be highlighted that the seaweed species can display diverse behaviors depending on the treatments used, emphasizing the necessity for rapid investigation of this new preservation strategy [93].
Furthermore, the presence of hazardous chemicals, such as toxins or heavy metals, in seaweeds must be carefully monitored [94]. In this situation, it was discovered that solar-drying, boiling, and seaweed dehydration, as well as other methods of processing (such as washing or cooking), lowered the toxins and the concentrations of other volatile chemicals (such as iodine or arsenic) in seaweeds [95,96,97,98]. Thus, it is critical to have scientific data about the heat treatment process as well as the chemical characterization of the resulting product in order to obtain the best method of maintaining the important properties of seaweed; it is also critical to find new methods of heat treatment that do not destroy the nutritional value of seaweeds.
The safety of seaweeds and the quality starts not in biochemicals but in the certifications and guidelines for the food industry due to be a crucial point to guarantee the security of the seaweed to the food industry, without exposing it to external risks between cultivation and the final product.
4.4. Guidelines for Food Safety in Industry
Ensuring food safety in the food industry entails following norms and regulations to avoid foodborne diseases and protect consumers. The handling and packing of seaweed by the food industry chain, for example, can be a hotspot for cross-contamination with viruses, bacteria, fungus, protozoa, organic compounds such as prions, natural poisons, and persistent organic pollutants [13,99]. If preservation measures are inadequate, for example, after harvesting the seaweed, contaminating organisms may proliferate. Guidelines for preventing these contaminations, such as Hazard Analysis Critical Control Points (HACCPs) and Good Manufacturing Practices (GMP), are, nevertheless, thoroughly defined in food security standards. Food safety can also be enhanced by the application of ISO2200. After-harvest biomass management might avoid product deterioration while ensuring a low level of pollutants. The major critical point of danger in food safety is mostly due to man-handling the seaweed from the harvest until the seaweed consumption.
4.4.1. Good Manufacturing Practices (GMPs)
The food business is one of the world’s most significant industries. As the world’s population grows, so does the need for food goods. However, as demand grows, so does the responsibility for ensuring the safety and quality of food items. Implementing Good Manufacturing Practices (GMPs) is a critical step in ensuring the safety and quality of seaweed [100]. GMPs define the operating conditions and regulations required to ensure cleanliness across the food chain and in its manufacturing. GMPs are a set of standards that outline management and handling activities with the goal of guaranteeing safe food production conditions. They are also important in the design and operation of facilities, as well as in the creation of food-related processes and products. The Codex Alimentarius designed GMPs with the primary goal of protecting customers. It comprises numerous essential operating criteria and processes that food firms must follow [101].
GMPs are norms and processes that are meant to ensure the safety and quality of food items. These principles and practices apply to all areas of food production, including food product manufacturing, processing, packaging, and storage. The primary goal of GMPs is to decrease the risks involved with food manufacturing while also ensuring that food products are safe and of high quality [100].
GMP compliance is critical for the food business for the following reasons:
- Ensure food safety: The fundamental goal of GMPs is to ensure food product safety. GMPs ensure that food items are free of contamination, adulteration, and other potentially dangerous chemicals [100].
- Meeting regulatory requirements: GMP implementation is a legal obligation in several countries. Companies that do not follow GMPs face legal action, penalties, and closure [100].
- Improving brand reputation: Companies that use GMPs are seen as responsible and dependable. This boosts the company’s reputation and consumer trust [100].
- Improving efficiency: Using GMPs may help businesses improve efficiency by decreasing waste, minimizing downtime, and increasing overall output [100].
- Improving product quality: Good Manufacturing Practices (GMPs) guarantee that food items are of high quality and fulfill customer expectations. This can boost consumer happiness and loyalty [100].
4.4.2. HACCPs
Seaweeds contain a variety of important and hazardous components, and humans have no control over their existence in the wild. For seaweeds to be edible in terms of the mineral concentration, precautions must be taken to prevent overdosage, such as adhering to the DRI (Daily Recommended Intake) guidelines for seaweeds. These numbers indicate that a daily intake of one product will not impair one’s health [54].
To reduce the danger of mineral overdosage, analysis can be performed to define and quantify those components in specific goods. There is, however, the HACCP (Hazard Analysis Critical Control Point) system, which is a methodology for establishing food safety processes and procedures [102]. The primary purpose of implementing this system is to prevent, for example, harm to humans resulting from food hazards, whether physical, biological, or chemical hazards, or to reduce them to an acceptable level [103]. Implementing this approach in the seaweed area is yet another way to safeguard consumers from mineral overdosage. Analyzing the essential control points entails identifying particular processes in the seaweed-processing process that are most likely to cause harm and measuring the three major risks. For physical risks, it is necessary to check to see whether there is anything that should not be there, such as plastics. Analyses must be performed to detect the presence of bacteria, viruses, or parasites in biological risks. Chemical analysis is required for chemical dangers, which include minerals and heavy metals, to ensure that the limits have not been exceeded. All of the procedures mentioned above must be paired with an investigation of the water in which the seaweeds were formed, because the environment in which they grow is also important for their composition [102,103].
By analyzing the risks and hazards in food, HACCP implementation protects both consumers and the industries that use it. Once these businesses are HACCP-certified, they can assure people that the food they produce is safe for customers [103].
4.4.3. ISO 22000
ISO 22000 is an international standard that provides rules for food safety management systems. The standard is intended to assist organizations in implementing efficient food safety management systems and ensuring that food items are safe to consume. The standard is based on the concepts of Hazard Analysis Critical Control Points (HACCPs) and integrates GMP features [104].
While GMP requirements ensure safe and sanitary food manufacturing, ISO 22000 takes a broader approach to food safety management by covering the whole food supply chain, from raw material acquisition to final product delivery [101,105].
Companies must take a systematic approach to food safety management, which involves identifying possible risks, establishing control measures, and assessing the efficacy of those measures. GMP implementation is a critical step in guaranteeing the safety and quality of food products. GMPs establish norms and procedures for all areas of food production, including the manufacture, processing, packing, and storage of food items. ISO 22000 establishes a framework for GMP implementation in the food business [100].
It is critical to regulate storage and manufacturing environments using the essential features of ISO 22000: The standard requires the implementation of an FSMS (Food Safety Management System) that addresses food safety hazards at every stage of the food chain (from production until the consumer’s home), basic conditions and activities necessary for maintaining a hygienic environment throughout the food chain, a systematic approach to identifying, evaluating, and controlling food safety hazards, an increased focus on upper management’s involvement in establishing and upholding the FSMS, and continuous attempts to improve. ISO 22000 adheres to the high-level structure (HLS) typical of other ISO management system standards, which includes understanding the internal and external factors that can impact the FSMS, the role of top management and quality control experts in establishing food safety policies and the objectives for that specific industry, risk management, objectives, and planning to achieve food safety in that targeted company. There is a need for support (mostly, general awareness, communication and scientific evaluations). In addition, frequent performance evaluations are required to monitor, measure, analyze, and evaluate performance, as well as internal and external audits and management reviews to enhance performance and rectify nonconformances [105,106].
Through all the steps that need to be addressed, there is a key element related to human welfare: “Seaweed can be a real keystone to the human food chain? And how we can enhance the food safety?”.
5. Seaweed Food’s Real Potential: How Can Be Checked?
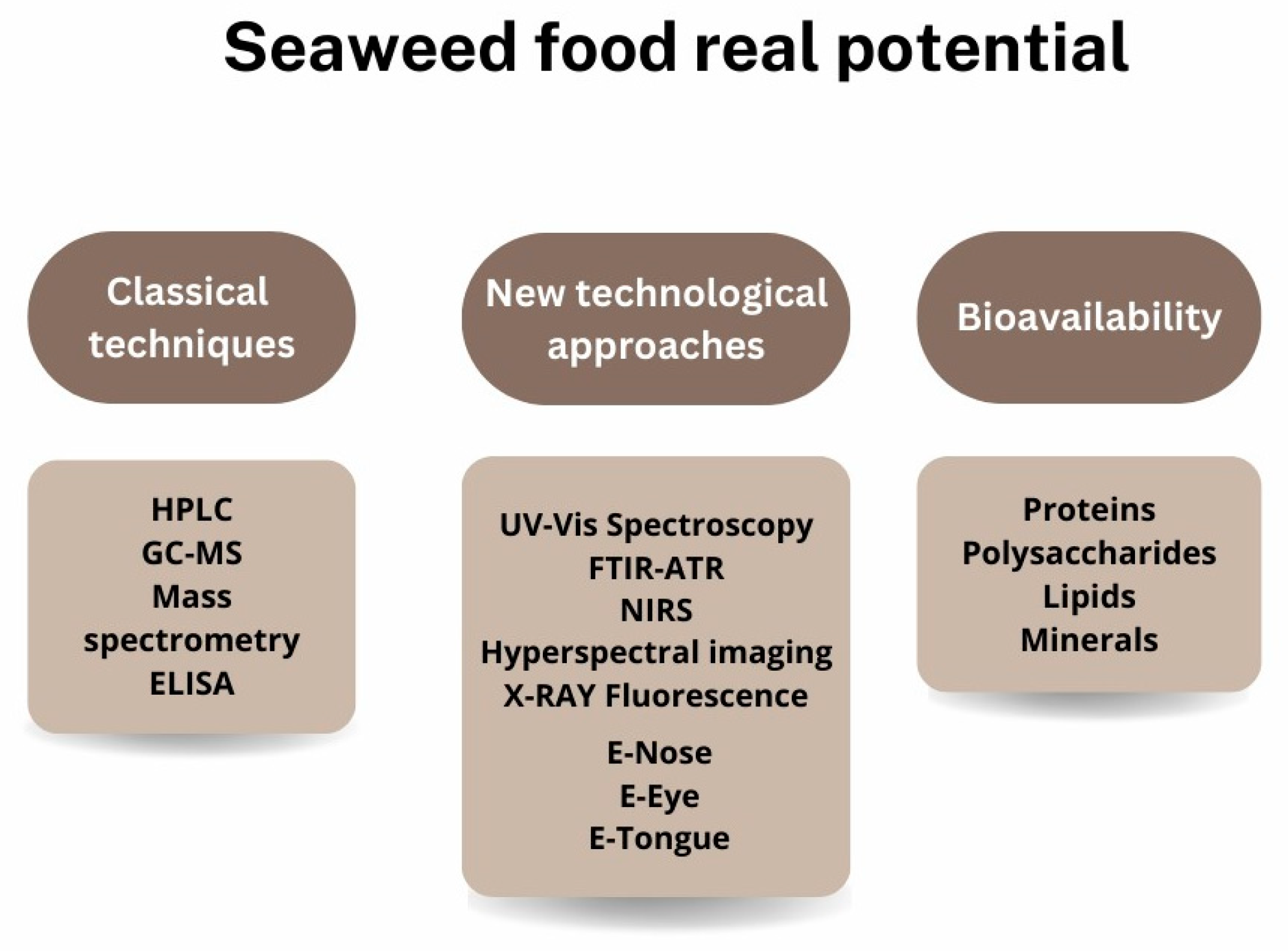
There is a requirement in the food sector to ensure product safety and quality; however, seaweeds live in areas where it is more difficult to ensure their safety and quality using just typical procedures for plants and vegetables [4]. As a result, the safety of seaweeds’ consumption and understanding of their quality is currently a hot topic in order to ensure a “new” food source that can substitute the plants’ part in the human diet, thereby reducing the pressure on the terrestrial ecosystem [10,107]. Still, the seaweed safety check is similar to terrestrial vegetables, being only necessary for the nutritional composition and bacterial analysis (Figure 6).
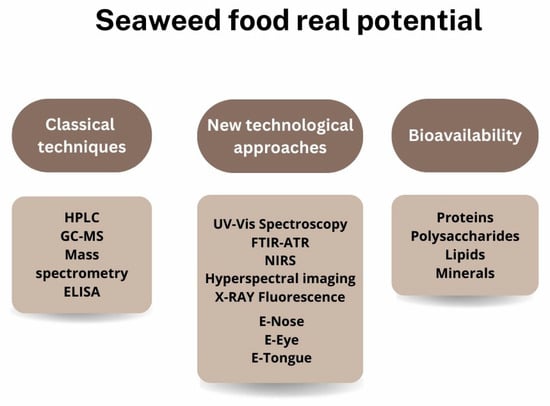
Figure 6.
Standard methodologies for determining the nutritional composition of seaweeds and possible new technological steps toward food safety in seaweed consumption.
To evaluate the bioavailability of seaweed nutrients and bioactive substances in simple raw material, in food or in meals, it is necessary to understand their content, structure, interactions with other dietary components, and destiny in the human body after ingestion. Thus, to observe all the data above and to fully understand the complex chemical reaction, there is a need to develop a system that can support the food agencies regarding the ingredients and their real potential [8,108].
5.1. Chemical and Biochemical Techniques: New Approaches
The methodologies and approaches used for seaweed nutritional composition as direct food are based on the Association of Official Analytical Chemists (AOAC) international standards, similar to other food sources, which are based on standard procedures [109]. As a result, the nutritional content analysis of seaweed is the same as that of other foods, such as vegetables. However, seaweeds include substances other than necessary nutrients, such as phenolic compounds, pigments, fatty acids, minerals, and perhaps heavy metals [10]. As a result, seaweed can absorb potentially dangerous metals or pollutants [10]. Other types of study are needed to provide additional assurance that seaweed is an excellent and safe dietary source [110]. Food safety concerns have become critical in promoting public health safety and the financial viability of global food companies throughout the global food supply chain [110]. Despite the newest technology, detection methods, legislation, and consumer education on food safety and quality, there is still an increase in foodborne disease outbreaks throughout the world, despite the latest food standards and customer expectations [110].
Different lab-scale techniques like microbial analysis, microscopic examination, gas chromatography-mass spectrometry, liquid chromatography, differential scanning calorimetry, and nuclear magnetic resonance are used, but most of these techniques are costly, time-consuming and required technical experts [111]. Biological techniques, such as culture-dependent microbiological techniques to quantify viable bacteria, nucleic acid detection technology (e.g., multiplex PCR), biochemical detection techniques, and immunological detection techniques are examples of common traditional microbial detection techniques, which can be prone to errors and also take some time for analysis when talking about fresh or perishable food [112].
5.1.1. Classical Techniques
By utilizing sensing platforms, the great majority of food contaminations, such as heavy metals, pathogens, mycotoxins, pesticides, veterinary medications, herbicides, and unlawful additions, may be examined [113]. HPLC (high-performance liquid chromatography), GC-MS (gas chromatography-mass spectrometer), MS (mass spectrometry), and ELISA (enzyme-linked immunosorbent assay) are common conventional detection methods in food science that can provide high sensitivity and selectivity in terms of sensing approaches for the detection of various types of food contaminations [114,115]. Traditional sensing platforms, on the other hand, need professional operators to deploy samples and expensive devices, which is time-consuming and costly [116]. Furthermore, well-developed sensing technologies are frequently used in the lab, which cannot meet the critical need of on-site determination, which necessitates portable sensors to obtain the result instantly [117]. As a result, simple, rapid, economical, and portable analytical approaches for determining different food contaminations have received considerable attention [113,118].
