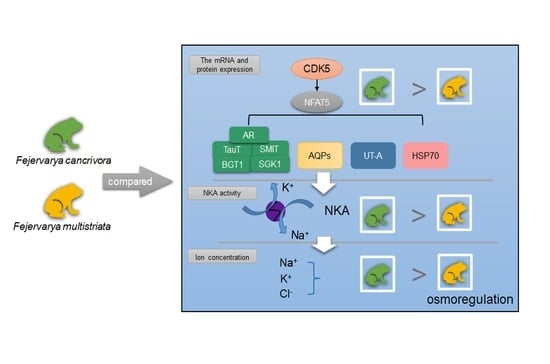
CDK5/NFAT5-Regulated Transporters Involved in Osmoregulation in Fejervarya cancrivora
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sampling
2.2. Biochemical Analysis
2.3. NKA Enzyme Measurement
2.4. Total RNA Extraction and cDNA Synthesis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
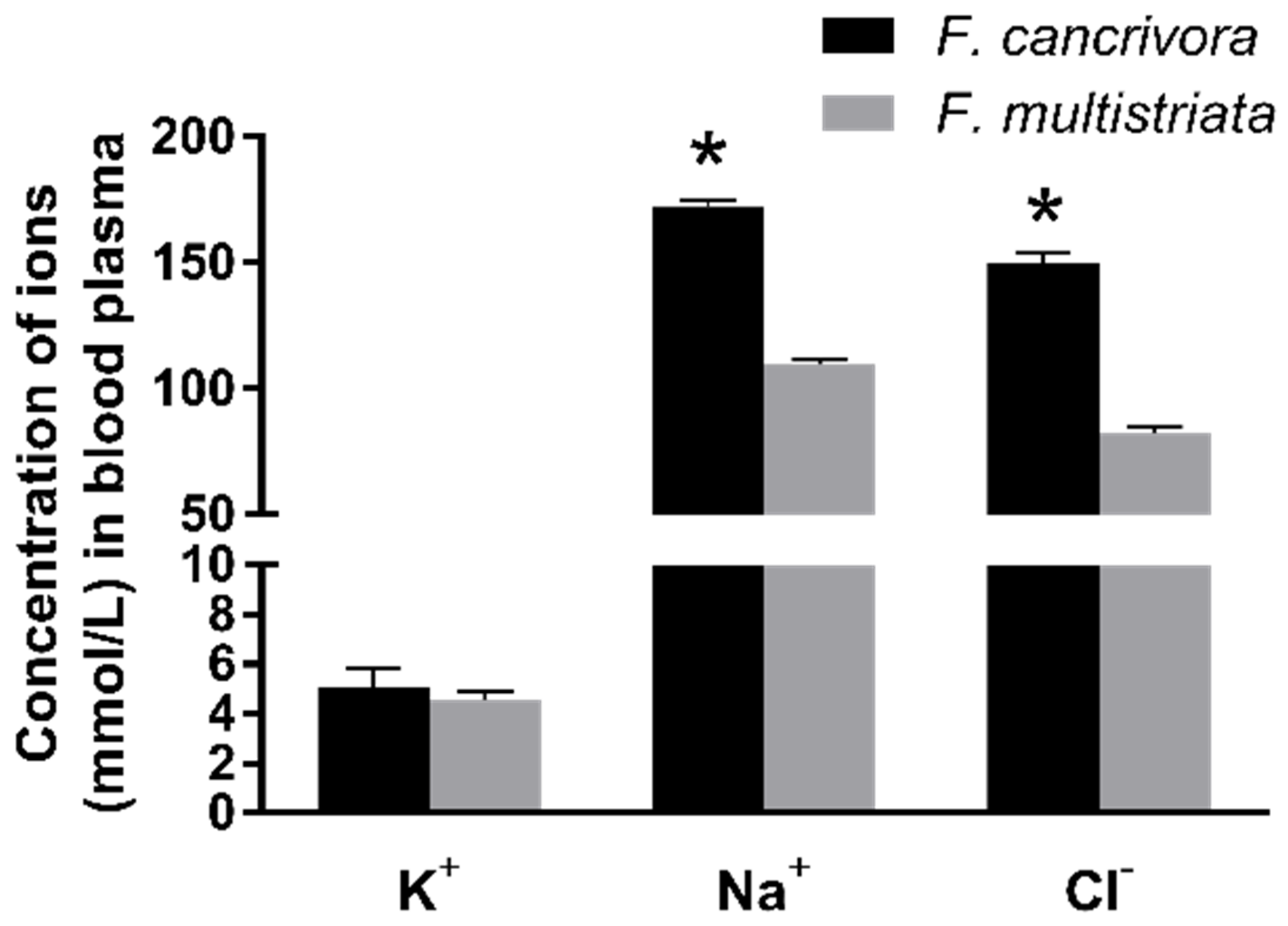
3.1. Ion Concentration in Plasma
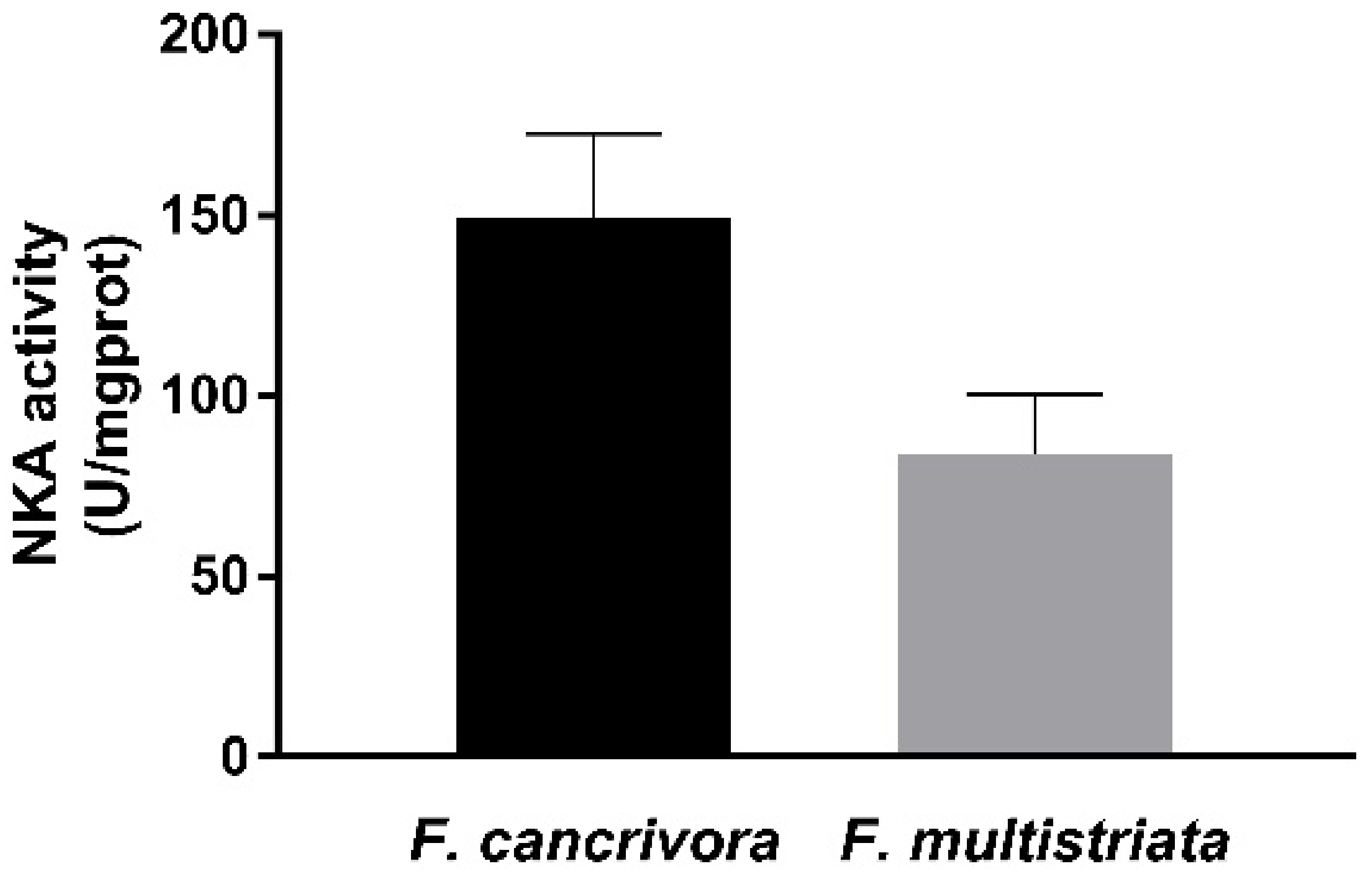
3.2. NKA Enzyme Activity
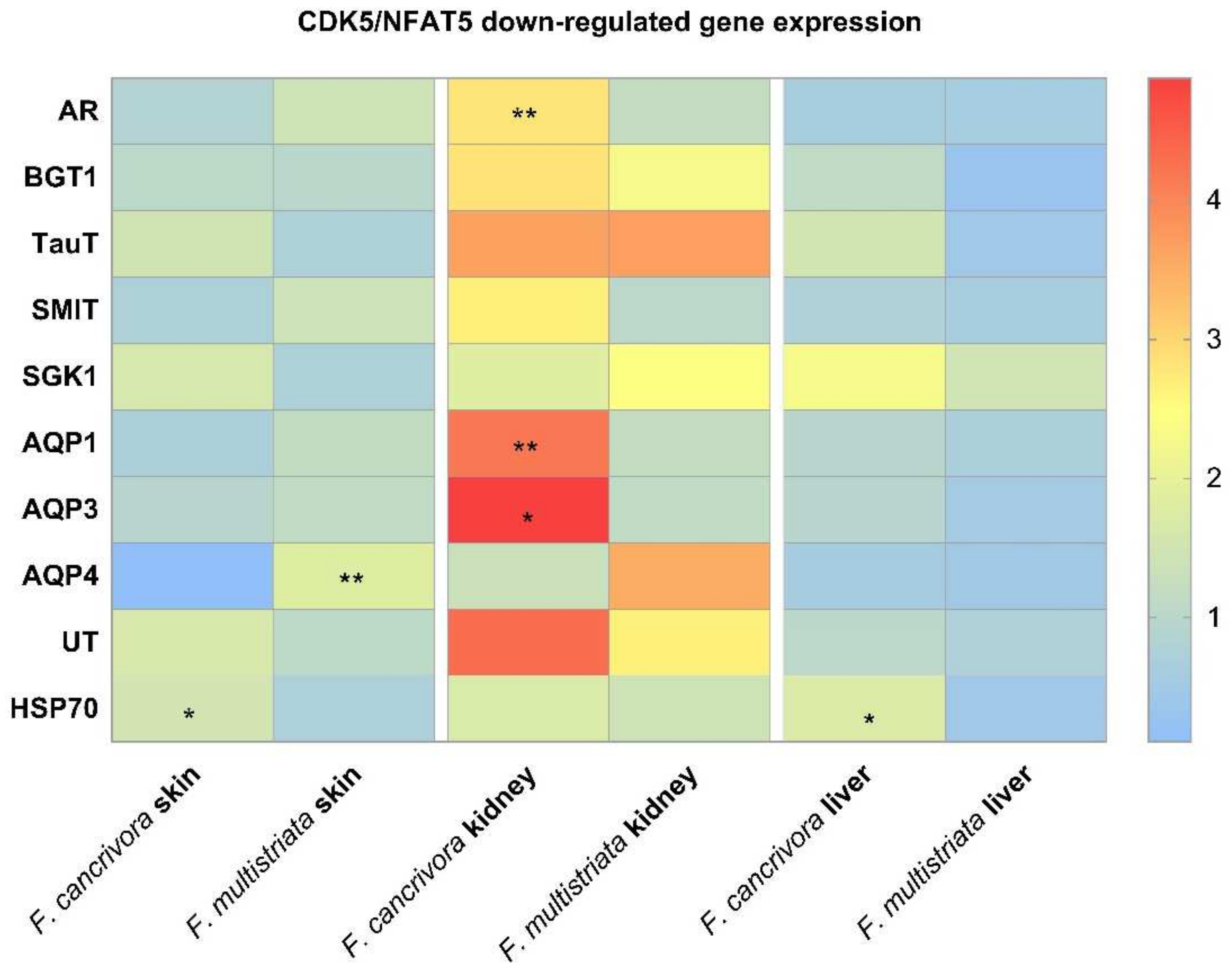
3.3. The mRNA Expression of Osmoregulative Genes
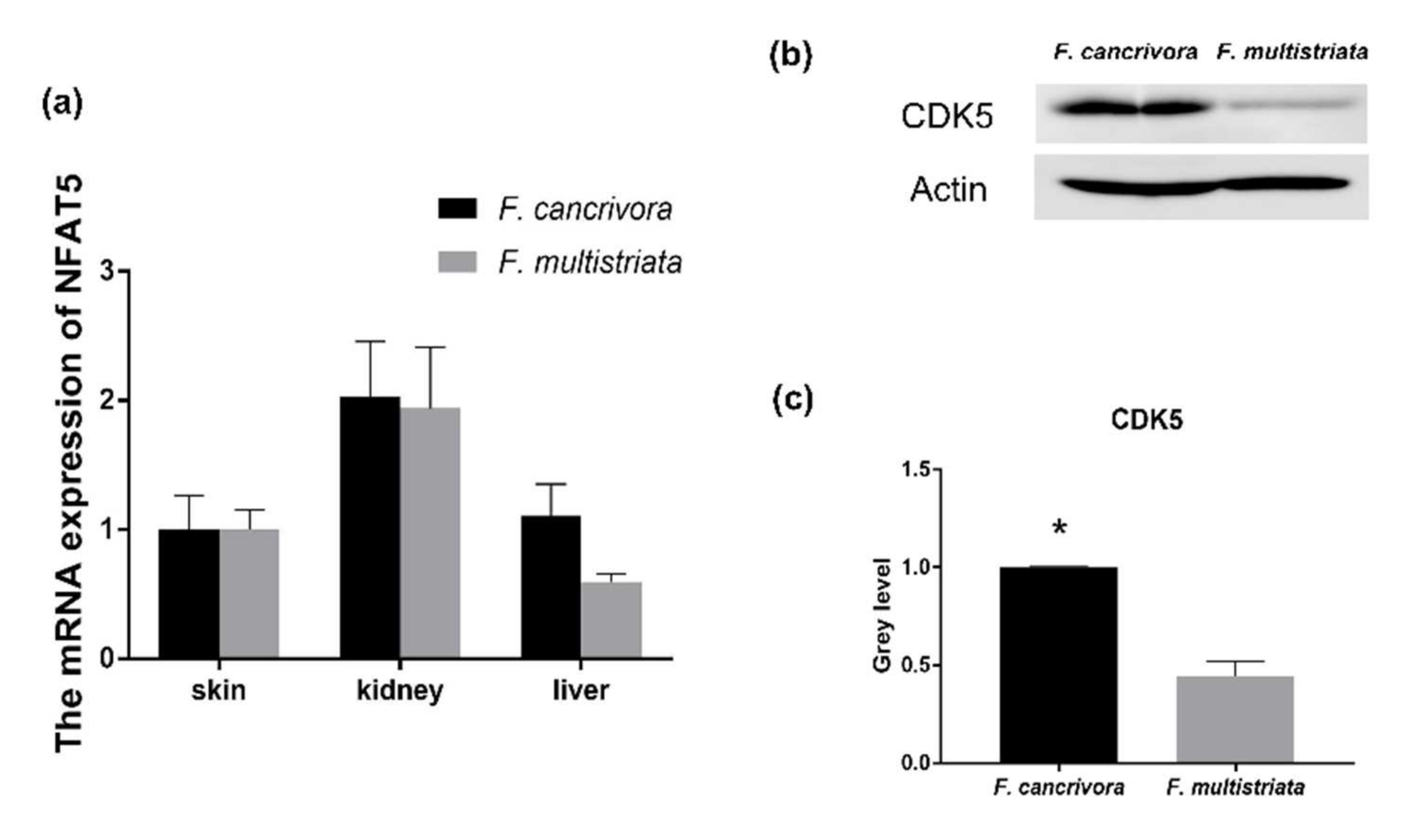
3.4. The Expression of NFAT5 and CDK5
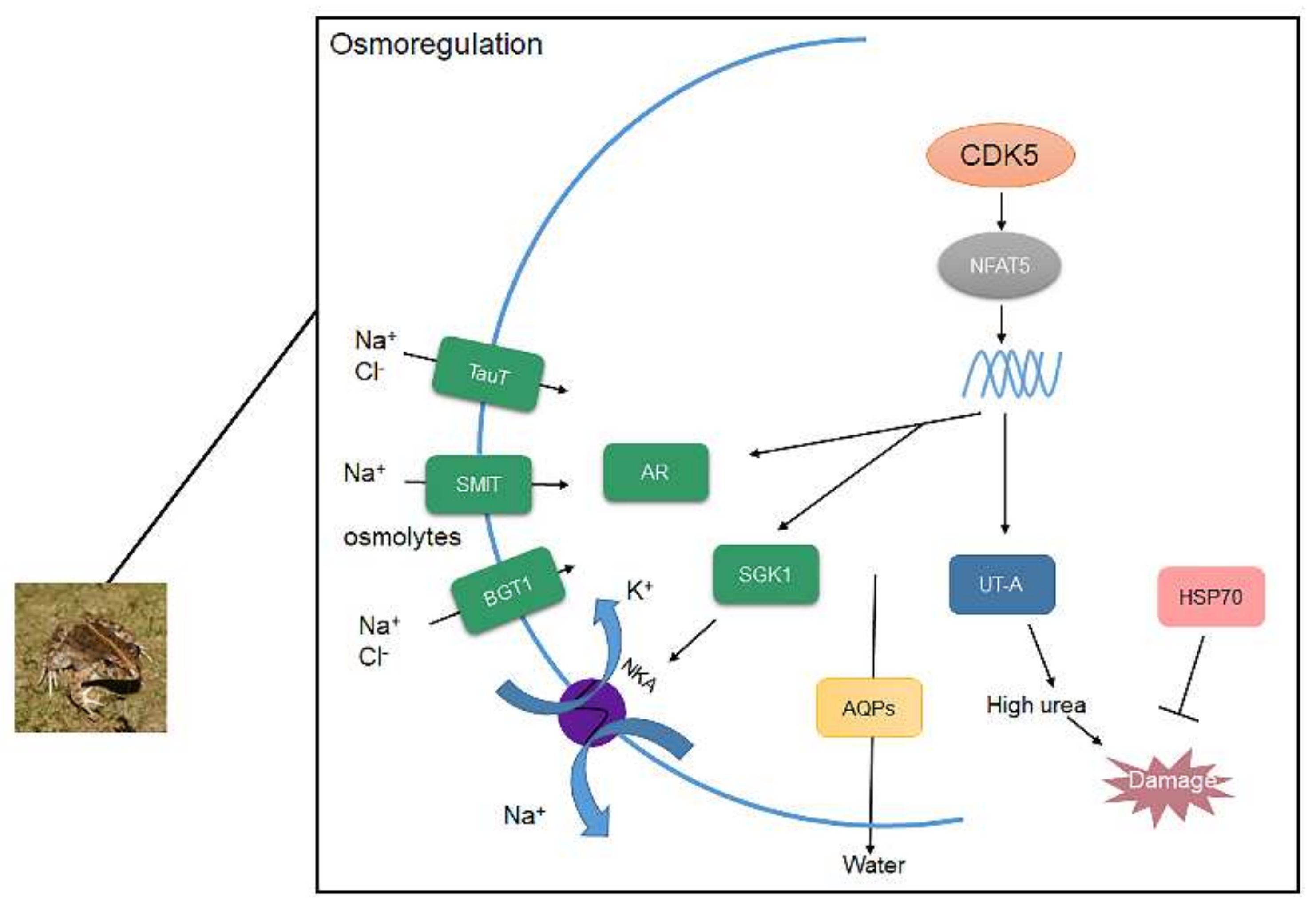
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balinsky, J.B. Adaptation of nitrogen metabolism to hyperosmotic environment in amphibia. J. Exp. Zool. Part A 1980, 215, 335–350. [Google Scholar] [CrossRef]
- Kurniawan, N.; Djong, T.H.; Islam, M.M.; Nishizawa, T.; Belabut, D.M.; Sen, Y.H.; Wanichanon, R.; Yasir, I.; Sumida, M. Taxonomic status of three types of Fejervarya cancrivora from Indonesia and other Asian countries based on morphological observations and crossing experiments. Zool. Sci. 2011, 28, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Schmidtnielsen, K.; Kelly, M. Osmotic regulation in the crab-eating frog (Rana cancrivora). J. Exp. Biol. 1961, 38, 659–678. [Google Scholar] [CrossRef]
- Gordon, M.S.; Tucker, V.A. Osmotic regulation in tadpoles of crab-eating frog (Rana cancrivora). J. Exp. Biol. 1965, 42, 437–445. [Google Scholar] [CrossRef]
