The Interventricular Septum: Structure, Function, Dysfunction, and Diseases
Abstract
:1. Introduction
2. IVS Structure
3. IVS Function
3.1. Ventricular Interdependence
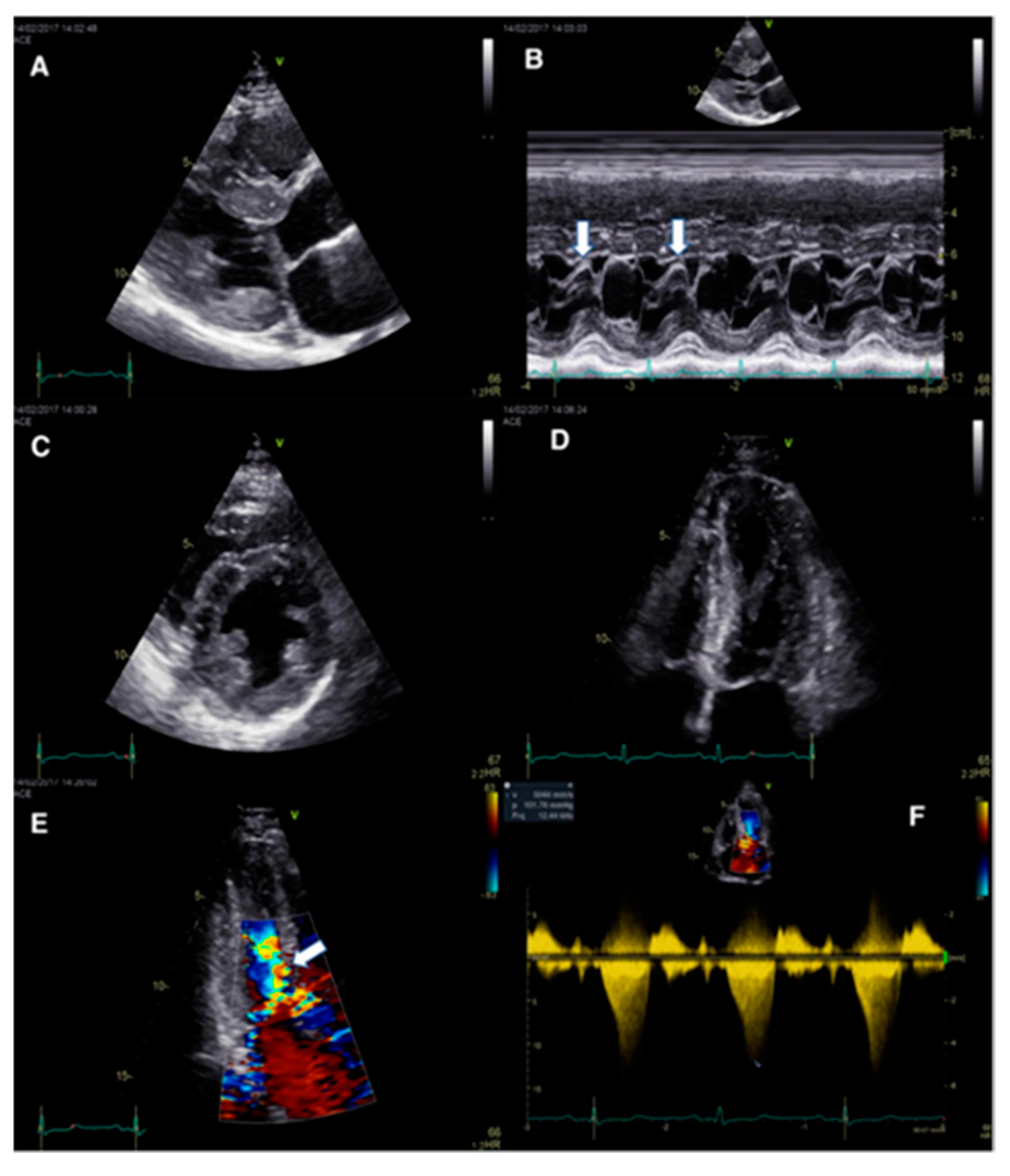
3.2. Abnormal IVS Motion
4. Disorders and Diseases Affecting the IVS
4.1. Hypertrophic Cardiomyopathy
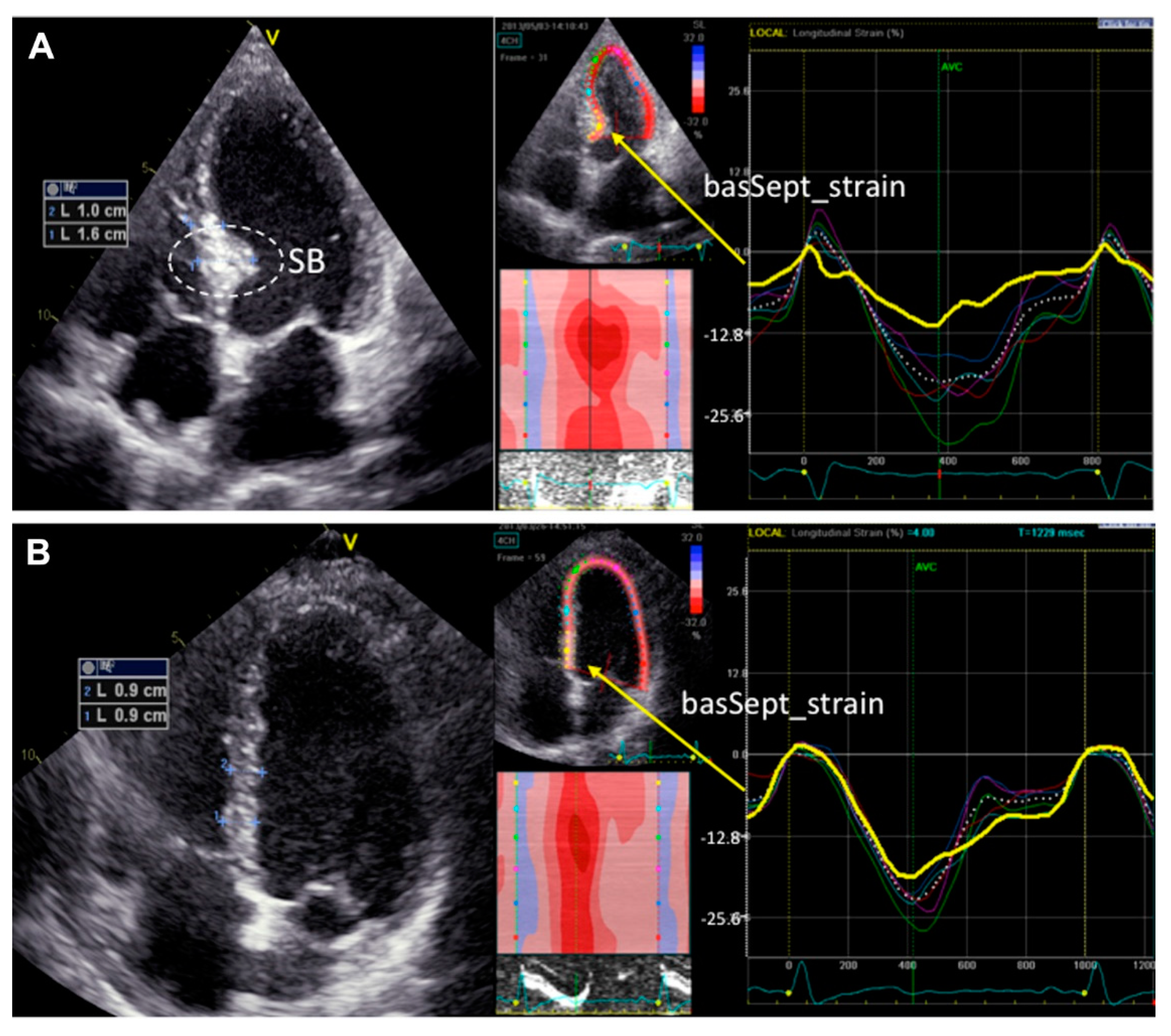
4.2. Basal Septal Hypertrophy (Upper Septal Hypertrophy or ‘Sigmoid Septum’)
4.3. Ventricular Septal Defects
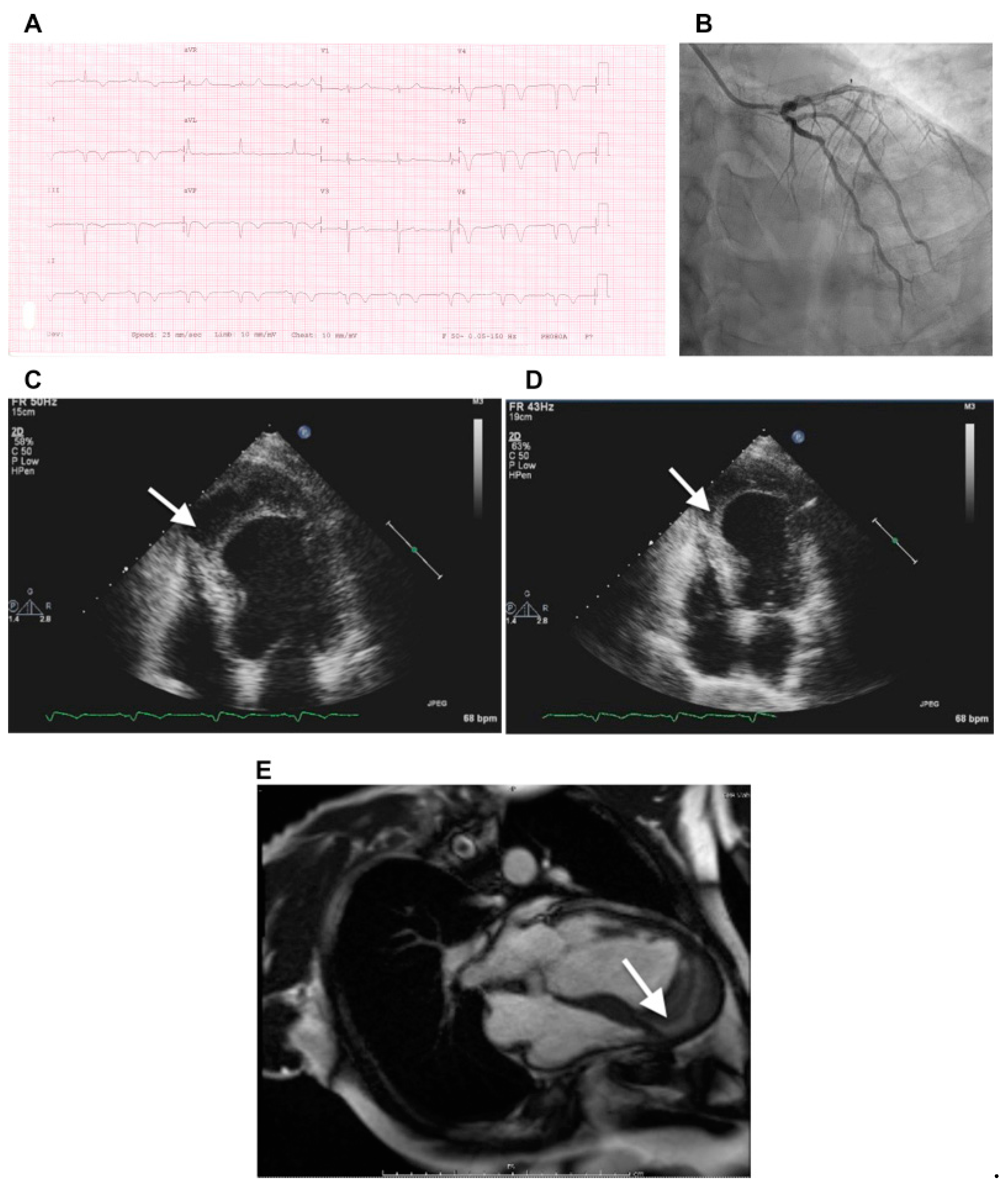
4.4. Septal Infarction
4.5. Ventricular Arrhythmias and Conduction Abnormalities
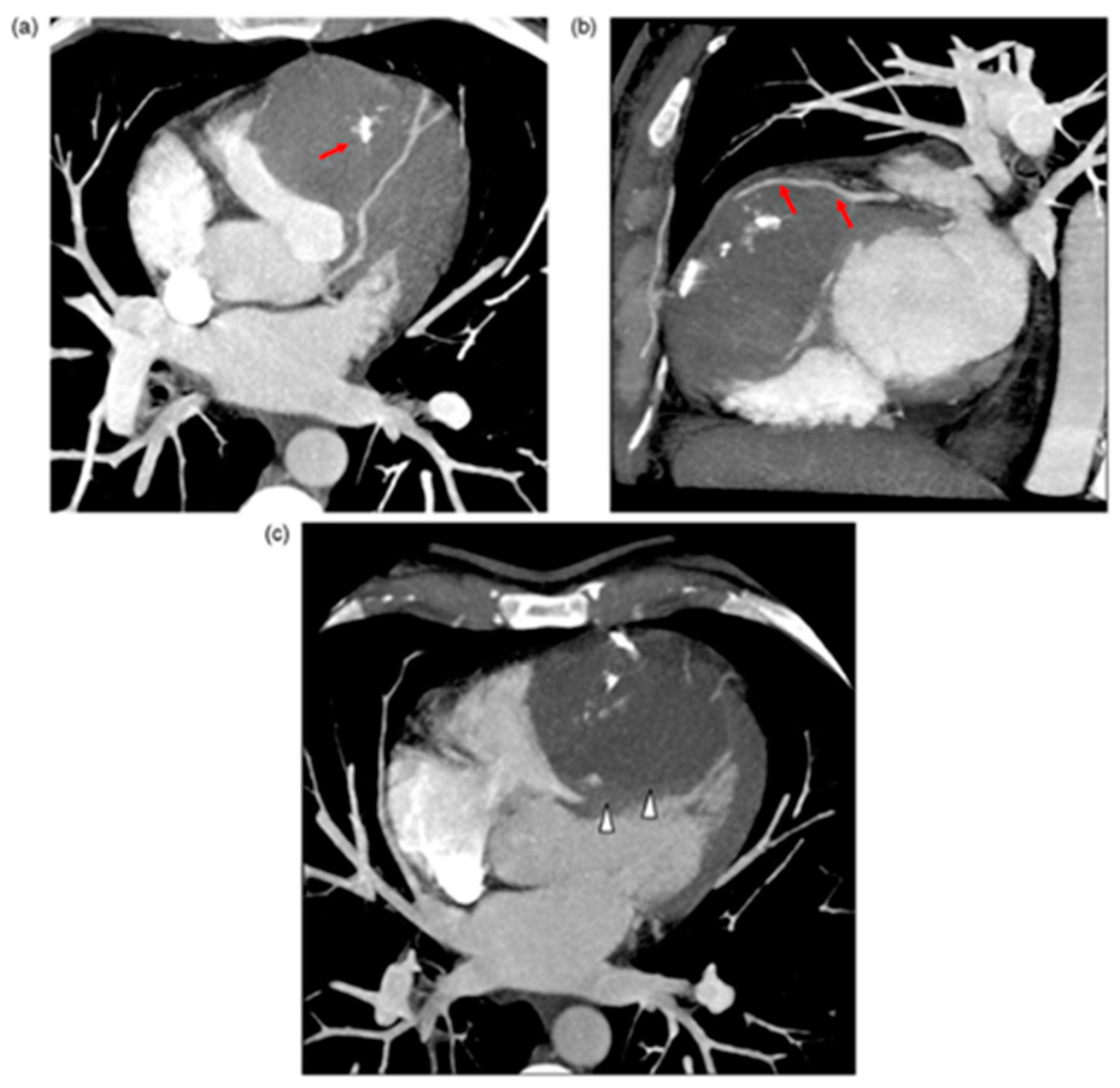
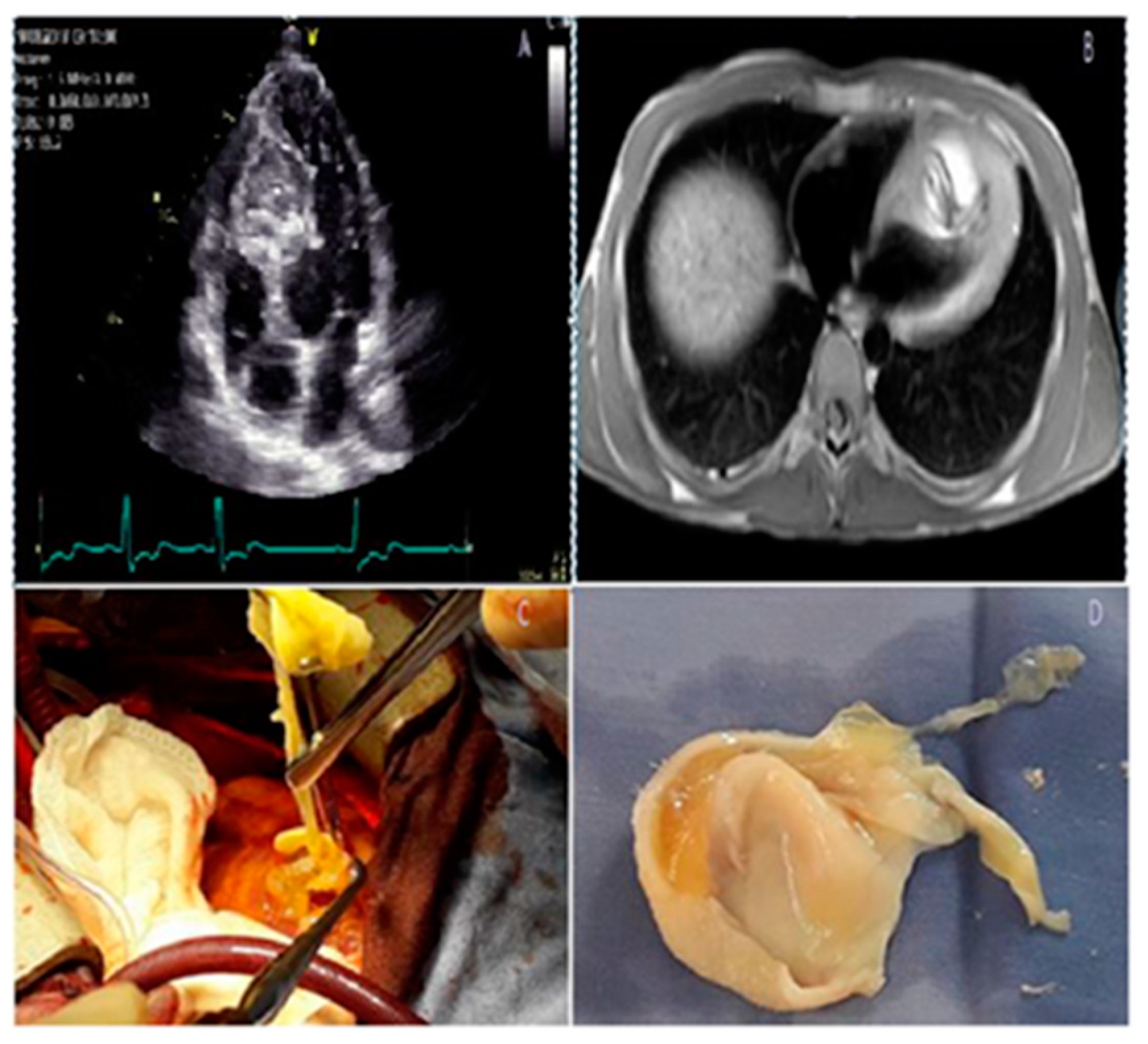
4.6. Other
5. Treatment Modalities Targeting Diseases of the IVS
5.1. Cardiac Resynchronization Therapy (CRT)
5.2. Mavacamten
5.3. Septal Reduction Therapy (SRT)
5.4. VSD Closure
5.5. IVS Ablation of VT
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katano, W.; Moriyama, Y.; Takeuchi, J.K.; Koshiba-Takeuchi, K. Cardiac septation in heart development and evolution. Dev. Growth Differ. 2019, 61, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Rojas, C.A.; Jaimes, C.; Abbara, S. Ventricular septal defects: Embryology and imaging findings. J. Thorac. Imaging 2013, 28, W28–W34. [Google Scholar] [CrossRef]
- Buckberg, G.; Hoffman, J.I. Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J. Thorac. Cardiovasc. Surg. 2014, 148, 3166–3171. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S. The interventricular septum in health and disease. Am. Heart J. 1986, 112, 568–581. [Google Scholar] [CrossRef]
- Daubert, M.A.; Tailor, T.; James, O.; Shaw, L.J.; Douglas, P.S.; Koweek, L. Multimodality cardiac imaging in the 21st century: Evolution, advances and future opportunities for innovation. Br. J. Radiol. 2021, 94, 20200780. [Google Scholar] [CrossRef] [PubMed]
- Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-Liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, M.C.; Sanchez-Quintana, D.; Anderson, R.H. The membranous septum revisited: A glimpse of our anatomical past. Clin. Anat. 2021, 34, 178–186. [Google Scholar] [CrossRef]