5.1.2. New Technological Approaches
The evaluation of quality in the food sector is a significant problem due to the need for high-cost equipment and lengthy analysis to ensure that products reaching customers are safe and of the highest quality. Existing technologies often require significant resources, skilled individuals, and sophisticated analytical techniques, which has generated a desire for quick and cost-effective solutions [111]. The use of current analytical systems ensures that the utilized technique is sensitive, linear, and repeatable. This is particularly important for method development and validation, as it provides the foundation for future studies (Table 2).

Table 2.
New technological approaches and their demonstrated potential.
UV/VIS Spectroscopy
Absorption spectroscopy in the ultraviolet and visible (UV/VIS) range is a rapid method used for the qualitative and quantitative assessment of sample substances. This approach has been applied in numerous fields of study in food science and food processing companies due to its simplicity and dependability [119]. UV/VIS spectroscopy is a technique used to monitor and measure the interactions of UV and visible light with various chemical substances in the wavelength range of 200 to 780 nm. The approach takes advantage of the physical reactions of light and analytes in the sample, such as absorption, scattering, diffraction, refraction, and reflection. UV and visible light absorption are limited to certain chromophores and chemical species with specified molecular functional groups. When the electrons within chromophores are stimulated, they produce distinct absorption spectra for individual molecules. UV/VIS spectroscopy applies to solid, liquid, and gaseous materials. However, UV absorption solute analysis is only feasible in homogeneous solutions. Nonhomogeneous samples often show significant interference within the spectra, especially when solid particles are present. This is due to the absorption and light-scattering effects of individual particles. The benefits and limitations of this technique include the sensitivity, equipment cost, remote-sampling capabilities, and the ability to analyze liquid, solid, and slurry samples with chemical resolution. The path length can also have an influence [120,121].
The utilization of UV/VIS spectroscopy stands out prominently in ensuring food safety, as evidenced by several recent studies across diverse food industries [122].
In the assessment of grape-must caramel in balsamic vinegar from Modena and Spanish PDO wine vinegar, UV/VIS spectroscopy emerges as a rapid analytical method, offering the potential for the quantification of the caramel content below legal limits. This underscores its crucial role in verifying compliance and authenticity within regulated food products [123].
Similarly, UV/VIS spectroscopy, coupled with chemometrics, demonstrates its efficacy in evaluating the impact of heating on various edible oils. By discerning the characteristic spectral changes indicative of oil degradation, UV/VIS spectroscopy enables the precise determination of the acid value, thus ensuring the quality and safety of heated oils [124].
Moreover, in the quantitative analysis of edible blend oils, UV/VIS spectroscopy is integrated with weighted multiscale support vector regression, providing enhanced accuracy in predicting the oil composition. This innovative approach showcases the instrumental role of UV/VIS spectroscopy in addressing complex compositional challenges, thereby bolstering food safety standards [125].
In one study, the Irish seaweed Himanthalia elongata (Phaeophyceae) was investigated as a natural source of antioxidant compounds [126]. The ethyl acetate subfraction showed high scavenging capacities for DPPH and lipid peroxidation, indicating strong antioxidant activity. LC-DAD-ESI-MS/MS analysis identified eight phenolic compounds, including some not previously reported in H. elongata. These results suggest the potential use of purified subfractions in food, pharmaceutical and cosmetic applications for health promotion due to their antioxidant properties [126]. Overall, this study highlights the importance of UV/VIS spectroscopy in the characterization of compounds from seaweeds and their potential contribution to food safety and health.
UV/VIS spectroscopy plays a vital role in food safety, enabling rapid and accurate analysis of the compounds present in both conventional foods and marine algae. By providing insight into the chemical composition of these products, UV/VIS spectroscopy helps to identify and quantify substances that may affect their safety and quality. This analytical technique makes a significant contribution to ensuring the integrity of food products and marine algae-based dietary supplements, thereby protecting public health and enhancing consumer confidence.
FTIR-ATR
Fourier transform infrared (FTIR) spectroscopy is a very effective method of analysis that does not damage the material and provides a “fingerprint” of the compounds present. The spectral peaks correspond to the vibration frequencies between the bonds of each atom in each chemical present in the sample, and they indicate the absorption of the IR beam. FTIR procedures are efficient, dependable, and easy to use, requiring no sample pre-treatment. Such approaches offer a simple and consistent way to handle a wide range of foods with non-destructive analysis, with the entire sample and analysis process often taking less than five minutes from start to finish. Previous research has demonstrated that FTIR-based methods, when combined with other chemometric techniques, can be effectively utilized in food industry processes to identify chemicals that may compromise the quality of the food or have been added to falsely claim that a food item is something other than what it is [127].
FTIR-ATR (Fourier-transform infrared spectroscopy–attenuated total reflectance) is a low-cost technique that analyses the chemical bonds of dried materials. This technique is an improvement over previous techniques that required a liquid extract solution [128]. It employs infrared light to vibrate chemical bonds and can be used to analyze the polysaccharides, pigments, phenolic fractions, compound oxidation, and microplastics in seaweeds before commercialization [128,129,130,131]. Compared to chromatography, the FTIR approach is less expensive and easier to use. However, it is less sensitive to biochemical quantification and quality analysis [107,132].
Food safety is a growing concern worldwide as consumers become increasingly aware of the risks associated with food contamination, adulteration, and deterioration. In this context, the use of FTIR-ATR is proving to be a powerful and versatile tool for ensuring the quality and authenticity of food.
The application of FTIR-ATR in food safety has been demonstrated in several recent studies. For example, researchers have used the technique to detect the presence of mycotoxins in foods such as sultanas, helping to prevent risks to human health [133]. FTIR-ATR has also been used to detect adulteration in dairy products such as cheese, ensuring the authenticity and quality of these foods [134].
Another important aspect is monitoring the quality and authenticity of meat and meat products, where FTIR-ATR has proven to be a valuable tool. This technique allows the detection of adulteration and the assessment of the deterioration status, and it ensures compliance with food safety standards quickly and efficiently [135].
In addition, FTIR-ATR has been used to identify pathogenic bacteria rapidly and accurately in a wide range of foods, providing an effective alternative to traditional methods of microbiological analysis. This rapid identification capability is critical in preventing outbreaks of foodborne illness [136].
The technique has also been used to quantify the sugars in products such as honey, monitor pesticide residues in crops and to detect adulteration in a wide range of foods [137,138].
In this study, FTIR-ATR was used to extract and characterize polysaccharides from macroalgae including Eucheuma denticulatum, Solieria chordalis (Rhodophyta) and Sargassum muticum (Phaeophyceae) [139]. The research focused on the identification of cell-wall polysaccharides such as carrageenans, fucoidans and alginates, highlighting the potential of these compounds for various applications in food and biotechnology.
Comparison of purified extracts with commercial solutions of polysaccharides showed strong similarities in the spectra, validating the extraction methods and confirming FTIR-ATR as a reliable technique for polysaccharide analysis. In addition, the study investigated seasonal variations in the polysaccharide composition, revealing differences in fucoidans, alginates and carrageenans depending on the time of harvest [139]. This information can guide the optimization of extraction processes and improve the utilization of macroalgal polysaccharides in different economic sectors.
The research also provided insights into the structural characteristics of carrageenans in specific algal species, such as the presence of iota-carrageenan at mature stages of development [139]. This knowledge can facilitate better control of the extraction methods and enable targeted applications of these compounds.
In a related study, ulvan, an edible sulphated polysaccharide extracted from Ulva lactuca (Chlorophyta), was used for the biosynthesis of silver nanoparticles (Ag-NPs) to produce bio-nanocomposite films for active food packaging. FTIR-ATR spectroscopy was used to confirm the formation of these films, which exhibited antimicrobial and antioxidant properties, making them potential replacements for conventional food-packaging materials [140].
FTIR-ATR is proving to be a valuable tool for ensuring food safety, particularly in the analysis of both traditional and novel food sources such as marine algae.
NIRS
Near-infrared spectroscopy (NIRS) is a promising approach for nondestructive and easy food safety inspection and control. It offers great benefits, such as speed, noninvasive measurement, ease of use, and low sample preparation requirements [141]. NIRS has been widely demonstrated to be effective in this field.
When radiation enters the sample, it is either reflected, absorbed by molecular bonds, or transmitted, resulting in changes in the light energy. These changes may reflect certain chemical bonds and, therefore, the properties of the tested items. To conduct scientific studies, it is important to select appropriate equipment and apparatus with better configurations, such as high spectral resolution, extensive scanning ranges, and adjustable scanning speed. Although NIRS systems are used to monitor manufacturing lines, they should be explicitly developed for commercial use as the application is typically specified [142]. However, it is important to consider the impact of the characteristics of NIRS instrumentation components on the overall performance when creating such a system. In recent years, advancements in hardware and software have made NIRS sensors more portable and practical [143]. The method of sample collection that is most suitable for the elements to be examined can have a significant impact on the overall outcome of the endeavor. The IRS for evaluating food quality involves collecting the spectra of tested items and constructing calibration models to correlate the spectral fingerprints with the sample attributes [143,144]. Nonetheless, NIR spectra contain enormous amounts of data that need to be processed, so chemometrics (a mixture of statistical and quantitative sciences) is often used to extract usable information that can significantly improve the potential applications of NIRS. Furthermore, to prevent fraudulent situations, NIRS can be used to perform qualitative and quantitative analyses of harmful compounds in food. The food industry has effectively demonstrated the potential of NIRS. Although more research has been conducted on food quality analysis, the use of NIRS in food safety evaluation and control is also increasing. This will be explored in detail in the following sections. The following sections show the number of recently published publications cited in this study, covering several application disciplines (such as freshness assessment, authenticity and adulteration, toxin detection and unlawful treatments) [141].
The NIRS technique has been widely studied in the food industry, particularly in detecting meat adulteration and assessing fish quality and fishery products [145,146]. However, its potential is not limited to these sectors alone, as it offers numerous possibilities in other areas of the food industry. For example, Kurz et al. [147] identified and analyzed the constituents in fruits, Kuligowski et al. [148] in oils, and Balabin and Smirnov [149] in milk products. Studies have demonstrated that NIRS can accurately discriminate and quantify adulterated oils [150].
NIR technology has been successfully applied in various areas, such as evaluating beef freshness, determining tomato maturity and textural properties, and analyzing rheological parameters in wheat grains [151,152,153]. These examples highlight the versatility and effectiveness of NIRS in the food industry, providing a valuable tool to ensure food quality, authenticity, and safety [141].
NIRS is a promising tool for ensuring food safety and quality in traditional food products and macroalgae. Recent studies have shown that NIRS can be applied to various aspects of seaweed analysis, including monitoring microbiological growth, determining the protein concentration, and assessing the nutritional composition [154,155,156]. For example, NIRS has proven effective in predicting the microbial counts in stored seaweed samples, providing rapid and real-time assessment of the product quality [154]. Furthermore, NIRS has been successfully used for on-site detection of the protein concentration in red seaweed, offering a non-destructive and precise alternative to traditional laboratory-based methods [155]. Furthermore, NIRS has demonstrated potential in evaluating the nutritional value and digestibility of brown seaweeds [156]. However, further validation on larger datasets may be required to assess its robustness.
NIRS shows potential as a versatile and efficient tool for enhancing food safety and quality assurance in conventional and emerging food sources like macroalgae. Its non-destructive nature, rapid analysis capabilities, and real-time assessments make it valuable for monitoring and ensuring food safety throughout the production and supply chain. Although NIRS shows considerable potential, further research and validation are required to fully establish its reliability and accuracy in different food matrices, including macroalgae. Nevertheless, with ongoing advancements and refinement, NIRS is poised to play a crucial role in advancing food safety and quality standards in the future.
E-Nose
Electrochemical (EC) methods, including impedance spectroscopy, voltammetry, potentiometry, and coulometry, have made significant contributions to food analysis. It is important to note that these methods provide objective and precise measurements of food components. EC techniques directly convert chemical processes occurring at the electrode/electrolyte interface into quantifiable electronic signals, such as altered conductive properties (conductometric), current (amperometry), and potential or charge accumulation (potentiometric) [111,157].
The electronic nose, as a non-invasive technology for identifying volatile substances, has been applied to food safety and quality analysis. The use of the E-Nose for pathogen identification has been proven to be successful and superior to traditional approaches. The E-Nose is a non-invasive and rapid approach that requires little or no sample preparation, making it perfect for use as an online monitoring tool. An E-Nose is a device that combines a chemical sensor array system with partial specificity and an appropriate pattern recognition system to distinguish simple or complex odors. It can analyze volatile organic compounds (VOCs) generated by microorganisms and is used as an alternative approach to identify and classify various chemicals and bacteria [110].
In the food industry, the E-Nose is used to detect food spoilage bacteria, total volatile basic nitrogen, trimethylamine, and fungal infections [158,159,160,161,162,163,164]. The E-Nose has several advantages over traditional and non-invasive approaches, such as vibrational spectroscopy and hyperspectral imaging. Despite these benefits, E-Noses are currently used in a limited number of applications in the food industry. The growth of E-Nose systems in the food industry has been rapid despite challenges in terms of sensor selection and the difficulty of implementing pattern recognition algorithms in low-cost hardware components. However, the necessity for such algorithms has hindered progress [111]. It is important to maintain objectivity when discussing these limitations.
E-Nose technology has a significant application in classifying the degree of contamination in leftover cooked foods, as demonstrated by research conducted in Malaysia. The odor characteristics of local leftover cooked meals are analyzed by E-Nose devices equipped with sensor arrays, which effectively classify the contamination levels with high accuracy rates ranging from 90% to 100%. This article highlights the potential of E-Nose technology in ensuring the safety and quality of prepared foods worldwide [165].
E-Nose technology has proven to be valuable in monitoring fermentation processes. Studies on the fermentation of Tremella aurantialba (fungi) have exemplified this. By detecting volatile compounds associated with fermentation, E-Nose devices allow for real-time analysis and prediction of the fermentation phases. This technology offers precise monitoring and control, ensuring the quality and safety of fermented food products [166].
In postharvest scenarios, E-Nose technology coupled with machine vision provides a rapid and non-destructive method for detecting the freshness and spoilage of perishable food items such as spinach [167]. By analyzing odor and image data, E-Nose devices accurately classify the freshness levels, facilitating timely quality assessments during storage [168]. E-Nose technology has been used to detect microbial spoilage in canned foods by analyzing volatile organic compounds (VOCs), providing early indicators of spoilage [169].