- Albecker, M.A.; McCoy, M.W. Adaptive responses to salinity stress across multiple life stages in anuran amphibians. Front. Zool. 2017, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an Anuran amphibian to osmotically stressful environments. Evolution 2003, 57, 1889–1899. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mestre, I.; Tejedo, M. Contrasting patterns of quantitative and neutral genetic variation in locally adapted populations of the natterjack toad. Evolution 2004, 58, 2343–2352. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.S.; Tucker, V.A. Further observations on the physiology of salinity adaptation in the crab-eating frog (Rana cancrivora). J. Exp. Biol. 1968, 48, 185–193. [Google Scholar] [CrossRef]
- Katz, U.; Degani, G.; Gabbay, S. Acclimation of the euryhaline toad Bufo viridis to hyperosmotic solution (Nacl, Urea and Mannitol). J. Exp. Biol. 1984, 108, 403–409. [Google Scholar] [CrossRef]
- Liggins, G.W.; Grigg, G.C. Osmoregulation of the cane toad, Bufo marinus, in salt water. Comp. Biochem. Physiol. A Comp. Physiol. 1985, 82, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Bernabò, I.; Bonacci, A.; Coscarelli, F.; Tripepi, M.; Brunelli, E. Effects of salinity stress on Bufo balearicus and Bufo bufo tadpoles: Tolerance, morphological gill alterations and Na(+)/K(+)-ATPase localization. Aquat. Toxicol. 2013, 132–133, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Na, K-ATPase. Curr. Opin. Nephrol. Hypertens. 1997, 6, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Scheiner-Bobis, G. The sodium pump-its molecular properties and mechanics of ion transport. Eur. J. Biochem. 2002, 269, 2424–2433. [Google Scholar] [CrossRef]
- Ko, B.C.; Ruepp, B.; Bohren, K.M.; Gabbay, K.H.; Chung, S.S. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 1997, 272, 16431–16437. [Google Scholar] [CrossRef] [Green Version]