- Nguyen-Truong, M.; Liu, W.; Doherty, C.; LeBar, K.; Labus, K.M.; Puttlitz, C.M.; Easley, J.; Monnet, E.; Chicco, A.; Wang, Z. The Interventricular Septum Is Biomechanically Distinct from the Ventricular Free Walls. Bioengineering 2021, 8, 216. [Google Scholar] [CrossRef]
- Kim, S.D. Anatomy of the septal perforating arteries of the heart. Anat. Cell Biol. 2019, 52, 236–241. [Google Scholar] [CrossRef]
- Kassem, M.W.; Lake, S.; Roberts, W.; Salandy, S.; Loukas, M. Cardiac veins, an anatomical review. Transl. Res. Anat. 2021, 23, 100096. [Google Scholar] [CrossRef]
- Duraes Campos, I.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell Cardiol. 2018, 119, 1–9. [Google Scholar] [CrossRef]
- De Almeida, M.C.; Mori, S.; Anderson, R.H. Three-dimensional visualization of the bovine cardiac conduction system and surrounding structures compared to the arrangements in the human heart. J. Anat. 2021, 238, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Meilhac, S.M.; Christoffels, V.M.; Kispert, A.; Buckingham, M.; Kelly, R.G. Left and right ventricular contributions to the formation of the interventricular septum in the mouse heart. Dev. Biol. 2006, 294, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Allen, J.; Hu, L.; Caruthers, S.D.; Wickline, S.A.; Chen, J. Cardiomyocyte architectural plasticity in fetal, neonatal, and adult pig hearts delineated with diffusion tensor MRI. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H246–H252. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.J.; McLean, A.; Slama, M.; Orde, S.R. Paradoxical septal motion: A diagnostic approach and clinical relevance. Australas J. Ultrasound Med. 2018, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Santamore, W.P.; Dell’Italia, L.J. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog. Cardiovasc. Dis. 1998, 40, 289–308. [Google Scholar] [CrossRef]
- Wanner, P.M.; Filipovic, M. The Right Ventricle-You May Forget it, but It Will Not Forget You. J. Clin. Med. 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlaug, B.A.; Reddy, Y.N.V. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- Naeije, R.; Badagliacca, R. The overloaded right heart and ventricular interdependence. Cardiovasc. Res. 2017, 113, 1474–1485. [Google Scholar] [CrossRef]