E-Nose technology is being used to detect fraud in food products, specifically extra virgin olive oil, by analyzing the VOCs emitted by olive oil samples [170]. This technology helps distinguish between authentic and adulterated or fraudulent products. Additionally, E-Nose devices are used to monitor the oxidation process of frying oils, ensuring quality control and preventing the consumption of degraded or rancid oils [171].
The various applications of E-Nose technology in food safety highlight its importance in modern food production and distribution systems. E-Nose devices offer versatile solutions for enhancing food safety and quality control practices, from detecting contamination and spoilage to monitoring fermentation processes and ensuring product authenticity. As technology advances, research and implementation of E-Nose technology are poised to redefine food safety standards. This will benefit both consumers and the food industry.
Electronic nose (E-Nose) technology provides a quick and efficient method for evaluating the quality and shelf life of food products, including macroalgae. In a recent study, the shelf life of different types of macroalgae was evaluated using an E-Nose over a storage period of 150 days. The E-Nose system detected the release of volatile organic compounds (VOCs) from the macroalgae, which are indicative of food deterioration [172].
The E-Nose recorded significant changes in the values for certain types of macroalgae during the storage period. These changes in the sensor values were positively correlated with the physical, microbiological, and Fourier-transform infrared (FTIR) spectroscopy parameters, providing comprehensive insights into the quality and shelf life of the macroalgae samples [172].
E-Nose technology has the potential to enhance food safety and quality assurance in both traditional food products and emerging food sources such as macroalgae. Its ability to detect changes in food quality quickly and accurately makes it a valuable tool for the food industry, facilitating timely interventions to maintain product freshness and safety.
E-Eye
Color is a crucial aspect of food quality as it is closely linked to perceptions of freshness, maturity, attractiveness, and safety. Customers often examine the color of food when making purchases [173]. Color is the perceptual response to the visible spectrum of the light that is reflected or emitted by an object. This response is generated by the interaction of light with receptors in the retina, which then sends a signal to the brain via the optic nerve. Color perception is influenced not only by the object itself but also by the surrounding lighting conditions [174]. Therefore, color analysis is crucial for categorizing items such as meat, peas, maize, canola, rice, and wheat for human and animal consumption [173].
The E-Eye is a detection system that utilizes visual information identification and analysis to assess food quality [175]. Color differentiation is achieved by comparing wavelengths. There are various color spaces available, such as HSI (hue, saturation, intensity), HSV (hue, saturation, value), HSL (hue, saturation, lightness), and HSB (hue, saturation, brightness), which differ in sensitivity [174]. The E-Eye has advantages for use in food quality evaluation due to its low cost, portability, and ease of implementation on a large scale [176].
This technology can be powered by colorimetry, spectrophotometry, or computer vision. Using appropriate equipment can result in a more accurate color description [177]. The E-Eye can detect the appearance-related characteristics of samples but not the flavor- or aroma-related components [178]. Therefore, combining multiple technologies such as the E-Nose and E-Tongue has become an alternative to detect the various characteristics of samples. Spectrophotometers are devices used to measure color that analyze the spectrum distribution of a sample’s transmittance or reflectance. They offer a spectral analysis of the reflectance wavelength and/or transmission qualities of objects [179]. Near-infrared reflectance (NIR) spectrophotometers are commonly used in the food industry to evaluate the chemical composition of items, particularly in cases where color is a crucial factor. This includes assessing the levels of proteins, oil, starch, fiber, and moisture. It is important to maintain objectivity in the evaluation of these elements [173,180].
Several research studies have demonstrated the applicability of E-Eye technology to various food products and safety scenarios. For example, in the characterization of edible olive oils, E-Eye devices were used alongside an electronic nose (E-Nose) and electronic tongue (E-Tongue) to assess the oil quality deterioration during storage [181]. Through innovative data-processing techniques and mid-level data fusion approaches, E-Eye technology facilitated the classification of olive oil samples based on freshness, demonstrating high classification accuracy rates.
E-Eye technology has also been instrumental in the fight against food fraud, particularly in the case of Italian lentils. Using red–green–blue (RGB) imaging and discriminant classifiers, E-Eye devices were able to classify lentils according to the harvest year and origin, as well as to discriminate between expired and edible samples. These results highlight the effectiveness of E-Eye technology in detecting subtle visual changes associated with food quality and authenticity [182].
The integration of E-Eye technology into food safety practices offers significant benefits, enabling rapid and non-destructive assessment of external food characteristics. From assessing food coloring in Chinese medicine to distinguishing between expired and edible lentils, E-Eye devices show immense potential for improving food safety measures. As research continues to explore the capabilities of E-Eye technology, its adoption is expected to grow, providing valuable tools to ensure the integrity and safety of food products worldwide [175,182].
Although macroalgae are vulnerable to spoilage, they offer numerous health-promoting compounds, making them a valuable food source. Due to the increasing demand for food quality and safety, sensitive and rapid analytical technologies are needed in the seafood industry. When applying the E-Eye technology to macroalgae safety, it is expected to yield similar benefits. The E-Eye system can effectively assess the quality and authenticity of macroalgae products by utilizing rapid and non-destructive techniques, such as spectroscopic methods. This approach offers advantages such as speed, minimal sample preparation, and the ability to monitor products in real time, which enhances food safety practices [183].
E-Eye technology shows potential for enhancing food safety and quality assessment for both conventional food products and sources such as macroalgae. Its quick and non-invasive nature makes it a valuable tool for monitoring freshness and authenticity, thereby contributing to consumer confidence and industry standards.
E-Tongue
Taste is the perception of chemicals that stimulate the taste buds on the tongue [184]. The E-Tongue has advanced in terms of the sensitivity, selectivity, and multiplexing capabilities of current biosensors, enabling accurate and rapid quality prediction of samples. This has led to its use in various industries, including pharmaceuticals, cosmetics, and food [185].
The term “E-Tongue”, which is an analogy to the human tongue, was first introduced in the 1990s and has since been extensively researched. It is a technology that uses a collection of sensors to detect chemical solutions. The system consists of an electrochemical cell (sensor array), a measuring module, and a pattern recognition algorithm capable of distinguishing between the simple and complex chemical systems that make up the flavor [180,184].
By examining various components of a sample, the electronic tongue extracts a signal signature [186]. The E-Tongue can describe the flavor of complex liquids or samples that have been transformed into liquid form [187]. The main purpose of this technology is to analyze meals using a collection of sensors, such as ion-selective electrodes with specific features, followed by statistical analysis. This allows for the collection of information on the freshness and maturity levels [187,188]. An E-Tongue sensor array provides multidimensional information in a short amount of time [189]. This technology distinguishes distinct patterns of classes of molecules responsible for flavor. The complicated information obtained by E-Tongue measurement is processed using multivariate statistical analysis. In contrast, human taste and flavor perception involves matching signals from taste receptors with memories to determine taste and flavor [187]. Additionally, an E-Tongue’s sensor suite can include a wide range of chemical sensors, such as electrochemical, optical, mass, and enzymatic sensors [180,189]. The combination of these techniques can improve seaweed quality characterization and safety.
One application of the E-Tongue is the detection of tetracycline residues in milk samples [190]. The E-Tongue successfully identified the presence of tetracycline residues in milk samples, providing a rapid and reliable method for contaminant detection without the need for sample pre-treatment.
Similarly, studies have demonstrated the superiority of E-Tongue technology coupled with advanced machine-learning algorithms such as back-propagation neural networks (BP-NN) in assessing the microbiological quality of fish samples [191]. These approaches outperformed traditional microbiological plating methods in predicting the total viable counts (TVCs), demonstrating the high accuracy and reliability of E-Tongue-based models in assessing microbiological quality parameters and improving food safety assurance measures.
In addition, the E-Tongue technology was instrumental in the sensory analysis of vegetable milk [192]. The results underlined the potential feasibility of using electronic tongues for simple, rapid, and effective sensory evaluation to ensure quality and consumer satisfaction.
E-Tongue technology was also explored for the express evaluation of quality parameters in vegetable oils [193]. Investigations into the use of multi-sensor systems have shown promising results. The application of these tools has demonstrated the potential for the development of rapid and simple methods for express quality assessment of vegetable oils, further highlighting the versatility and effectiveness of E-Tongue technology in improving food safety and quality assurance measures.
The diverse applications of E-Tongue technology underscore its importance in ensuring food safety, quality, and consumer satisfaction in various sectors of the food industry. From contaminant detection to sensory analysis and quality assessment, E-Tongue technology continues to play a key role in advancing food safety measures and maintaining high food quality standards.
The use of E-Tongue technology in food safety, specifically in relation to macroalgae, presents potential for quality assessment and product development. By utilizing the E-Tongue, researchers can examine the taste profile and chemical composition of macroalgae-based products, aiding in the creation of innovative food formulations and substitutes.
In the development of a low-sodium salt substitute, the E-Tongue can evaluate the taste characteristics of aqueous extracts from seaweed and other marine sources. By assessing taste parameters such as saltiness, bitterness, and umami, the E-Tongue can identify extracts with desirable flavor profiles. This helps researchers identify potential candidates for salt substitute formulations [194]. In addition, the E-Tongue can aid in comprehending the intricate interplay of salty constituents in macroalgae extracts. This can lead to the creation of improved salt substitutes that emulate the flavor of conventional sodium-based salts. By scrutinizing the sensory characteristics of macroalgae extracts, researchers can refine formulations to attain the desired taste profiles while diminishing the sodium content. This can help address health issues linked to excessive salt consumption [194].
The use of E-Tongue technology shows potential to improve food safety and quality assessment for both traditional food products and innovative macroalgae-based formulations. The E-Tongue provides rapid and accurate sensory analysis, allowing researchers to evaluate taste characteristics, identify flavor compounds, and optimize product formulations. This contributes to the development of healthier and more sustainable food alternatives.
Hyperspectral Imaging
Hyperspectral imaging (HSI) is a non-destructive technology used in food safety to identify adulterations, as well as microbiological, chemical, and physical contamination. HSI combines conventional imaging with spectroscopy to obtain a spectrum for each point (i.e., pixel) in an area of interest in the material being examined [195]. HSI’s spectroscopic component can cover spectral ranges from ultraviolet (UV) to terahertz. However, the most commonly used ranges are visible (VIS) and near-infrared (NIR). Studies have shown that when integrated with chemometrics and machine learning, HSI can identify fungal contamination in food. HSI detected fungal infections on grains at an early stage, indicating the possibility of early identification and removal of affected sections to minimize or prevent the development of fungal diseases. The use of HSI has been documented in identifying pollutants in fruits, vegetables, and meat products in the VIS-NIR or NIR range [195]. This method has also been found feasible in detecting faults and features related to the composition of meat products [196]. Physical contamination of food poses two primary difficulties, namely fecal contamination and the presence of foreign elements in food matrices. Fecal contamination is a significant food safety concern as it can introduce harmful germs into fruits, vegetables, and meat, leading to potential cases of food poisoning. It is associated with various fungal and bacterial pollutants [197,198].
Hyperspectral imaging has been investigated by researchers for its potential to ensure food safety. Studies have shown that hyperspectral-imaging systems are effective in detecting fecal contamination on fresh produce surfaces and in poultry-processing facilities [199]. These systems can differentiate between fecal contaminants and food surfaces, providing valuable insights for preventing foodborne illnesses.
Investigations have shown that hyperspectral imaging is highly versatile in various food safety applications. It can detect chemical residues and contaminants, as well as assess food quality attributes such as freshness and ripeness [198]. Hyperspectral imaging provides a non-destructive and efficient means of monitoring food safety throughout the supply chain.
Additionally, studies have highlighted its potential for rapid and accurate detection of foodborne pathogens. Hyperspectral-imaging systems can identify specific microbial strains and assess their presence in food products, enabling proactive measures to prevent foodborne outbreaks and ensure consumer safety [198].
The research conducted in this field demonstrates the significant potential of hyperspectral imaging in enhancing food safety practices and standards. As technology advances, hyperspectral-imaging systems are expected to play a crucial role in shaping the future of food safety in the food industry.
Hyperspectral imaging shows potential for improving food safety, especially in the context of macroalgae. By using advanced imaging techniques and innovative algorithms, researchers can enhance the accuracy and efficiency of foreign object detection, ensuring the quality and safety of seaweed products [200]. Furthermore, hyperspectral imaging can be applied to a range of food products, making it a versatile and powerful tool for ensuring food quality across multiple industries.
X-ray Fluorescence
Mineral analysis is a critical aspect of assessing seaweed’s impact on human health. However, it is a complicated and time-consuming technique. To reduce the time and expense associated with Inductively Coupled Plasma (ICP) techniques, a faster methodology suitable for frequent and numerous sample testing is required. A beam of primary X-rays, produced by an X-ray tube in X-ray fluorescence (XRF), bombards the material. The collimator restricts the cross-section of the main beam, allowing for specific excitation of the sample. This stimulation results in the release of fluorescence radiation. An energy-dispersive detector is used to measure the energy distribution of the fluorescence light. It allows for the identification and calculation of the relative concentrations of elements present in a sample. X-ray fluorescence spectroscopy (XRF) is a non-destructive and continuous technique that has become viable for generating high-resolution elemental data. The adjusted operating conditions improve the minimal detection limits and detection efficiency for several evaluated items. X-ray fluorescence spectrometry offers advantages over other multi-element methods, such as ICP-MS/ICP-OES. It requires limited sample preparation, provides non-destructive analysis, has a higher total speed, generates less hazardous waste, and has lower operating costs [201,202].
The gamma X-ray (XRF) technology was tested and calibrated against wet samples of seaweed to determine the relative and quantitative levels of elements. XRF is commonly used in geology and material sectors to evaluate the amounts of valuable or dangerous metals in solid or manufactured materials. However, it has not been widely accepted in the biological sciences, despite its potential [203].
The use of XRF has enabled frequent testing of seaweed biomass products, resulting in a developing record of range measurements for each element. This has allowed for the development of a profile of elemental ranges for nutrition and safety purposes for a specific type of seaweed. The nutritional value and safety of seaweed are determined by its elemental profile, which varies across different species. Therefore, it is crucial to thoroughly test all seaweeds and provide the results to the user. It is important to note that some seaweeds, such as Kombu, contain quantities of trace elements that exceed the recommended daily intake and even the upper limit. Hence, Kombu should only be consumed in very small doses [203].
A recent research review examined the application of XRF in the elemental analysis of milk [204]. Different configurations of XRF spectrometers were evaluated for their ability to quantify minerals and trace elements in milk samples with the accuracy and reproducibility required for food products. The study highlighted practical examples of the use of XRF techniques to determine the mineral composition of milk, emphasizing the importance of instrumentation, sample preparation, calibration and quantification procedures to ensure reliable results [204].