- Miyakawa, H.; Rim, J.S.; Handler, J.S.; Kwon, H.M. Identification of the second tonicity-responsive enhancer for the betaine transporter (BGT1) Gene. Biochim. Biophys. Acta 1999, 1446, 359–364. [Google Scholar] [CrossRef]
- Rim, J.S.; Atta, M.G.; Dahl, S.C.; Berry, G.T.; Handler, J.S.; Kwon, H.M. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5’-flanking region. J. Biol. Chem. 1998, 273, 20615–20621. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Fujio, Y.; Hirata, M.; Takatani, T.; Matsuda, T.; Muraoka, S.; Takahashi, K.; Azuma, J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (tone-binding protein) pathway and contributes to cytoprotection in Hepg2 cells. Biochem. J. 2004, 382, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Grigsby, C.L.; Law, C.S.; Ni, X.; Nekrep, N.; Olsen, K.; Humphreys, M.H.; Gardner, D.G. Tonicity-dependent induction of sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J. Clin. Investig. 2009, 119, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Pearce, D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol. Metab. 2001, 12, 341–347. [Google Scholar] [CrossRef]
- Stockand, J.D. New ideas about aldosterone signaling in epithelia. Am. J. Physiol. Renal. Physiol. 2002, 282, 559–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez de la Rosa, D.; Gimenez, I.; Forbush, B.; Canessa, C.M. SGK1 Activates Na+-K+-ATPase in amphibian renal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, 492–498. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Renal. Physiol. 2020, 318, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hasegawa, T.; Ogushi, Y.; Tanaka, S. Amphibian aquaporins and adaptation to terrestrial environments: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 72–81. [Google Scholar] [CrossRef]