- Paridon, S.M.; Mitchell, P.D.; Colan, S.D.; Williams, R.V.; Blaufox, A.; Li, J.S.; Margossian, R.; Mital, S.; Russell, J.; Rhodes, J.; et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J. Am. Coll. Cardiol. 2008, 52, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresca, A.M.; Mongiardi, C.; Corso, R.; Robustelli Test, L.; Lippi, A.; Montalbetti, L.; Campiotti, L.; Moretti, S.; Tandurella, N.; Agostinis, M.; et al. Right ventricular remodelling in mild hypertensive patients: Role of left ventricular morpho-functional parameters. J. Hum. Hypertens. 2020, 34, 293–300. [Google Scholar] [CrossRef]
- Stankovic, I.; Prinz, C.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Belmans, A.; Neskovic, A.N.; et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). Eur. Heart J. Cardiovasc. Imaging 2016, 17, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Mele, D.; Luisi, G.A.; Malagu, M.; Laterza, A.; Ferrari, R.; Bertini, M. Echocardiographic evaluation of cardiac dyssynchrony: Does it still matter? Echocardiography 2018, 35, 707–715. [Google Scholar] [CrossRef]
- Calle, S.; Delens, C.; Kamoen, V.; De Pooter, J.; Timmermans, F. Septal flash: At the heart of cardiac dyssynchrony. Trends Cardiovasc. Med. 2020, 30, 115–122. [Google Scholar] [CrossRef]
- Agarwal, J.B.; Yamazaki, H.; Bodenheimer, M.M.; Banka, V.S.; Helfant, R.H. Effects of isolated interventricular septal ischemia on global and segmental function of the canine right and left ventricle. Am. Heart J. 1981, 102, 654–658. [Google Scholar] [CrossRef]
- Braile-Sternieri, M.; Mustafa, E.M.; Ferreira, V.R.R.; Braile Sabino, S.; Braile Sternieri, G.; Buffulin de Faria, L.A.; Sbardellini, B.C.; Vianna Queiroz, C.O.; Braile, D.M.; Zotarelli Filho, I.J. Main Considerations of Cardiogenic Shock and Its Predictors: Systematic Review. Cardiol. Res. 2018, 9, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Palau-Caballero, G.; Walmsley, J.; Van Empel, V.; Lumens, J.; Delhaas, T. Why septal motion is a marker of right ventricular failure in pulmonary arterial hypertension: Mechanistic analysis using a computer model. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H691–H700. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, R.J.; Haddad, F.; Couture, E.J.; Hansmann, G.; de Jesus Perez, V.A.; Denault, A.Y.; de Man, F.S.; Amsallem, M. Mechanics of right ventricular dysfunction in pulmonary arterial hypertension and heart failure with preserved ejection fraction. Cardiovasc. Diagn. Ther. 2020, 10, 1580–1603. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Nishimura, R.A. Constrictive pericarditis in the modern era: A diagnostic dilemma. Heart 2001, 86, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Welch, T.D. Constrictive pericarditis: Diagnosis, management and clinical outcomes. Heart 2018, 104, 725–731. [Google Scholar] [CrossRef]
- Stanley, A.; Athanasuleas, C. Paradoxical Septal Motion after Uncomplicated Cardiac Surgery: A Consequence of Altered Regional Right Ventricular Contractile Patterns. Curr. Cardiol. Rev. 2022, 18, 9. [Google Scholar] [CrossRef]
- Hung, J.; Uren, R.F.; Richmond, D.R.; Kelly, D.T. The mechanism of abnormal septal motion in atrial septal defect: Pre- and postoperative study by radionuclide ventriculography in adults. Circulation 1981, 63, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.R.; Kingma, I.; MacDonald, R.P.; Belenkie, I.; Tyberg, J.V.; Smith, E.R. Transseptal pressure gradient and diastolic ventricular septal motion in patients with mitral stenosis. Circulation 1987, 76, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Maron, M.S.; Hellawell, J.L.; Lucove, J.C.; Farzaneh-Far, R.; Olivotto, I. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy in the United States. Am. J. Cardiol. 2016, 117, 1651–1654. [Google Scholar] [CrossRef]
- Walsh, R.; Offerhaus, J.A.; Tadros, R.; Bezzina, C.R. Minor hypertrophic cardiomyopathy genes, major insights into the genetics of cardiomyopathies. Nat. Rev. Cardiol. 2022, 19, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Veselka, J.; Anavekar, N.S.; Charron, P. Hypertrophic obstructive cardiomyopathy. Lancet 2017, 389, 1253–1267. [Google Scholar] [CrossRef]