Another study investigated the use of low-power XRF systems, including energy-dispersive X-ray fluorescence (EDXRF) and micro-XRF (μ-XRF), for multi-element analysis and chemical imaging of various edible plant species [205]. The research demonstrated the feasibility of these XRF methods in analyzing plant material without the need for complex sample preparation, offering advantages in terms of simplicity and cost-effectiveness compared to traditional methods. The results underlined the potential of XRF techniques to increase knowledge of the impact of environmental factors, such as irrigation with treated wastewater, on the elemental composition of food products [205].
Another study focused on the rapid determination of cadmium in rice using energy-dispersive X-ray fluorescence spectrometry. The research evaluated the detection efficiency, periodic stability, and detection limits of different XRF instruments and demonstrated their applicability for the detection of cadmium in rice samples. The study highlighted the sensitivity of XRF spectrometers in meeting the rapid detection requirements with minimal impact from variations in the rice morphology. However, the research also identified challenges related to false negative and false positive results, highlighting the importance of careful calibration and validation procedures in XRF analysis for food safety applications [206].
The elemental composition of algae-based dietary supplements is crucial for assessing their safety and nutritional value. Energy-dispersive X-ray fluorescence (XRF) was used in a study evaluating 15 algae-based supplements commonly sold on the Portuguese market [207]. Despite the beneficial iodine content found in most of the kelp samples, there were concerns about potential excess iodine intake, which can lead to thyroid dysfunction. In addition, the presence of lead in various seaweed supplements posed significant health risks due to the potential for high daily doses. There was also considerable variability in the arsenic levels, raising further concerns about the safety of these supplements. These findings highlight the importance of informed consumption and regulatory oversight to mitigate the health risks associated with algae-based products.
Another study focused on XRF analysis of kelp supplement powders and capsules for heavy metal contamination [208]. While no significant levels of lead or mercury were found in the 17 samples analyzed, all the samples had detectable levels of arsenic exceeding the recommended reference doses. This highlights the importance of stringent quality control measures to ensure the safety of dietary supplements containing algae-based ingredients.
In Kenya, where seaweed is not widely consumed, there is growing interest in its potential as a food and medicine [209]. However, concerns remain about marine pollution and heavy metal contamination. In a study, XRF was used to analyze the trace metal concentrations in seaweed samples collected from different sites along the Kenyan coast. The results showed relatively high concentrations of essential trace elements such as calcium, manganese, iron, copper, and zinc, while toxic elements such as arsenic and lead were within acceptable limits according to the EPA/WHO regulations. This suggests that direct consumption of edible seaweed can be encouraged as it poses minimal health risks. In addition, due to their ability to accumulate trace metals, seaweeds serve as valuable indicators of marine pollution, making them essential for environmental health monitoring.
Overall, X-ray fluorescence spectroscopy plays a crucial role in assessing the safety and quality of algae-based dietary supplements and edible seaweeds, thereby ensuring consumer protection and environmental sustainability in the food industry.
5.2. Bioavailability
After conducting mandatory biochemical profiling of commercially available food, a major question remains: “The nutritional profile of seaweed and its true nutritional benefit to humans?”. Scientific evidence supports the fact that the nutritional value of vegetables differs from their real nutritional value due to the synergistic behavior of the vegetable matrix compounds [4,13,52,210]. Therefore, it is extremely necessary to conduct bioavailability assays to fully understand the true nutritional power of seaweed.
Bioavailability is a two-stage process consisting of bioaccessibility and bioactivity. Bioaccessibility refers to the amount of a consumed component that is released from its food matrix and available for absorption in the colon [211]. The biological activity of a medicine or food component involves transporting the component to the target tissue, interacting with other biomolecules, undergoing biotransformation and/or metabolism, and inducing a physiological response [108,212].
Thus, bioavailability is closely associated with the human digestive system and the food matrix. In vitro digestion methods reveal that food matrix effects have a significant impact on bioaccessibility, which is the fraction of the ingested substance available for absorption and is frequently the rate-limiting factor determining the overall bioavailability. Ideally, test methodologies should accurately predict the behavior of loaded food matrices in real-world settings, such as human consumption, and produce reproducible findings across laboratories [213]. In vivo human-feeding experiments provide the most precise approximation of real-world settings, but they are time-consuming and expensive to conduct. In vivo animal experiments can be used as an alternative, but they are time-consuming, expensive, and raise ethical concerns about animal confinement and sacrifice. Additionally, due to differences between the gastrointestinal tract of test animals and humans, the data obtained cannot be accurately extrapolated. As a result, researchers are developing in vitro digestion procedures to simulate the human gastrointestinal system. These approaches are especially useful as screening tools for determining the influence of certain food matrix effects on the bioavailability [214,215,216].
Over the last decade, several in vitro digestive models have been developed to investigate the bioavailability of bioactive compounds in foods and medicines [217,218,219]. However, in recent years, various approaches have been created to understand the nutritional and nutraceutical potential of seaweed. The INFOGEST static digestive simulator has become the most extensively used in vitro digestion model for foods due to its ability to precisely replicate the human gut while being simple to operate [219]. This method was developed through a COST action to promote a more accurate model and has gained popularity for its effectiveness [213]. This approach exposes food samples to sequential oral, gastric, and intestinal digestion. Factors such as the electrolytes, enzymes, bile, dilution, pH, and digestion time are dependent on the available physiological data. The method has been modified and enhanced from the INFOGEST 2.0 digesting technique by including the oral phase and using gastric lipase. The method described here assesses the food digestion endpoints by analyzing digestion products such as peptides, amino acids, fatty acids, and simple sugars, as well as quantifying micronutrient release from the food matrix. It is important to note that this method is not static and can be improved to provide more information based on real human digestion and its complexity. The procedure is divided into three steps that simulate the oral, gastric, and small intestine phases of digestion in vivo. At each step, the text describes the length, physical and biochemical environment, and rationale for the selection of the steps [220].
5.2.1. Seaweed Bioavailability Analysis
Understanding the kinetics of seaweed vs. stability may be critical for gaining important insights into the impacts of seaweed in the digestive process, ensuring improved food safety levels, and retaining a comparable product with stable chemicals that can be bioavailable to humans, thereby promoting human welfare. However, there is a general lack of studies on the bioavailability of seaweeds, and most of them use isolated compounds, mainly seaweed polysaccharides [52,53].
Raw seaweed biomass contains proteins, polysaccharides, lipids, and minerals. However, due to the variable intrinsic composition of seaweeds, some of these components may not be accessible for biological functions in the human body. In vitro research includes laboratory-based mimics/models for one or more circumstances faced by these substances in the digestive system, such as the mouth cavity, esophagus, stomach, and small and large intestines. In vivo investigations aim to evaluate the precise digestion and fermentation pathways of seaweed-isolated proteins, polysaccharides, and lipids by the gut microbiota [31,221].
Seaweed Proteins
Protein digestibility is a crucial factor in determining protein availability. The protein content of seaweed varies significantly. Digestive enzymes can easily break down seaweed proteins, making them readily absorbable by humans. However, the high soluble fiber content of seaweed can lead to inadequate protein digestibility, limiting protein digestion and reducing the accessibility of proteolytic enzymes. There is an inverse correlation between protein digestibility and the polysaccharide content in seaweed [222].
A recent study determined the in vitro protein digestibility values of six different Irish seaweeds using a rapid method. This methodology assessed the protein quality and bioavailability in seaweeds based on their amino acid profiles and protein content [223]. Furthermore, the study found that seaweeds are rich in essential amino acids and taurine, which are integral to human health. This suggests that seaweeds have the potential to serve as valuable protein sources in human nutrition, providing a diverse array of essential and non-essential amino acids to consumers [223].
The study emphasizes the significance of evaluating the protein bioavailability in seaweeds. This enables the identification of appropriate alternative protein sources for human diets, contributing to sustainable food systems and addressing the increasing demand for protein in a resource-efficient manner.
Seaweed Polysaccharides
Seaweed polysaccharides and fibers typically range from 33% to 62% (dry weight basis) and are generally indigestible by the human stomach and intestinal enzymes. They act as prebiotic food in the human body [224]. Seaweed is abundant in soluble fibers, which are known for their ability to retain water and their hydrocolloid properties. Hydrocolloids are used as functional additives in food formulation to improve food consistency. They act as thickening, stabilizing and emulsifying agents, and they enhance the sensory quality of food products [225].
Most seaweed polysaccharides are resistant to digestion in the stomach and small intestine. Oral digestion of marine algae has shown no breakdown of marine polysaccharides or release of oligosaccharides due to saliva enzymatic reactions. In vitro evidence suggests that no monosaccharides are identified in the gastric juice, indicating that they are not digested in the stomach. Similarly, no monosaccharides or oligosaccharides were detected in the small intestine, indicating that seaweed polysaccharides were not degraded there. Seaweed polysaccharides are carbohydrate polymers that are neither digested nor absorbed in the small intestine. Instead, they are fermented by bacteria in the colon, which influences and modifies bacterial populations in the gut. The human large intestine contains a diverse range of bacterial species that play a crucial role in various metabolic and regulatory processes related to human health [31,221].
Recent studies have investigated the in vitro digestibility and fermentability of polysaccharides extracted from two types of seaweed: Ascophyllum nodosum (AnPs) (Phaeophyceae) [226] and Pyropia haitanensis (formerly Porphyra haitanensis) (PHPs) (Rhodophyta) [227]. The findings suggest that these polysaccharides have potential as functional food components with implications for human health.
AnPs showed significant resistance to enzymatic digestion by salivary amylase, gastric juice, and intestinal juice. However, fermentation by the gut microbiota resulted in a notable reduction in the molecular weight and reduced the sugar content of AnPs. This process also caused significant changes in the composition of the gut microbiota, particularly by increasing the abundance of Bacteroidetes and Firmicutes, which are associated with metabolic health benefits. Furthermore, the fermentation of AnPs led to a significant rise in the overall content of short-chain fatty acids (SCFAs), suggesting potential advantages for gut health and overall well-being [226].
Polysaccharides extracted from PHPs using water extraction and alcohol precipitation methods showed a promising yield with a distinctive molar ratio of galactose, glucose, and fucose. These polysaccharides were resistant to digestion by alpha-amylase, indicating their potential as prebiotics that can selectively stimulate the growth and activity of beneficial gut microbiota. Furthermore, PHPs have demonstrated significant antioxidant activity, making them attractive candidates for applications in the food and cosmetic industries [227].
The investigation into the digestibility and bioavailability of polysaccharides from macroalgae highlights their potential as functional food ingredients with diverse health-promoting properties. Further research is needed to elucidate their physiological effects and explore their applications in human nutrition and well-being.
Seaweed Lipids
Macroalgae, also known as seaweeds, are being considered as potential candidates for improving the nutritional value of various food products. This is due to their rich composition of lipids and diverse bioactive compounds. Studies examining the lipid content and availability of lipids in different species of seaweeds provide valuable insights into their potential as functional food ingredients [228].
Seaweeds have a diverse nutritional composition, with lipids being a key component. The lipid content of seaweeds usually ranges from 0.2% to 2.5%, with variations observed among different species and environmental factors. Seaweeds are valued for their unique lipid profiles and bioactive lipid compounds, despite their relatively low lipid content compared to other food sources [228,229].
Analysis of the fatty acid profiles in seaweeds reveals that they contain a predominance of polyunsaturated fatty acids (PUFAs), including omega-3 and omega-6 fatty acids, which are essential for human health. Seaweeds have higher levels of PUFAs compared to saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs), making them a valuable source of healthy fats [228].
The study conducted by Demarco et al. [222] investigated the lipid and fatty acid contents of brown seaweed Fucus spiralis (Phaeophyceae) before and after in vitro modeling of the human digestive process. The results showed that F. spiralis has a low lipid content of 3.5% dry weight, with a lipid bioaccessibility of about 12.1%. The most abundant −3 fatty acid found was eicosatetraenoic acid, with a bioaccessibility percentage of 13.0%. The low lipid bioaccessibility may be because most of the internal lipids in seaweed are phospholipids and glycolipids, which are associated with cell membranes [222].
An investigation found that different seaweed species have a wide range of total lipid content, ranging from 0.7 ± 0.1% in Chondrus crispus (Rhodophyta) to 3.8 ± 0.6% in Ulva spp. (Chlorophyta). This variability highlights the diverse lipid compositions present in seaweeds.
Analysis of the fatty acid profiles revealed varying proportions of polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), and saturated fatty acids (SFAs) across different species. Ulva species exhibited the highest content of polyunsaturated fatty acids (PUFAs), while C. crispus and Gracilaria species were rich in saturated fatty acids (SFAs) [230].
Seaweed Minerals
Minerals are chemical elements that organisms require as essential nutrients for survival. They are primarily obtained through the consumption of plants, animals, or water. The human body contains five primary minerals: calcium (Ca), phosphorus (P), potassium (K), sodium (Na), and magnesium (Mg). Sulfur (S), iron (Fe), chloride (Cl), cobalt (Co), copper (Cu), zinc (Zn), manganese (Mn), molybdenum (Mo), iodine (I), and selenium (Se) are trace elements with unique metabolic functions in the human body. The bioavailability of these metals is strongly related to the carbohydrate and dietary fiber concentrations [22]. Samples with high carbohydrate or dietary fiber concentrations have high metal bioavailability ratios. A negative association was found between the bioavailability percentages of certain metals and proteins. This is because during in vitro digestion, proteins are hydrolyzed into amino acids. Most soluble amino acids carry positive or negative charges at a physiological pH, which increases the ionic strength in the aqueous phase. This results in lower metal solubility via the salting-out effect [231]. Metals and lipids have a negative correlation, which is due to metals not being emulsified by bile extracts during in vitro digestion and being introduced into the liquid phase before becoming available for absorption [22,222].
One study has shown that certain seaweeds, such as nori and sea lettuce, contain high levels of bioavailable iron. However, the absorption efficiency of iron from seaweeds varies, with some species exhibiting lower absorption rates compared to spinach. Factors such as the vitamin C content can enhance the iron bioavailability in specific seaweeds, but high levels of arsenic or other minerals may limit their regular consumption as a safe iron source [232].
The absorption of magnesium from seaweeds differs based on their fiber content and solubility. Seaweeds like Aosa (Chlorophyta) and Kombu (Phaeophyceae) have been identified as good sources of magnesium due to their high absorbable magnesium concentration. Despite variations in the magnesium solubility after in vitro digestion, factors beyond solubility alone influence magnesium absorption from seaweeds [233].
The bioavailability of minerals in seaweeds can be affected by cooking methods. Some metals, such as chromium, iron, cobalt, nickel, and zinc, are released into the cooking water during heat treatment, while others, such as copper, iron, and zinc, are retained in the seaweed [210].
Understanding the bioavailability and digestibility of minerals in edible seaweeds is crucial for maximizing their nutritional benefits. Seaweeds offer a diverse range of minerals, but variations in the absorption rates and interactions with food constituents highlight the need to consider these factors when incorporating seaweeds into the diet. Further research is needed to optimize the bioavailability of minerals from seaweeds and ensure their safe and effective use as valuable sources of essential nutrients [52,53,95,107].