- Nakayama, Y.; Peng, T.; Sands, J.M.; Bagnasco, S.M. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 2000, 275, 38275–38280. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.K.; Kwon, H.M. Adaptation of kidney medulla to hypertonicity: Role of the transcription factor TonEBP. Int. Rev. Cytol. 2002, 215, 189–202. [Google Scholar]
- Woo, S.K.; Lee, S.D.; Kwon, H.M. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002, 444, 579–585. [Google Scholar]
- Ito, T.; Fujio, Y.; Takahashi, K.; Azuma, J. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J. Biol. Chem. 2007, 282, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Jeon, U.S.; Kim, J.A.; Sheen, M.R.; Kwon, H.M. How tonicity regulates genes: Story of TonEBP transcriptional activator. Acta Physiol. 2006, 187, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, H.; Woo, S.K.; Dahl, S.C.; Handler, J.S.; Kwon, H.M. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA. 1999, 96, 2538–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kültz, D.; Chakravarty, D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA. 2001, 98, 1999–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, T.J. Ion-selective electrodes. Postgrad. Med. J. 1999, 75, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 28–47. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.C.; Faria, S.C. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod crustacea: A review. J. Comp. Physiol. B 2012, 182, 997–1014. [Google Scholar] [CrossRef]
- Uchiyama, M.; Konno, N. Hormonal regulation of ion and water transport in anuran amphibians. Gen. Comp. Endocrinol. 2006, 147, 54–61. [Google Scholar] [CrossRef]