- Mandeş, L.; Roşca, M.; Ciupercă, D.; Popescu, B.A. The role of echocardiography for diagnosis and prognostic stratification in hypertrophic cardiomyopathy. J. Echocardiogr. 2020, 18, 137–148. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Roberts, W.C. Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am. J. Cardiol. 1992, 70, 1333–1340. [Google Scholar] [CrossRef]
- Daniels, M.J.; Fusi, L.; Semsarian, C.; Naidu, S.S. Myosin Modulation in Hypertrophic Cardiomyopathy and Systolic Heart Failure: Getting Inside the Engine. Circulation 2021, 144, 759–762. [Google Scholar] [CrossRef]
- Spudich, J.A. Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflug. Arch. 2019, 471, 701–717. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Toepfer, C.N. Cardiac myosin super relaxation (SRX): A perspective on fundamental biology, human disease and therapeutics. Biol. Open 2021, 10, bio057646. [Google Scholar] [CrossRef] [PubMed]
- Vander Roest, A.S.; Liu, C.; Morck, M.M.; Kooiker, K.B.; Jung, G.; Song, D.; Dawood, A.; Jhingran, A.; Pardon, G.; Ranjbarvaziri, S.; et al. Hypertrophic cardiomyopathy β-cardiac myosin mutation (P710R) leads to hypercontractility by disrupting super relaxed state. Proc. Natl. Acad. Sci. USA 2021, 118, e2025030118. [Google Scholar] [CrossRef]
- Ommen, S.R.; Semsarian, C. Hypertrophic cardiomyopathy: A practical approach to guideline directed management. Lancet 2021, 398, 2102–2108. [Google Scholar] [CrossRef]
- Brosnan, M.J.; Rakhit, D. Differentiating Athlete’s Heart from Cardiomyopathies—The Left Side. Heart Lung. Circ. 2018, 27, 1052–1062. [Google Scholar] [CrossRef]
- Loncaric, F.; Nunno, L.; Mimbrero, M.; Marciniak, M.; Fernandes, J.F.; Tirapu, L.; Fabijanovic, D.; Sanchis, L.; Doltra, A.; Cikes, M.; et al. Basal Ventricular Septal Hypertrophy in Systemic Hypertension. Am. J. Cardiol. 2020, 125, 1339–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzetti, E.; Tastet, L.; Annabi, M.S.; Capoulade, R.; Shen, M.; Bernard, J.; Garcia, J.; Le Ven, F.; Arsenault, M.; Bedard, E.; et al. Effect of Regional Upper Septal Hypertrophy on Echocardiographic Assessment of Left Ventricular Mass and Remodeling in Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 62–71. [Google Scholar] [CrossRef]