The seaweed food matrix can be optimized to improve the bioaccessibility by modifying its content and structure. Seaweed compounds can interact positively or negatively, affecting the bioavailability of nutrients. In vitro digestion models are powerful tools for modifying the food matrix and improving the bioavailability of bioactive compounds, resulting in potentially healthier food products [52,53,95,107].
Furthermore, there is a need to further develop and study this topic due to its vital importance for human health. The nutritional value of seaweed is currently undervalued, despite being a key factor in human welfare and a potential tool for future food nutraceuticals. With the evolution of technology, it is possible to develop a food safety system using new technologies such as the E-Nose, FTIR-ATR, and NIRS, coupled with the INFOGEST system. This can enhance food safety and improve understanding of the real nutrient availability through pro analysis component analysis and logarithms. It is important to develop a new technological safety system to improve human welfare quality while reducing the costs associated with classical assays.
6. Future Road into Seaweed Food Safety and Nutraceutical Potential
Even though each biochemical and biological study has both positive and negative implications for seaweed quality certification, these analytical techniques are currently in development due to the complexity of seaweeds’ composition. The additional expenses that these studies may incur for businesses might be reflected in the final product pricing. In contrast, seaweed characterization is required to provide quality control and customer safety. Thus, the framework and regulations for regulating seaweed food quality management are continuously evolving, with specialized analysis for seaweeds considering the chemical diversity and compound complexity [4]. It is still unknown how the gut microbiota influences dietary component metabolism and how much this differs across people (because humans differ as to their digestive system, with different microbiomes). Many kinds of food substances, such as complex polysaccharides, lipids, proteins, and phytochemicals, are transformed by gut microorganisms, and these metabolic processes are associated with a variety of health advantages as well as disease susceptibility. Furthermore, the gut microbiota changes the toxicities and lives of industrial chemicals and pollutants in the body [234]. There is currently a scarcity of information on the interactions and metabolic regulation of the gut microbiota and seaweeds’ components. Due to the inherent heterogeneity of the gut microbiome from individual to individual, it is critical to understand the functions of gut bacteria in the processing of components acquired from macroalgae sources, most creating a robust gastrointestinal model (INFOGEST). The gut microbiota’s direct and indirect metabolic effect on the chemical alterations of a wide variety of chemicals might possibly have consequences for host health [107,235].
As a novel food product, the seaweed sector for human consumption confronts a number of obstacles, including a lack of standard quality and safety processes, as well as a lack of regulatory rules. There are now various analytical techniques available for evaluating the nutritional value of seaweeds, which can guarantee a better quality and safety check of seaweed-based products; however, advancements to standardize the methodology and findings are urgently needed. Actually, a desire to collaborate with the scientific community to establish rules and laws applicable to seaweed farmers and industry for the food sector is rising throughout the Asian, European, and American continents [107].
Seaweeds are a promising novel food source that can help address global food security challenges. However, ensuring the safety of seaweed consumption is of the utmost importance. A systematic review was conducted to assess the microbiological, chemical, physical, and allergenic risks associated with seaweed consumption. The review found no significant hazards for microbiological, allergenic, and physical risks. However, there are concerns regarding chemical risks, particularly the accumulation of heavy metals, when harvesting seaweed in polluted areas. To mitigate this risk, seaweed cultivation in controlled environments is recommended. It is also necessary to periodically monitor the heavy metal levels in final products. Establishing safety standards for seaweed consumption is crucial as its popularity continues to grow worldwide [236].
The seaweed industry has great potential for sustainable ocean economies and aligns with the UN Decade of Ocean Science for Sustainable Development. Seaweed production accounts for over 50% of the total global marine production and supports the livelihoods of millions. However, the industry faces significant challenges, including the need to enhance biosecurity, traceability, and pest management, as well as to promote technological innovation and policy coordination, to ensure its long-term sustainability. Paying attention to small-scale farmers and processors is crucial to ensure inclusive growth and resilience while addressing key Sustainable Development Goals [237].
The expansion of the seaweed market in Australia provides a valuable example for Europe as it incorporates seaweed into its food systems. Australia’s initiatives, including significant investments in research, development, and commercialization, serve as a blueprint for Europe to follow [238].
In Australia, concerted efforts have been made to sustainably cultivate native seaweed species, tapping into the country’s rich biodiversity. This approach is in line with Europe’s increasing interest in using local resources and promoting biodiversity conservation. European countries can explore the cultivation of indigenous seaweed species suited to their coastal environments, thus reducing their dependence on imported seaweeds by adopting similar strategies [238].
Additionally, Australia’s emphasis on innovation and technology in seaweed cultivation and processing provides valuable insights for Europe. By embracing advancements in cultivation techniques, biorefinery processes, and product development, Europe’s emerging seaweed industry can enhance its efficiency and sustainability [238].
Collaboration between scientists, government agencies, industry stakeholders and local communities has been instrumental in the growth of the seaweed sector in Australia. Similarly, to overcome regulatory hurdles, address food safety concerns, and ensure consumer acceptance of seaweed-based products, fostering interdisciplinary collaborations and stakeholder engagement will be crucial for Europe [238].
To ensure the safety of seaweed for food and feed purposes, a comprehensive assessment of the potential hazards is necessary. Major hazards, such as heavy metal accumulation and microbial contamination, pose significant risks to human health. It is essential to address data gaps and prioritize monitoring programs to identify and mitigate hazards. The hazard presence is influenced by factors such as the seaweed type, cultivation environment, and processing methods. Prioritizing monitoring efforts based on the hazard severity and knowledge gaps can inform risk management strategies and ensure the safety of seaweed-based products [97,239]. There needs to be developed a new system based on the HACCP, GMP and ISO 2200 and linked to the new characterization technologies and bioavailability analysis to evolve human food into a new step toward human safe nutraceutical food [102,104,105].
Despite the need for responses to growing health concerns and a secure and stable food supply, it is critical to adopt new rules that improve the quality and safety of seaweeds and their sub-products for human use. Furthermore, promoting and improving the required human daily nutrition dosage is critical for improving overall health conditions. Thus, there is a need to observe if the different types of seaweed will need a different type of safety analysis protocol or can an intermediate protocol be used for all. Furthermore, there is need to develop a control of seaweed quality and a handbook of best practices for the culture that enhances sustainably and healthy seaweed aquaculture. A handbook focusing on the methods to analyze the quality and safety of seaweeds and their sub-products for human use, including microbial, heavy metals and nutritional methods, is required, as is bioavailability assays to be applied around the globe, and clarifying if different types of algae need different approaches due to brown seaweeds having different compounds from red and green seaweed [4,23,52,240].
7. Conclusions
There is a new wave of research to find a new natural food source and ingredient, which is being setting on seaweeds. However, there still a lot to do to verify seaweeds’ real potential at a food solution for humankind’s future. Still, the principal features and discoveries demonstrated that seaweeds are a future key for human nutrition and healthcare solution.
Food safety is becoming an international problem for human health worldwide. Common food contaminants, including but not limited to antibiotics, veterinary medications, pesticides, mycotoxins, heavy metals, foodborne diseases, unlawful additives, and allergies, can constitute a substantial hazard to human health. Therefore, to have a positive impact on human health, there is need to correctly certify seaweeds’ quality as a food ingredient by the regulations and principles with which food raw sources are normally accessed. Thus, a variety of analytical approaches were used to check food safety, but each had practical limits or analytical flaws. There are still margins to develop even more technological tools to have more data to check and enhance seaweed in relations to food safety and security.
More assays concerning seaweed compounds’ bioavailability are needed to promote seaweed foods and their safety for human welfare, using standard methods which can be validated by diverse food agencies. This is one of the most important topics, which continues without a theoretical and practical recommended and applied method for the studies of nutraceutical compounds and foods. Thus, bioavailability analysis of seaweeds and their compounds/products requires an integrated and comprehensive approach that includes in vitro, in silico and in vivo studies, new modern analytical methods (connect to the classical techniques), and evaluation of the many affecting variables. Understanding the bioavailability of seaweed-derived nutrients and bioactive compounds will help with their best application in functional and healthy meals, nutritional supplements, and therapeutic uses, being a keystone to the future of seaweeds as a safe nutraceutical source.
Author Contributions
Writing—original draft preparation, J.C., J.O.T. and R.S.; writing—review and editing, J.C., J.O.T., R.S. and L.P.; visualization, J.C. and J.O.T.; supervision, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FCT—Fundação para a Ciência e Tecnologia, I.P. by project reference UIDB/04004/2020 and DOI identifier 10.54499/UIDB/04004/2020 (https://doi.org/10.54499/UIDB/04004/2020). This work was supported by FCT—Fundação para a Ciência e Tecnologia, I.P. by project reference UIDP/04004/2020 and DOI identifier 10.54499/UIDP/04004/2020 (https://doi.org/10.54499/UIDP/04004/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Due to the extensive work, we created a list of abbreviations:
| % | Percentage |
| Ag-NPs | Silver nanoparticles |
| AnPs | Ascophyllum nodosum |
| AOAC | Association of Official Analytical Chemists |
| BP-NN | Back-propagation neural networks |
| Ca | Calcium |
| Cl | Chloride |
| Co | Cobalt |
| CO2 | Carbon dioxide |
| Cu | Copper |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| E401 | Sodium alginate |
| E407a | Transformed Eucheuma algae |
| EAAs | Essential amino acids |
| EC | Commission Regulation |
| EC | Electrochemical |
| EDXRF | Energy-dispersive X-ray fluorescence |
| ELISA | Enzyme-linked immunosorbent assay |
| EPA | Environmental Protection Agency |
| EU | European Union |
| FA | Fatty acids |
| FAO | Food and Agriculture Organization of the United Nations |
| Fe | Iron |
| FSMS | Food Safety Management System |
| FTIR-ATR | Fourier-transform infrared spectroscopy |
| GC-MS | Gas chromatography-mass spectrometer |
| GMP | Good Manufacturing Practices |
| HACCP | Hazard Analysis Critical Control Point |
| HIS | Hue, saturation, intensity |
| HLS | High-level structure |
| HPLC | High-performance liquid chromatography |
| HSB | Hue, saturation, brightness |
| HSI | Hyperspectral imaging |
| HSL | Hue, saturation, lightness |
| HSV | Hue, saturation, value |
| I | Iodine |
| ICP | Inductively coupled plasma |
| ICP-MS/ICP-OES | Inductively coupled plasma mass/spectroscopy inductively coupled plasma atomic emission spectroscopy |
| INFOGEST | COST INFOGEST network standardized protocol for human digestion assay |
| K | Potassium |
| LC-DAD-ESI-MS/MS | Liquid chromatography coupled to diode array detection and electrospray ionization tandem mass spectrometry |
| Mg | Magnesium |
| Mn | Manganese |
| Mo | Molybdenum |
| MS | Mass spectrometry |
| MUFAs | Monounsaturated fatty acids |
| Na | Sodium |
| NEAAs | Non-essential amino acids |
| NIRS | Near-infrared spectroscopy |
| P | Phosphorus |
| PCR | Polymerase chain reaction |
| PDO | Protected designation of origin |
| pH | Potential of hydrogen |
| PHPs | Pyropia haitanensis |
| PUFAs | Polyunsaturated fatty acids |
| RGB | Red–green–blue |
| S | Sulfur |
| SCFAs | Short-chain fatty acids |
| Se | Selenium |
| SFAs | Saturated fatty acids |
| TVC | Total viable count |
| UV-Vis | Absorption spectroscopy in ultraviolet and visible |
| VOCs | Volatile organic compounds |
| WHO | World Health Organization |
| WWII | World War II |
| XRF | X-ray fluorescence spectroscopy |
| Zn | Zinc |
| μ-XRF | Micro X-ray fluorescence spectroscopy |
| ω | Ômega |
References
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal Proteins: Production Strategies and Nutritional and Functional Properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Dopelt, K.; Radon, P.; Davidovitch, N. Environmental Effects of the Livestock Industry: The Relationship between Knowledge, Attitudes, and Behavior among Students in Israel. Int. J. Environ. Res. Public Health 2019, 16, 1359. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.M.; Herrero, M.; Tuomisto, H.; Valin, H.; Van Middelaar, C.E.; et al. The Potential of Future Foods for Sustainable and Healthy Diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Sultana, F.; Wahab, M.A.; Nahiduzzaman, M.; Mohiuddin, M.; Iqbal, M.Z.; Shakil, A.; Mamun, A.-A.; Khan, M.S.R.; Wong, L.; Asaduzzaman, M. Seaweed Farming for Food and Nutritional Security, Climate Change Mitigation and Adaptation, and Women Empowerment: A Review. Aquac. Fish. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Ross, F.W.R.; Boyd, P.W.; Filbee-Dexter, K.; Watanabe, K.; Ortega, A.; Krause-Jensen, D.; Lovelock, C.; Sondak, C.F.A.; Bach, L.T.; Duarte, C.M.; et al. Potential Role of Seaweeds in Climate Change Mitigation. Sci. Total Environ. 2023, 885, 163699. [Google Scholar] [CrossRef] [PubMed]
- Klnc, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; InTech: London, UK, 2013. [Google Scholar]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as Nutraceuticals for Health and Nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An Overview on the Nutritional and Bioactive Components of Green Seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- da Costa, E.; Silva, J.; Mendonça, S.; Abreu, M.; Domingues, M. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in Food: A General Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 240–250. [Google Scholar]
- Ferrara, L. Seaweeds: A Food for Our Future. J. Food Chem. Nanotechnol. 2020, 6, 56–64. [Google Scholar] [CrossRef]
- FAO; WHO. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives; FAO: Rome, Italy, 2022; ISBN 9789251365908. [Google Scholar]
- FAO. New Report Urges Food Safety Guidance on Seaweed. In Inocuidad y Calidad de los Alimentos; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/food-safety/news/news-details/es/c/1607078/ (accessed on 20 November 2023).