- Shui, C.; Zhang, H.M.; Shi, Y.H.; Xie, Y.D.; Liu, Y.S.; Lu, G.H.; Xu, J.B. Effects of salinity on growth, osmophysiology and body composition of juvenile Soiuy liza haematocheila. J. Dalian Ocean. Univ. 2015, 30, 634–640. (In Chinese) [Google Scholar]
- Zhao, F.; Zhuang, P.; Zhang, L.Z.; Huang, X.R.; Tian, H.J.; Zhang, T.; Feng, G.P. The influence of salinity acclimation on activity of Na+/K+-ATPase in branchial epithelium, concentration of ions and osmolarity in serum of Acipenser schrenckii. J. Fish. China 2006, 4, 444–449. (In Chinese) [Google Scholar]
- Mcenroe, M.; Cech, J.J. Osmoregulation in juvenile and adult white sturgeon, Acipenser transmontanus. Environ. Biol. Fish. 1985, 14, 23–30. [Google Scholar] [CrossRef]
- Pillans, R.D.; Good, J.P.; Anderson, W.G.; Hazon, N.; Franklin, C.E. Freshwater to seawater acclimation of juvenile bull sharks (Carcharhinus leucas): Plasma osmolytes and Na+/K+-ATPase Activity in gill, rectal gland, kidney and intestine. J. Comp. Physiol. B 2005, 175, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wheatly, M.G.; Gannon, A.T. Ion regulation in crayfish: Freshwater adaptations and the problem of molting. Am. Zool. 1995, 35, 49–59. [Google Scholar] [CrossRef]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, T.; Garcia-Perez, A.; Burg, M.B. Osmotic regulation of aldose reductase protein synthesis in renal medullary cells. J. Biol. Chem. 1989, 264, 16810–16814. [Google Scholar] [CrossRef]
- Smardo, F.L., Jr.; Burg, M.B.; Garcia-Perez, A. Kidney aldose reductase gene transcription is osmotically regulated. Am. J. Physiol. 1992, 262, 776–782. [Google Scholar] [CrossRef]
- Grunewald, R.W.; Wagner, M.; Schubert, I.; Franz, H.E.; Müller, G.A.; Steffgen, J. Rat renal expression of mRNA coding for aldose reductase and sorbitol dehydrogenase and its osmotic regulation in inner medullary collecting duct cells. Cell Physiol. Biochem. 1998, 8, 293–303. [Google Scholar] [CrossRef]