- Gaudron, P.D.; Liu, D.; Scholz, F.; Hu, K.; Florescu, C.; Herrmann, S.; Bijnens, B.; Ertl, G.; Stork, S.; Weidemann, F. The septal bulge—An early echocardiographic sign in hypertensive heart disease. J. Am. Soc. Hypertens. 2016, 10, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, S.; Edvardsen, T. Basal septal hypertrophy in hypertension; about time to introduce an objective and reproducible quantification. J. Hypertens. 2021, 39, 1316–1318. [Google Scholar] [CrossRef]
- Yalcin, F.; Yalcin, H.; Kucukler, N.; Arslan, S.; Akkus, O.; Kurtul, A.; Abraham, M.R. Basal Septal Hypertrophy as the Early Imaging Biomarker for Adaptive Phase of Remodeling Prior to Heart Failure. J. Clin. Med. 2021, 11, 75. [Google Scholar] [CrossRef]
- Alashi, A.; Smedira, N.G.; Popovic, Z.B.; Fava, A.; Thamilarasan, M.; Kapadia, S.R.; Wierup, P.; Lever, H.M.; Desai, M.Y. Characteristics and Outcomes of Elderly Patients with Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e018527. [Google Scholar] [CrossRef]
- Wu, M.H.; Lu, C.W.; Chen, H.C.; Kao, F.Y.; Huang, S.K. Adult Congenital Heart Disease in a Nationwide Population 2000–2014: Epidemiological Trends, Arrhythmia, and Standardized Mortality Ratio. J. Am. Heart Assoc. 2018, 7, e007907. [Google Scholar] [CrossRef] [Green Version]
- Mark, S.D.; Prasanna, V.; Ferrari, V.A.; Herrmann, H.C. Percutaneous Ventricular Septal Defect Closure after Sapien 3 Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2015, 8, e109–e110. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Badheka, A.O.; Bokhari, S.S.; Ghersin, E.; Clark, P.M.; O’Neill, W.W. Retrograde percutaneous closure of a ventricular septal defect after myectomy for hypertrophic obstructive cardiomyopathy. Tex. Heart Inst. J. 2013, 40, 468–471. [Google Scholar]
- Dagnegard, H.H.; Ugander, M.; Liska, J.; Kallner, G.G. Ventricular Septal Perforation Caused by the Strut of a Mitral Valve Bioprosthesis. Ann. Thorac. Surg. 2016, 101, 1164–1166. [Google Scholar] [CrossRef] [Green Version]