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Giercksky, E.; Doumeizel, V. Seaweed Revolution: A Manifesto for a Sustainable Future; Lloyd’s Register Foundation: London, UK, 2020. [Google Scholar]
- Sloth, J.J.; Holdt, S.L. Setting the Standards for Seaweed Analysis. In New Food; Russell Publishing Ltd.: Kent, UK, 2021. [Google Scholar]
- Cian, R.; Drago, S.; De Medina, F.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Domínguez-González, R.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. Trace Metals in Marine Foodstuff: Bioavailability Estimation and Effect of Major Food Constituents. Food Chem. 2012, 134, 339–345. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Guiry, M.D.M.; Guiry, M.D.M. AlgaeBase; World-Wide Electronic Publication; National Univerity of Ireland: Galway, Ireland, 2021. [Google Scholar]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Webb, P.; Somers, N.K.; Thilsted, S.H. Seaweed’s Contribution to Food Security in Low- and Middle-Income Countries: Benefits from Production, Processing and Trade. Glob. Food Sec 2023, 37, 100686. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-Animal Proteins as Cutting-Edge Ingredients to Reformulate Animal-Free Foodstuffs: Present Status and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6390–6420. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Hayes, M. Red and Green Macroalgae for Fish and Animal Feed and Human Functional Food Development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- van der Heide, M.E.; Stødkilde, L.; Værum Nørgaard, J.; Studnitz, M. The Potential of Locally-Sourced European Protein Sources for Organic Monogastric Production: A Review of Forage Crop Extracts, Seaweed, Starfish, Mussel, and Insects. Sustainability 2021, 13, 2303. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as a Fermentation Substrate: A Challenge for the Food Processing Industry. Processes 2021, 9, 1953. [Google Scholar] [CrossRef]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429154041. [Google Scholar]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; A Science Publishers Book; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315152844. [Google Scholar]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An Opportunity for Wealth and Sustainable Livelihood for Coastal Communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional Quality of Some Wild and Cultivated Seaweeds: Nutrient Composition, Total Phenolic Content and In Vitro Digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Collins, K.; Fitzgerald, G.; Stanton, C.; Ross, R. Looking Beyond the Terrestrial: The Potential of Seaweed Derived Bioactives to Treat Non-Communicable Diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional Value of Proteins from Edible Seaweed Palmaria Palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid Composition, Fatty Acids and Sterols in the Seaweeds Ulva Armoricana, and Solieria Chordalis from Brittany (France): An Analysis from Nutritional, Chemotaxonomic, and Antiproliferative Activity Perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Ng, W.-K. Evaluation of the Proximate, Fatty Acid and Mineral Composition of Representative Green, Brown and Red Seaweeds from the Persian Gulf of Iran as Potential Food and Feed Resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef]
- Polat, S.; Ozogul, Y. Biochemical Composition of Some Red and Brown Macro Algae from the Northeastern Mediterranean Sea. Int. J. Food Sci. Nutr. 2008, 59, 566–572. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO: Rome, Italy, 2003; ISBN 9251049580. [Google Scholar]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Narayan, B.; Kumar, C.S.; Sashima, T.; Maeda, H.; Hosokawa, M.; Miyashita, K. Composition, Functionality and Potential Applications of Seaweed Lipids. In Biocatalysis and Bioenergy; Wiley: Hoboken, NJ, USA, 2008; pp. 463–490. [Google Scholar]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae Food Products as a Healthcare Solution. Mar. Drugs 2023, 21, 578. [Google Scholar] [CrossRef]
- Cotas, J.; Lomartire, S.; Gonçalves, A.M.M.; Pereira, L. From Ocean to Medicine: Harnessing Seaweed’s Potential for Drug Development. Int. J. Mol. Sci. 2024, 25, 797. [Google Scholar] [CrossRef]
- Circuncis, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Batty, L. Metal Content of Seaweeds in the Vicinity of Acid Mine Drainage in Amlwch, North Wales, U.K. Indian. J. Mar. Sci. 2013, 42, 16–20. [Google Scholar]
- Vellinga, R.E.; Sam, M.; Verhagen, H.; Jakobsen, L.S.; Ravn-Haren, G.; Sugimoto, M.; Torres, D.; Katagiri, R.; Thu, B.J.; Granby, K.; et al. Increasing Seaweed Consumption in the Netherlands and Portugal and the Consequences for the Intake of Iodine, Sodium, and Exposure to Chemical Contaminants: A Risk-Benefit Study. Front. Nutr. 2022, 8, 792923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace Elements and the Thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- American Cancer Society. Arsenic and Cancer Risk. Available online: https://www.cancer.org/cancer/risk-prevention/chemicals/arsenic.html#:~:t (accessed on 15 December 2023).
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M. Biosorption of Heavy Metal Ions from Aqueous Solution by Red Macroalgae. J. Hazard. Mater. 2011, 192, 1827–1835. [Google Scholar] [CrossRef]
- Banach, J.L.; van den Burg, S.W.K.; van der Fels-Klerx, H.J. Food Safety during Seaweed Cultivation at Offshore Wind Farms: An Exploratory Study in the North Sea. Mar. Policy 2020, 120, 104082. [Google Scholar] [CrossRef]
- Grebe, G.S.; Byron, C.J.; St. Gelais, A.; Kotowicz, D.M.; Olson, T.K. An Ecosystem Approach to Kelp Aquaculture in the Americas and Europe. Aquac. Rep. 2019, 15, 100215. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Gianello, D.; Ávila-Hernández, E.; Aguer, I.; Crettaz-Minaglia, M.C. Water Quality Assessment of a Temperate Urban Lagoon Using Physico-Chemical and Biological Indicators. SN Appl. Sci. 2019, 1, 470. [Google Scholar] [CrossRef]
- Rahikainen, M.; Yang, B. Macroalgae as Food and Feed Ingredients in the Baltic Sea Region—Regulation by the European Union; Growing Algae Sustainably in the Baltic Sea (GRASS) Project, University of Turku: Turku, Finland, 2020. [Google Scholar]
- Augyte, S.; Yarish, C.; Redmond, S.; Kim, J.K. Cultivation of a Morphologically Distinct Strain of the Sugar Kelp, Saccharina Latissima Forma Angustissima, from Coastal Maine, USA, with Implications for Ecosystem Services. J. Appl. Phycol. 2017, 29, 1967–1976. [Google Scholar] [CrossRef]
- Concepcion, A.; DeRosia-Banick, K.; Balcom, N. Seaweed Production and Processing in Connecticut: A Guide to Understanding and Controlling Potential Food Safety Hazards; NOAA: Washington, DC, USA, 2020.
- Campbell, I.; Kambey, C.S.B.; Mateo, J.P.; Rusekwa, S.B.; Hurtado, A.Q.; Msuya, F.E.; Stentiford, G.D.; Cottier-Cook, E.J. Biosecurity Policy and Legislation for the Global Seaweed Aquaculture Industry. J. Appl. Phycol. 2020, 32, 2133–2146. [Google Scholar] [CrossRef]
- WHO. Food Safety; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- Suutari, M.; Leskinen, E.; Spilling, K.; Kostamo, K.; Seppälä, J. Nutrient Removal by Biomass Accumulation on Artificial Substrata in the Northern Baltic Sea. J. Appl. Phycol. 2017, 29, 1707–1720. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino Acid Composition, Protein Content, and Nitrogen-to-Protein Conversion Factors of 21 Seaweed Species from Norwegian Waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Guo, J.; Qi, M.; Chen, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Macroalgae-Derived Multifunctional Bioactive Substances: The Potential Applications for Food and Pharmaceuticals. Foods 2022, 11, 3455. [Google Scholar] [CrossRef] [PubMed]
- Desideri, D.; Cantaluppi, C.; Ceccotto, F.; Meli, M.A.; Roselli, C.; Feduzi, L. Essential and Toxic Elements in Seaweeds for Human Consumption. J. Toxicol. Environ. Health A 2016, 79, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.A. Biosorption of Heavy Metals by Marine Algae. Curr. Microbiol. 2000, 41, 232–238. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Effects of Phlorotannins on Organisms: Focus on the Safety, Toxicity, and Availability of Phlorotannins. Foods 2021, 10, 452. [Google Scholar] [CrossRef]
- Nutrition and Food Safety (NFS). Basic Food Safety for Health Workers; Nutrition and Food Safety (NFS): Geneva, Switzerland, 1999. [Google Scholar]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in Edible Seaweed: Health Benefits and Food Safety Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Klemetsdal, G.; Ergon, Å. Cultivar Development of Kelps for Commercial Cultivation—Past Lessons and Future Prospects. Front. Mar. Sci. 2020, 8, 110. [Google Scholar] [CrossRef]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Roleda, M.Y.; Hurd, C.L. Seaweed Nutrient Physiology: Application of Concepts to Aquaculture and Bioremediation. Phycologia 2019, 58, 552–562. [Google Scholar] [CrossRef]
- Hafting, J.T.; Critchley, A.T.; Cornish, M.L.; Hubley, S.A.; Archibald, A.F. On-Land Cultivation of Functional Seaweed Products for Human Usage. J. Appl. Phycol. 2012, 24, 385–392. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and Challenges for Industrial Production of Seaweed Bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Snethlage, J.S.; de Koning, S.; Giesbers, E.; Veraart, J.A.; Debrot, A.O.; Harkes, I.; van den Burg, S.W.K.; Hamon, K.G. Knowledge Needs in Realising the Full Potential of Seaweed for World Food Provisioning. Glob. Food Secur. 2023, 37, 100692. [Google Scholar] [CrossRef]
- Farkas, J. Physical Methods of Food Preservation. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2007; pp. 685–712. [Google Scholar]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods; Wiley: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Skonberg, D.I.; Fader, S.; Perkins, L.B.; Perry, J.J. Lactic Acid Fermentation in the Development of a Seaweed Sauerkraut-style Product: Microbiological, Physicochemical, and Sensory Evaluation. J. Food Sci. 2021, 86, 334–342. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Yoha, K.S.; Moses, J.A. Drying of Seaweed: Approaches, Challenges and Research Needs. Trends Food Sci. Technol. 2023, 138, 153–163. [Google Scholar] [CrossRef]
- Regal, A.L.; Alves, V.; Gomes, R.; Matos, J.; Bandarra, N.M.; Afonso, C.; Cardoso, C. Drying Process, Storage Conditions, and Time Alter the Biochemical Composition and Bioactivity of the Anti-Greenhouse Seaweed Asparagopsis Taxiformis. Eur. Food Res. Technol. 2020, 246, 781–793. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of Different Drying Temperatures on the Moisture and Phytochemical Constituents of Edible Irish Brown Seaweed. LWT—Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Leite, B.; Antunes, R.; Cotas, J.; Martins, N.; Costa, N.; Noronha, J.P.; Mata, P.; Diniz, M. Modified Atmosphere Packaging (MAP) for Seaweed Conservation: Impact on Physicochemical Characteristics and Microbiological Activity. Foods 2023, 12, 2736. [Google Scholar] [CrossRef]
- Moreira Leite, B.S. Novas Alternativas Para o Uso de Macroalgas Da Costa Portuguesa Em Alimentação. Master’s Thesis, Universidade Nova de Lisboa, Lisboa, Portugal, 2017. [Google Scholar]
- Cascais, M.; Monteiro, P.; Pacheco, D.; Cotas, J.; Pereira, L.; Marques, J.C.; Gonçalves, A.M.M. Effects of Heat Treatment Processes: Health Benefits and Risks to the Consumer. Appl. Sci. 2021, 11, 8740. [Google Scholar] [CrossRef]
- Vucko, M.J.; Magnusson, M.; Kinley, R.D.; Villart, C.; de Nys, R. The Effects of Processing on the in Vitro Antimethanogenic Capacity and Concentration of Secondary Metabolites of Asparagopsis Taxiformis. J. Appl. Phycol. 2017, 29, 1577–1586. [Google Scholar] [CrossRef]
- Banach, J.L.; Hoek-van den Hil, E.F.; van der Fels-Klerx, H.J. Food Safety Hazards in the European Seaweed Chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- Nitschke, U.; Stengel, D.B. Quantification of Iodine Loss in Edible Irish Seaweeds during Processing. J. Appl. Phycol. 2016, 28, 3527–3533. [Google Scholar] [CrossRef]
- WHO. Cyanobacterial Toxins: Anatoxin-a and Analogues; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Wright, A. What Are Good Manufacturing Practices in the Food Industry? Available online: https://www.imsm.com/gb/blogs/what-are-good-manufacturing-practices-in-the-food-industry/ (accessed on 20 December 2023).
- NQA. GMP: Food Safety Management. Available online: https://www.nqa.com/en-gb/certification/standards/gmp (accessed on 20 December 2023).
- Chon, J.; Koo, R.; Song, K.; Kang, I.; Kim, D.; Bae, D.; Kim, H.; Kim, S.; Seo, K. Strategies for Expanding HACCP Certification Rate Using an Awareness Survey of Dairy Farmers. Int. J. Dairy. Technol. 2021, 74, 453–461. [Google Scholar] [CrossRef]
- Suherman, S.; Janitra, A.A.; Budhiary, K.N.S.; Pratiwi, W.Z.; Idris, F.A. Review on Hazard Analysis and Critical Control Point (HACCP) in the Dairy Product: Cheese. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012081. [Google Scholar] [CrossRef]
- Food Safety Management—ISO 22000:2018. Available online: https://www.iso.org/publication/PUB100430.html (accessed on 20 December 2023).
- NQA. Guide to ISO 22000. Available online: https://www.nqa.com/en-ca/resources/blog/february-2019/guide-to-iso-22000 (accessed on 20 December 2023).
- ISO 22000:2018—Food Safety Management Systems, a Practical Guide. Available online: https://www.iso.org/publication/PUB100454.html (accessed on 20 December 2023).
- Monteiro, P.; Cotas, J.; Pacheco, D.; Figueirinha, A.; da Silva, G.J.; Pereira, L.; Gonçalves, A.M.M. Seaweed as Food: How to Guarantee Their Quality? In Sustainable Global Resources of Seaweeds Volume 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 309–321. [Google Scholar]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- AOAC Scientific Standards & Methods—AOAC International. Available online: https://www.aoac.org/scientific-solutions/ (accessed on 20 November 2023).