- Garcia-Perez, A.; Burg, M.B. Renal medullary organic osmolytes. Physiol. Rev. 1991, 71, 1081–1115. [Google Scholar] [CrossRef]
- Nakanishi, T.; Uyama, O.; Sugita, M. Osmotically regulated taurine content in rat renal inner medulla. Am. J. Physiol. 1991, 261, 957–962. [Google Scholar] [CrossRef]
- Uchida, S.; Nakanishi, T.; Kwon, H.M.; Preston, A.S.; Handler, J.S. Taurine behaves as an osmolyte in Madin-Darby canine kidney cells. protection by polarized, regulated transport of taurine. J. Clin. Investig. 1991, 88, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Yamauchi, A.; Preston, A.S.; Kwon, H.M.; Handler, J.S. Medium tonicity regulates expression of the Na(+)- and Cl(−)-dependent betaine transporter in Madin-Darby canine kidney cells by increasing transcription of the transporter gene. J. Clin. Investig. 1993, 91, 1604–1607. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, A.; Uchida, S.; Preston, A.S.; Kwon, H.M.; Handler, J.S. Hypertonicity stimulates transcription of gene for Na(+)-myo-inositol cotransporter in MDCK Cells. Am. J. Physiol. 1993, 264, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Montrose, M.H. Osmotic regulation of renal betaine transport: Transcription and beyond. Pflugers Arch. 2004, 449, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cowley, B.D., Jr.; Ferraris, J.D.; Carper, D.; Burg, M.B. In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am. J. Physiol. 1990, 258, 154–161. [Google Scholar] [CrossRef]