- Narita, M.; Sakakura, K.; Ohashi, J.; Ibe, T.; Yamamoto, K.; Wada, H.; Momomura, S.I.; Fujita, H. Medically Treated Ventricular Septal Perforation Caused by Takotsubo Cardiomyopathy. Int. Heart J. 2019, 60, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Tobler, D.; Greutmann, M. Simple cardiac shunts in adults: Atrial septal defects, ventricular septal defects, patent ductus arteriosus. Heart 2020, 106, 307–314. [Google Scholar] [CrossRef]
- Jones, B.M.; Kapadia, S.R.; Smedira, N.G.; Robich, M.; Tuzcu, E.M.; Menon, V.; Krishnaswamy, A. Ventricular septal rupture complicating acute myocardial infarction: A contemporary review. Eur. Heart J. 2014, 35, 2060–2068. [Google Scholar] [CrossRef] [Green Version]
- Crenshaw, B.S.; Granger, C.B.; Birnbaum, Y.; Pieper, K.S.; Morris, D.C.; Kleiman, N.S.; Vahanian, A.; Califf, R.M.; Topol, E.J. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000, 101, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Yokokawa, M.; Good, E.; Chugh, A.; Pelosi, F., Jr.; Crawford, T.; Jongnarangsin, K.; Latchamsetty, R.; Oral, H.; Morady, F.; Bogun, F. Intramural idiopathic ventricular arrhythmias originating in the intraventricular septum: Mapping and ablation. Circ. Arrhythm. Electrophysiol. 2012, 5, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Yokokawa, M.; Desjardins, B.; Good, E.; Oral, H.; Chugh, A.; Pelosi, F.; Morady, F.; Bogun, F. Septal involvement in patients with post-infarction ventricular tachycardia: Implications for mapping and radiofrequency ablation. J. Am. Coll. Cardiol. 2011, 58, 2491–2500. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Seitz, A.; Herter, D.; Gunther, F.; Probst, S.; Bekeredjian, R.; Gawaz, M.; Sechtem, U.; Mahrholdt, H. Long-term risk of sudden cardiac death in hypertrophic cardiomyopathy: A cardiac magnetic resonance outcome study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 732–741. [Google Scholar] [CrossRef]
- Liang, J.J.; D’Souza, B.A.; Betensky, B.P.; Zado, E.S.; Desjardins, B.; Santangeli, P.; Chik, W.W.; Frankel, D.S.; Callans, D.J.; Supple, G.E.; et al. Importance of the Interventricular Septum as Part of the Ventricular Tachycardia Substrate in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 1155–1162. [Google Scholar] [CrossRef]