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of Electronic Nose as a Non-Invasive Technique for Odor Fingerprinting and Detection of Bacterial Foodborne Pathogens: A Review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Anwar, H.; Anwar, T.; Murtaza, S. Review on Food Quality Assessment Using Machine Learning and Electronic Nose System. Biosens. Bioelectron. X 2023, 14, 100365. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Song, G.; Huang, K.; Luo, Y.; Liu, Q.; He, X.; Cheng, N. Intelligent Biosensing Strategies for Rapid Detection in Food Safety: A Review. Biosens. Bioelectron. 2022, 202, 114003. [Google Scholar] [CrossRef]
- Mahmudiono, T.; Olegovich Bokov, D.; Abdalkareem Jasim, S.; Kamal Abdelbasset, W.; Dinora, M. Khashirbaeva State-of-the-Art of Convenient and Low-Cost Electrochemical Sensor for Food Contamination Detection: Technical and Analytical Overview. Microchem. J. 2022, 179, 107460. [Google Scholar] [CrossRef]
- Kaufmann, A. The Current Role of High-Resolution Mass Spectrometry in Food Analysis. Anal. Bioanal. Chem. 2012, 403, 1233–1249. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldra, F. (Eds.) Food Analysis by HPLC; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429151620. [Google Scholar]
- Kholafazad Kordasht, H.; Hasanzadeh, M. Biomedical Analysis of Exosomes Using Biosensing Methods: Recent Progress. Anal. Methods 2020, 12, 2795–2811. [Google Scholar] [CrossRef]
- Huang, X.; Chalmers, A.N. Review of Wearable and Portable Sensors for Monitoring Personal Solar UV Exposure. Ann. Biomed. Eng. 2021, 49, 964–978. [Google Scholar] [CrossRef]
- Kholafazad-Kordasht, H.; Hasanzadeh, M.; Seidi, F. Smartphone Based Immunosensors as next Generation of Healthcare Tools: Technical and Analytical Overview towards Improvement of Personalized Medicine. TrAC Trends Anal. Chem. 2021, 145, 116455. [Google Scholar] [CrossRef]
- Haque, F.; Bubli, S.Y.; Khan, M.S. UV–Vis Spectroscopy for Food Analysis. In Techniques to Measure Food Safety and Quality; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–193. [Google Scholar]
- Karoui, R. Spectroscopic Technique: Fluorescence and Ultraviolet-Visible (UV-Vis) Spectroscopies. In Modern Techniques for Food Authentication; Elsevier: Amsterdam, The Netherlands, 2018; pp. 219–252. [Google Scholar]
- Claßen, J.; Aupert, F.; Reardon, K.F.; Solle, D.; Scheper, T. Spectroscopic Sensors for In-Line Bioprocess Monitoring in Research and Pharmaceutical Industrial Application. Anal. Bioanal. Chem. 2017, 409, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Kharbach, M.; Alaoui Mansouri, M.; Taabouz, M.; Yu, H. Current Application of Advancing Spectroscopy Techniques in Food Analysis: Data Handling with Chemometric Approaches. Foods 2023, 12, 2753. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.; Callejón, R.M. Assessment of UV–Visible Spectroscopy as a Useful Tool for Determining Grape-Must Caramel in High-Quality Wine and Balsamic Vinegars. Food Chem. 2020, 323, 126792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, N.; Feng, Y.; Su, S.; Li, T.; Liang, B. A Unique Quantitative Method of Acid Value of Edible Oils and Studying the Impact of Heating on Edible Oils by UV–Vis Spectrometry. Food Chem. 2015, 185, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Bian, X.; Lin, E.; Wang, H.; Guo, Y.; Tan, X. Weighted Multiscale Support Vector Regression for Fast Quantification of Vegetable Oils in Edible Blend Oil by Ultraviolet-Visible Spectroscopy. Food Chem. 2021, 342, 128245. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and Characterization of Phenolic Antioxidant Compounds from Brown Irish Seaweed Himanthalia Elongata Using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Barnes, M.; Sulé-Suso, J.; Millett, J.; Roach, P. Fourier Transform Infrared Spectroscopy as a Non-Destructive Method for Analysing Herbarium Specimens. Biol. Lett. 2023, 19, 20220546. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13C-NMR Spectroscopy for Identification of Some Seaweed Phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Druzhinina, A.; Ovchinnikov, D.; Krasikov, V.; Khviyuzov, S. Physicochemical Characteristics of the Active Fractions of Polyphenols from Arctic Macrophytes. J. Appl. Phycol. 2020, 32, 4277–4287. [Google Scholar] [CrossRef]
- Cotas, J.; Figueirinha, A.; Pereira, L.; Batista, T. The Effect of Salinity on Fucus Ceranoides (Ochrophyta, Phaeophyceae) in the Mondego River (Portugal). J. Oceanol. Limnol. 2019, 37, 881–891. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the Commercial Seaweed Nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.F.; Lee, T.Z.E.; Mohd Irwan Lu, N.A.L.; Samling, B. Synchronized Analysis of FTIR Spectra and GCMS Chromatograms for Evaluation of the Thermally Degraded Vegetable Oils. J. Anal. Methods Chem. 2014, 2014, 271970. [Google Scholar] [CrossRef] [PubMed]
- Galvis-Sánchez, A.C.; Barros, A.; Delgadillo, I. FTIR-ATR Infrared Spectroscopy for the Detection of Ochratoxin A in Dried Vine Fruit. Food Addit. Contam. 2007, 24, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.I.N.; Pereira, C.G.; Andrade, J.; Vicentini, N.M.; Bell, M.J.V.; Anjos, V. FTIR-ATR Spectroscopy as a Tool for the Rapid Detection of Adulterations in Butter Cheeses. LWT 2019, 109, 63–69. [Google Scholar] [CrossRef]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and Quality Assessment of Meat Products by Fourier-Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- Al-Deen, R.B.; Azizieh, A.; Al-Ameer, L. Identification of Enterobacteriaceae Foodborne Bacteria in Syrian Foods by PCR and FTIR-ATR Techniques. Adv. Environ. Biol. 2014, 2014, 1233. [Google Scholar]
- Keshavarzi, Z.; Barzegari Banadkoki, S.; Faizi, M.; Zolghadri, Y.; Shirazi, F.H. Comparison of Transmission FTIR and ATR Spectra for Discrimination between Beef and Chicken Meat and Quantification of Chicken in Beef Meat Mixture Using ATR-FTIR Combined with Chemometrics. J. Food Sci. Technol. 2020, 57, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR Spectroscopy to the Quantification of Sugar in Honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Vandanjon, L.; Burlot, A.-S.; Zamanileha, E.F.; Douzenel, P.; Ravelonandro, P.H.; Bourgougnon, N.; Bedoux, G. The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Mar. Drugs 2023, 21, 482. [Google Scholar] [CrossRef]
- Amin, H.H. Safe Ulvan Silver Nanoparticles Composite Films for Active Food Packaging. Am. J. Biochem. Biotechnol. 2021, 17, 28–39. [Google Scholar] [CrossRef]
- Qu, J.-H.; Liu, D.; Cheng, J.-H.; Sun, D.-W.; Ma, J.; Pu, H.; Zeng, X.-A. Applications of Near-Infrared Spectroscopy in Food Safety Evaluation and Control: A Review of Recent Research Advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ying, Y. Theory and Application of near Infrared Spectroscopy in Assessment of Fruit Quality: A Review. Sens. Instrum. Food Qual. Saf. 2009, 3, 130–141. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Defraeye, T.; De Ketelaere, B.; Herremans, E.; Hertog, M.L.A.T.M.; Saeys, W.; Torricelli, A.; Vandendriessche, T.; Verboven, P. Nondestructive Measurement of Fruit and Vegetable Quality. Annu. Rev. Food Sci. Technol. 2014, 5, 285–312. [Google Scholar] [CrossRef]
- Pojić, M.M.; Mastilović, J.S. Near Infrared Spectroscopy—Advanced Analytical Tool in Wheat Breeding, Trade, and Processing. Food Bioproc Tech. 2013, 6, 330–352. [Google Scholar] [CrossRef]
- Morsy, N.; Sun, D.-W. Robust Linear and Non-Linear Models of NIR Spectroscopy for Detection and Quantification of Adulterants in Fresh and Frozen-Thawed Minced Beef. Meat Sci. 2013, 93, 292–302. [Google Scholar] [CrossRef]
- Tito, N.B.; Rodemann, T.; Powell, S.M. Use of near Infrared Spectroscopy to Predict Microbial Numbers on Atlantic Salmon. Food Microbiol. 2012, 32, 431–436. [Google Scholar] [CrossRef]
- Kurz, C.; Leitenberger, M.; Carle, R.; Schieber, A. Evaluation of Fruit Authenticity and Determination of the Fruit Content of Fruit Products Using FT-NIR Spectroscopy of Cell Wall Components. Food Chem. 2010, 119, 806–812. [Google Scholar] [CrossRef]
- Kuligowski, J.; Carrión, D.; Quintás, G.; Garrigues, S.; de la Guardia, M. Direct Determination of Polymerised Triacylglycerides in Deep-Frying Vegetable Oil by near Infrared Spectroscopy Using Partial Least Squares Regression. Food Chem. 2012, 131, 353–359. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Melamine Detection by Mid- and near-Infrared (MIR/NIR) Spectroscopy: A Quick and Sensitive Method for Dairy Products Analysis Including Liquid Milk, Infant Formula, and Milk Powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef]
- Mignani, A.G.; Ciaccheri, L.; Ottevaere, H.; Thienpont, H.; Conte, L.; Marega, M.; Cichelli, A.; Attilio, C.; Cimato, A. Visible and Near-Infrared Absorption Spectroscopy by an Integrating Sphere and Optical Fibers for Quantifying and Discriminating the Adulteration of Extra Virgin Olive Oil from Tuscany. Anal. Bioanal. Chem. 2011, 399, 1315–1324. [Google Scholar] [CrossRef]
- Prieto, N.; Andrés, S.; Giráldez, F.J.; Mantecón, A.R.; Lavín, P. Discrimination of Adult Steers (Oxen) and Young Cattle Ground Meat Samples by near Infrared Reflectance Spectroscopy (NIRS). Meat Sci. 2008, 79, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Flores, K.; Sánchez, M.-T.; Pérez-Marín, D.; Guerrero, J.-E.; Garrido-Varo, A. Feasibility in NIRS Instruments for Predicting Internal Quality in Intact Tomato. J. Food Eng. 2009, 91, 311–318. [Google Scholar] [CrossRef]
- Yuan, W.; Xiang, B.; Yu, L.; Xu, J. A Non-Invasive Method for Screening Sodium Hydroxymethanesulfonate in Wheat Flour by Near-Infrared Spectroscopy. Food Anal. Methods 2011, 4, 550–558. [Google Scholar] [CrossRef]
- Lytou, A.E.; Tsakanikas, P.; Lymperi, D.; Nychas, G.-J.E. Rapid Assessment of Microbial Quality in Edible Seaweeds Using Sensor Techniques Based on Spectroscopy, Imaging Analysis and Sensors Mimicking Human Senses. Sensors 2022, 22, 7018. [Google Scholar] [CrossRef] [PubMed]
- Tadmor Shalev, N.; Ghermandi, A.; Tchernov, D.; Shemesh, E.; Israel, A.; Brook, A. NIR Spectroscopy and Artificial Neural Network for Seaweed Protein Content Assessment In-Situ. Comput. Electron. Agric. 2022, 201, 107304. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Koidis, A.; Theodoridou, K. The Use of Near-Infrared and Mid-Infrared Spectroscopy to Rapidly Measure the Nutrient Composition and the in Vitro Rumen Dry Matter Digestibility of Brown Seaweeds. Anim. Feed. Sci. Technol. 2022, 285, 115239. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, F.; Xu, Y.; Cong, Z.; Wang, E. Applications of Electrochemical Techniques in Mineral Analysis. Talanta 2014, 127, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, S.; Zesiger, T.; Gustafsson, V.; Lundén, A.; Bosset, J. Determination of Trimethylamine in Milk Using an MS Based Electronic Nose. Eur. Food Res. Technol. 2002, 214, 163–167. [Google Scholar] [CrossRef]
- Lippolis, V.; Cervellieri, S.; Damascelli, A.; Pascale, M.; Di Gioia, A.; Longobardi, F.; De Girolamo, A. Rapid Prediction of Deoxynivalenol Contamination in Wheat Bran by MOS-based Electronic Nose and Characterization of the Relevant Pattern of Volatile Compounds. J. Sci. Food Agric. 2018, 98, 4955–4962. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and Growth Tracking of Fungi Contamination in Peaches Using Electronic Nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Sun, L.; Zhao, G.; Huang, X. Application of Electronic Nose for Measuring Total Volatile Basic Nitrogen and Total Viable Counts in Packaged Pork During Refrigerated Storage. J. Food Sci. 2016, 81, M906–M912. [Google Scholar] [CrossRef] [PubMed]
- Pallottino, F.; Costa, C.; Antonucci, F.; Strano, M.C.; Calandra, M.; Solaini, S.; Menesatti, P. Electronic Nose Application for Determination of Penicillium Digitatum in Valencia Oranges. J. Sci. Food Agric. 2012, 92, 2008–2012. [Google Scholar] [CrossRef]
- Pattarapon, P.; Zhang, M.; Bhandari, B.; Gao, Z. Effect of Vacuum Storage on the Freshness of Grass Carp ( Ctenopharyngodon Idella ) Fillet Based on Normal and Electronic Sensory Measurement. J. Food Process Preserv. 2018, 42, e13418. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Liu, T.; Liu, Y. Prediction of Total Viable Counts on Chilled Pork Using an Electronic Nose Combined with Support Vector Machine. Meat Sci. 2012, 90, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Azman, W.N.F.S.W.; Azir, K.N.F.b.K.; Khairuddin, A.b.M. E-Nose: Spoiled Food Detection Embedded Device Using Machine Learning for Food Safety Application. In Computing and Informatics; Springer: Singapore, 2024; pp. 221–234. [Google Scholar]
- Dai, C.; Huang, X.; Lv, R.; Zhang, Z.; Sun, J.; Aheto, J.H. Analysis of Volatile Compounds of Tremella Aurantialba Fermentation via Electronic Nose and HS-SPME-GC-MS. J. Food Saf. 2018, 38, e12555. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Xu, H.; Aheto, J.H.; Bonah, E.; Ma, M.; Wu, M.; Zhang, X. Rapid and Nondestructive Detection of Freshness Quality of Postharvest Spinaches Based on Machine Vision and Electronic Nose. J. Food Saf. 2019, 39, e12708. [Google Scholar] [CrossRef]
- Shen, H.; Tao, J. Applying Electronic Nose Based on Odour Classification and Identification Technology in Detecting the Shelf Life of Fresh Fruits. Chem. Eng. Trans. 2018, 68, 217–222. [Google Scholar] [CrossRef]
- Chongthanaphisut, P.; Seesaard, T.; Kerdcharoen, T. Monitoring of Microbial Canned Food Spoilage and Contamination Based on E-Nose for Smart Home. In Proceedings of the 2015 12th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology (ECTI-CON), Hua Hin, Thailand, 24–27 June 2015; IEEE: New York, NY, USA, 2015; pp. 1–5. [Google Scholar]
- Zarezadeh, M.R.; Aboonajmi, M.; Varnamkhasti, M.G.; Azarikia, F. Olive Oil Classification and Fraud Detection Using E-Nose and Ultrasonic System. Food Anal. Methods 2021, 14, 2199–2210. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Huang, J.; Gao, P.; Jin, Q.; Wang, X. Rapid Measuring Flavor Quality Changes of Frying Rapeseed Oils Using a Flash Gas Chromatography Electronic Nose. Eur. J. Lipid Sci. Technol. 2019, 121, 1800260. [Google Scholar] [CrossRef]