- Martial, S.; Price, S.R.; Sands, J.M. Regulation of aldose reductase, sorbitol dehydrogenase, and taurine cotransporter mRNA in rat medulla. J. Am. Soc. Nephrol. 1995, 5, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.C.; Turck, C.W.; Lee, K.W.; Yang, Y.; Chung, S.S. Purification, identification, and characterization of an osmotic response element binding protein. Biochem. Biophys. Res. Commun. 2000, 270, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, J.H.; Lee, W.O.; Dahms, H.U.; Han, K.N. Salinity changes in the anadromous river pufferfish, Takifugu obscurus, mediate gene regulation. Fish Physiol. Biochem. 2014, 40, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, V.H.; Mcclanahan, L.L. Evaporative water loss, nitrogen excretion and osmoregulation in phyllomedusine frogs. J. Comp. Phys. 1975, 100, 331–345. [Google Scholar] [CrossRef]
- Sands, J.M. Molecular approaches to urea transporte.ers. J. Am. Soc. Nephrol. 2002, 13, 2795–2806. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stokes, J., 3rd; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Shim, E.H.; Kim, J.I.; Bang, E.S.; Heo, J.S.; Lee, J.S.; Kim, E.Y.; Lee, J.E.; Park, W.Y.; Kim, S.H.; Kim, H.S.; et al. Targeted disruption of Hsp70.1 sensitizes to osmotic stress. EMBO Rep. 2002, 3, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Haramura, T.; Ikegami, T.; Wong, M.K.S.; Takei, Y. Preparatory mechanisms for salinity tolerance in two congeneric Anuran species inhabiting distinct osmotic habitats. Zool. Sci. 2019, 36, 215–222. [Google Scholar] [CrossRef] [PubMed]
| Gene | Fejervarya cancrivora | Fejervarya multistriata |
|---|---|---|
| NFAT5 | CGGTCCTTAACATTTCGCCA CACCCCCTTCATCTTGGCAT | CGGTCCTTAACATTTCGCCA CACCCCCTTCATCTTGGCAT |
| AQP1 | GTACATCATCGCCCAGTGCC CAAGGCCAGAGGTGATACCG | GTACATCATCGCCCAGTGCC CAAGGCCAGAGGTGATACCG |
| AQP3 | TGGGATCTTCGCCACCTTTC AGCGGTGCCAATAAACTGGT | TGGGATCTTCGCCACCTTTC AGCGGTGCCAATAAACTGGT |