- Singh, S.; Gaur, S. Development of Rapid and Non-Destructive Electric Nose (E-Nose) System for Shelf Life Evaluation of Different Edible Seeds. Food Chem. 2023, 426, 136562. [Google Scholar] [CrossRef]
- McCaig, T.N. Extending the Use of Visible/near-Infrared Reflectance Spectrophotometers to Measure Colour of Food and Agricultural Products. Food Res. Int. 2002, 35, 731–736. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Colour Measurements by Computer Vision for Food Quality Control—A Review. Trends Food Sci. Technol. 2013, 29, 5–20. [Google Scholar] [CrossRef]
- YongXia, C.; RuiXin, L.; ZhaoZhou, L.; PengJu, C.; LiLi, W.; YanLi, W.; SuiQing, C. Quality Evaluation Based on Color Grading: Quality Discrimination of the Chinese Medicine Corni Fructus by an E-Eye. Sci. Rep. 2019, 9, 17006. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Z.; Song, C.; Li, C.; Peng, Z.; Xu, W. E-Eye. In Proceedings of the 16th ACM Conference on Embedded Networked Sensor Systems, New York, NY, USA, 4–7 November 2018; ACM: New York, NY, USA, 2018; pp. 68–81. [Google Scholar]
- Calvini, R.; Pigani, L. Toward the Development of Combined Artificial Sensing Systems for Food Quality Evaluation: A Review on the Application of Data Fusion of Electronic Noses, Electronic Tongues and Electronic Eyes. Sensors 2022, 22, 577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Cui, Q.; Li, L.; Ning, J.; Zhang, Z. Monitoring the Withering Condition of Leaves during Black Tea Processing via the Fusion of Electronic Eye (E-Eye), Colorimetric Sensing Array (CSA), and Micro-near-Infrared Spectroscopy (NIRS). J. Food Eng. 2021, 300, 110534. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioproc Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Finardi, S.; de Souza, C.K.; Meinert, C.; Pateiro, M.; Hoffmann, T.G.; Domínguez, R.; Bertoli, S.L.; Kumar, M.; Lorenzo, J.M. Applications of Electronic Nose, Electronic Eye and Electronic Tongue in Quality, Safety and Shelf Life of Meat and Meat Products: A Review. Sensors 2023, 23, 672. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Malegori, C.; Benedetti, S.; Oliveri, P.; Giovanelli, G. E-Nose, e-Tongue and e-Eye for Edible Olive Oil Characterization and Shelf Life Assessment: A Powerful Data Fusion Approach. Talanta 2018, 182, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Foschi, M.; Di Maria, V.; D’Archivio, A.A.; Marini, F.; Biancolillo, A. E-Eye-Based Approach for Traceability and Annuality Compliance of Lentils. Appl. Sci. 2023, 13, 1433. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality Evaluation of Fish and Other Seafood by Traditional and Nondestructive Instrumental Methods: Advantages and Limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef]
- Titova, T.; Nachev, V. “Electronic Tongue” in the Food Industry. Food Sci. Appl. Biotechnol. 2020, 3, 71. [Google Scholar] [CrossRef]
- Surányi, J.; Zaukuu, J.-L.Z.; Friedrich, L.; Kovacs, Z.; Horváth, F.; Németh, C.; Kókai, Z. Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds. Foods 2021, 10, 2283. [Google Scholar] [CrossRef]
- Banerjee, R.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. A Review on Combined Odor and Taste Sensor Systems. J. Food Eng. 2016, 190, 10–21. [Google Scholar] [CrossRef]
- Kaya, Z.; Koca, İ. Gıda Mühendisliğinde Elektronik Dil Uygulamaları. Turk. J. Agric.—Food Sci. Technol. 2020, 8, 1463–1471. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic Tongues—A Review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Chiofalo, V. Electronic Noses and Tongues. In Chemical Analysis of Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 353–389. [Google Scholar]
- Scagion, V.P.; Mercante, L.A.; Sakamoto, K.Y.; Oliveira, J.E.; Fonseca, F.J.; Mattoso, L.H.C.; Ferreira, M.D.; Correa, D.S. An Electronic Tongue Based on Conducting Electrospun Nanofibers for Detecting Tetracycline in Milk Samples. RSC Adv. 2016, 6, 103740–103746. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Teye, E.; Gu, H. Quantitative Analysis of Fish Microbiological Quality Using Electronic Tongue Coupled with Nonlinear Pattern Recognition Algorithms. J. Food Saf. 2015, 35, 336–344. [Google Scholar] [CrossRef]
- Pascual, L.; Gras, M.; Vidal-Brotóns, D.; Alcañiz, M.; Martínez-Máñez, R.; Ros-Lis, J.V. A Voltammetric E-Tongue Tool for the Emulation of the Sensorial Analysis and the Discrimination of Vegetal Milks. Sens. Actuators B Chem. 2018, 270, 231–238. [Google Scholar] [CrossRef]
- Semenov, V.; Volkov, S.; Khaydukova, M.; Fedorov, A.; Lisitsyna, I.; Kirsanov, D.; Legin, A. Determination of Three Quality Parameters in Vegetable Oils Using Potentiometric E-Tongue. J. Food Compos. Anal. 2019, 75, 75–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Yao, Y.; Guo, X.; Wang, R.; Peng, Z. A Preliminary Study: Saltiness and Sodium Content of Aqueous Extracts from Plants and Marine Animal Shells. Eur. Food Res. Technol. 2014, 238, 565–571. [Google Scholar] [CrossRef]
- Gowen, A.; Odonnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral Imaging—An Emerging Process Analytical Tool for Food Quality and Safety Control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Al-Sarayreh, M.; Reis, M.M.; Yan, W.Q.; Klette, R. A Sequential CNN Approach for Foreign Object Detection in Hyperspectral Images. In Computer Analysis of Images and Patterns; Springer: Cham, Switzerland, 2019; pp. 271–283. [Google Scholar]
- Feng, Y.-Z.; Sun, D.-W. Application of Hyperspectral Imaging in Food Safety Inspection and Control: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef]
- Soni, A.; Dixit, Y.; Reis, M.M.; Brightwell, G. Hyperspectral Imaging and Machine Learning in Food Microbiology: Developments and Challenges in Detection of Bacterial, Fungal, and Viral Contaminants. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3717–3745. [Google Scholar] [CrossRef]
- Kim, M.S.; Chao, K.; Chen, Y.-R.; Chan, D.; Mehl, P.M. Hyperspectral Imaging System for Food Safety: Detection of Fecal Contamination on Apples. In Proc. SPIE 4206, Photonic Detection and Intervention Technologies for Safe Food; Chen, Y.-R., Tu, S.-I., Eds.; SPIE: Bellingham, WA, USA, 2001; pp. 174–184. [Google Scholar]
- Kwak, D.-H.; Son, G.-J.; Park, M.-K.; Kim, Y.-D. Rapid Foreign Object Detection System on Seaweed Using VNIR Hyperspectral Imaging. Sensors 2021, 21, 5279. [Google Scholar] [CrossRef]
- El Hosry, L.; Sok, N.; Richa, R.; Al Mashtoub, L.; Cayot, P.; Bou-Maroun, E. Sample Preparation and Analytical Techniques in the Determination of Trace Elements in Food: A Review. Foods 2023, 12, 895. [Google Scholar] [CrossRef]
- McComb, J.Q.; Rogers, C.; Han, F.X.; Tchounwou, P.B. Rapid Screening of Heavy Metals and Trace Elements in Environmental Samples Using Portable X-Ray Fluorescence Spectrometer, A Comparative Study. Water Air Soil. Pollut. 2014, 225, 2169. [Google Scholar] [CrossRef]
- Winberg, P.C. Best Practices for the Emerging Australian Seaweed Industry: Seaweed Quality Control Systems; AgriFutures: Wagga Wagga, NSW, Australia, 2017. [Google Scholar]
- Pashkova, G.V.; Smagunova, A.N.; Finkelshtein, A.L. X-Ray Fluorescence Analysis of Milk and Dairy Products: A Review. TrAC Trends Anal. Chem. 2018, 106, 183–189. [Google Scholar] [CrossRef]
- Gallardo, H.; Queralt, I.; Tapias, J.; Guerra, M.; Carvalho, M.L.; Marguí, E. Possibilities of Low-Power X-Ray Fluorescence Spectrometry Methods for Rapid Multielemental Analysis and Imaging of Vegetal Foodstuffs. J. Food Compos. Anal. 2016, 50, 1–9. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Xu, L.; Wang, S.; Zhou, M.; Yin, J.; Lu, A. Rapid Screening of Cadmium in Rice and Identification of Geographical Origins by Spectral Method. Int. J. Environ. Res. Public. Health 2018, 15, 312. [Google Scholar] [CrossRef]
- Reboredo, F.H.; Junior, W.; Pessoa, M.F.; Lidon, F.C.; Ramalho, J.C.; Leitão, R.G.; Silva, M.M.; Alvarenga, N.; Guerra, M. Elemental Composition of Algae-Based Supplements by Energy Dispersive X-Ray Fluorescence. Plants 2021, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Garshott, D.M.; MacDonald, E.A.; Murray, M.N.; Benvenuto, M.A.; Roberts-Kirchhoff, E.S. Elemental Analysis of a Variety of Dried, Powdered, Kelp Food Supplements for the Presence of Heavy Metals via Energy-Dispersive X-ray Fluorescence Spectrometry. In ACS Symposium Series; ACS Publications: Washington, DC, USA, 2011; pp. 123–133. [Google Scholar]
- Chesori, C.R. Determination of Elemental Concentrations in Edible Seaweeds, Sea Sediments and Seawater Samples from the Kenyan Coast Using X-Ray Fluorescence Techniques. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2015. [Google Scholar]
- García-Sartal, C.; Barciela-Alonso, M.d.C.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Study of Cooking on the Bioavailability of As, Co, Cr, Cu, Fe, Ni, Se and Zn from Edible Seaweed. Microchem. J. 2013, 108, 92–99. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent Updates on Bioaccessibility of Phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Alegría, A.; Garcia-Llatas, G.; Cilla, A. Static Digestion Models: General Introduction. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–12. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, R.; Zhou, H.; Liu, J.; Muriel Mundo, J.; Zhang, R.; McClements, D.J. Impact of Calcium Levels on Lipid Digestion and Nutraceutical Bioaccessibility in Nanoemulsion Delivery Systems Studied Using Standardized INFOGEST Digestion Protocol. Food Funct. 2020, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.R.; Li, Y.; Li, B. Influence of Anionic Alginate and Cationic Chitosan on Physicochemical Stability and Carotenoids Bioaccessibility of Soy Protein Isolate-Stabilized Emulsions. Food Res. Int. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in Vitro and in Vivo Data on Food Digestion. What Can We Predict with Static in Vitro Digestion Models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Review of in Vitro Digestion Models for Rapid Screening of Emulsion-Based Systems. Food Funct. 2010, 1, 32. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, H.; McClements, D.J. Application of Static in Vitro Digestion Models for Assessing the Bioaccessibility of Hydrophobic Bioactives: A Review. Trends Food Sci. Technol. 2022, 122, 314–327. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–22. [Google Scholar]
- Satoor, S.N.; Patil, D.P.; Kristensen, H.D.; Joglekar, M.V.; Shouche, Y.; Hardikar, A.A. Manipulation and Assessment of Gut Microbiome for Metabolic Studies. Methods Mol. Biol. 2014, 1194, 449–469. [Google Scholar]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, Bioaccessibility and Bioactivity of Compounds from Algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current Trends in Marine Algae Polysaccharides: The Digestive Tract, Microbial Catabolism, and Prebiotic Potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Qin, Y. Seaweed Hydrocolloids as Thickening, Gelling, and Emulsifying Agents in Functional Food Products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–152. [Google Scholar]
- Chen, L.; Xu, W.; Chen, D.; Chen, G.; Liu, J.; Zeng, X.; Shao, R.; Zhu, H. Digestibility of Sulfated Polysaccharide from the Brown Seaweed Ascophyllum Nodosum and Its Effect on the Human Gut Microbiota in Vitro. Int. J. Biol. Macromol. 2018, 112, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra Haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Nieto, G. Bioaccessibility, Digestibility and Nutritional Properties of Algae and Cyanophyceae as Basis of Their Potential as Functional Food Ingredients. Appl. Food Res. 2024, 4, 100404. [Google Scholar] [CrossRef]
- Rupérez, P.; Saura-Calixto, F. Dietary Fibre and Physicochemical Properties of Edible Spanish Seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Soares, C.; Sousa, S.; Machado, S.; Vieira, E.; Carvalho, A.P.; Ramalhosa, M.J.; Morais, S.; Correia, M.; Oliva-Teles, T.; Domingues, V.F.; et al. Bioactive Lipids of Seaweeds from the Portuguese North Coast: Health Benefits versus Potential Contamination. Foods 2021, 10, 1366. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.R.L.; Dobbs, J.; Dunn, M.A. Mineral Nutrient Content and Iron Bioavailability in Common and Hawaiian Seaweeds Assessed by an in Vitro Digestion/Caco-2 Cell Model. J. Food Compos. Anal. 2015, 43, 185–193. [Google Scholar] [CrossRef]
- Nakamura, E.; Yokota, H.; Matsui, T. The in Vitro Digestibility and Absorption of Magnesium in Some Edible Seaweeds. J. Sci. Food Agric. 2012, 92, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Chi, L.; Bodnar, W.; Zhang, Z.; Gao, B.; Bian, X.; Stewart, J.; Fry, R.; Lu, K. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Sandhu, K.V.; Griffin, B.T.; Dinan, T.G.; Cryan, J.F.; Hyland, N.P. Gut Reactions: Breaking Down Xenobiotic–Microbiome Interactions. Pharmacol. Rev. 2019, 71, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Lorini, C.; Garamella, G.; Bonaccorsi, G. Seaweeds as a “Palatable” Challenge between Innovation and Sustainability: A Systematic Review of Food Safety. Sustainability 2021, 13, 7652. [Google Scholar] [CrossRef]
- Cottier-Cook, E.J.; Nagabhatla, N.; Asri, A.; Beveridge, M.; Bianchi, P.; Bolton, J.; Bondad-Reantaso, M.G.; Brodie, J.; Buschmann, A.; Cabarubias, J.; et al. Ensuring the Sustainable Future of the Rapidly Expanding Global Seaweed Aquaculture Industry—A Vision; UNU Institute on Comparative Regional Integration Studies: Bruges, Belgium, 2021. [Google Scholar]
- Deepika, C.; Wolf, J.; Moheimani, N.; Hankamer, B.; von Herzen, B.; Rao, A.R. Utilisation of Seaweeds in the Australian Market—Commercialisation Strategies: Current Trends and Future Prospects. In Sustainable Global Resources Of Seaweeds Volume 1; Springer International Publishing: Cham, Switzerland, 2022; pp. 265–294. [Google Scholar]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Noriega Fernández, E. Seaweed Products for the Future: Using Current Tools to Develop a Sustainable Food Industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- Salido, M.; Soto, M.; Seoane, S. Seaweed: Nutritional and Gastronomic Perspective. A Review. Algal Res. 2024, 77, 103357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).