| AQP4 | CTGATGTTGCTGGAGGTTTGG TACCAGAAGACCGTGACCAG | CTGATGTTGCTGGAGGTTTGG TACCAGAAGACCGTGACCAG |
| BGT1 | CAGGACCGGGATTGGCTTT GAAGAAGAGGCAGGACCAGA | GTTACAGGACCGGGATTGGC GAAGAAGAGGCAGGACCAGAG |
| SMIT | TGGGTCAAACTCCAGCTTCT TTGCTGCCAAAACCCTTTGA | GCTGTGATGATTGCGGCTCT CAAGAGTGAAGATGGTGCTTGC |
| TAUT | TATTTCACGGCCACCTTCCC CAGTCGTGTGATGTTCGGGT | TATTTCACGGCCACCTTCCC CAGTCGTGTGATGTTCGGGT |
| SGK1 | GCCAAGCCATCAGACTTCCA ATCAGCTTTGTGTCGTGCCA | GCCAAGCCATCAGACTTCCA ATCAGCTTTGTGTCGTGCCA |
| HSP70 | GATGCCACACAAATCGCTGG CGAACACAACAATCCTCGGC | ATCGCTGGACTCAACTGTCT TGAATGTCCCATGTCCACGA |
| AR | GCTGCCAACCAATCTTCC ATGCCTTTGCCCACTTCA | GCTGCCAACCAATCTTCC ATGCCTTTGCCCACTTCA |
| UT-A | GCGTGGAGCATCTCAAGTCA GAGCCACCCATACCAGTCAC | GCGTGGAGCATCTCAAGTCA GAGCCACCCATACCAGTCAC |
| β-tubulin | CTGGCTGTCAACATGGTCCC TACTGTTGGCTACCACGGCT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, X.; Lan, T.; Lu, Y.; Hong, M.; Ding, L.; Wang, L. CDK5/NFAT5-Regulated Transporters Involved in Osmoregulation in Fejervarya cancrivora. Biology 2022, 11, 858. https://doi.org/10.3390/biology11060858
Li J, Wang X, Lan T, Lu Y, Hong M, Ding L, Wang L. CDK5/NFAT5-Regulated Transporters Involved in Osmoregulation in Fejervarya cancrivora. Biology. 2022; 11(6):858. https://doi.org/10.3390/biology11060858
Chicago/Turabian StyleLi, Jiao, Xinru Wang, Tian Lan, Yingnan Lu, Meiling Hong, Li Ding, and Lijun Wang. 2022. "CDK5/NFAT5-Regulated Transporters Involved in Osmoregulation in Fejervarya cancrivora" Biology 11, no. 6: 858. https://doi.org/10.3390/biology11060858
APA StyleLi, J., Wang, X., Lan, T., Lu, Y., Hong, M., Ding, L., & Wang, L. (2022). CDK5/NFAT5-Regulated Transporters Involved in Osmoregulation in Fejervarya cancrivora. Biology, 11(6), 858. https://doi.org/10.3390/biology11060858