- Anderson, K.R.; Murphy, J.G. The atrio-ventricular node artery in the human heart. Angiology 1983, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Bakr, L.; Al-Jadaan, M.; Younes, M. An interventricular membranous septal aneurysm obstructing the right ventricle outflow tract in a five-year-old boy: A case report. J. Cardiothorac. Surg. 2020, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Zealand, G.; Williams, M. Interventricular septal diverticulum and rheumatic mitral valve disease identified and managed concurrently in middle age. BMJ Case Rep. 2019, 12, e229298. [Google Scholar] [CrossRef]
- Subbaraman, S.; Rajan, S.C.; Veeraiyan, S.; Natarajan, P. Imaging Findings of Lipomatous Hypertrophy of the Interventricular Septum: A Case Report. Clin. Med. Insights Case Rep. 2021, 14, 11795476211024848. [Google Scholar] [CrossRef]
- Roslan, A.; Jauhari Aktifanus, A.T.; Hakim, N.; Megat Samsudin, W.N.; Khairuddin, A. Intramyocardial Dissecting Hematoma in Patients with Ischemic Cardiomyopathy: Role of Multimodality Imaging in Three Patients Treated Conservatively. Case 2017, 1, 159–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, L.; Kottam, A.; Reddy, V.; Penumetcha, A. Echocardiography in Infective Endocarditis: State of the Art. Curr. Cardiol. Rep. 2017, 19, 127. [Google Scholar] [CrossRef]
- Campisi, A.; Ciarrocchi, A.P.; Asadi, N.; Dell’Amore, A. Primary and secondary cardiac tumors: Clinical presentation, diagnosis, surgical treatment, and results. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stéphant, E.; Anac, S.; Philippeb, D. Inter-ventricular septal cardiac fibroma in an adult: MR and MDCT features with pathologic correlation. Eur. J. Radiol. Extra 2008, 67, e103–e106. [Google Scholar] [CrossRef]
- Delewi, R.; Zijlstra, F.; Piek, J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012, 98, 1743–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennira, S.; Kamoun, S.; Besbes, B.; Ben Mrad, I.; Zairi, I.; Ben Moussa, F.; Mzoughi, K.; Kraiem, S. Cardiac hydatid cyst in the interventricular septum: A literature review. Int. J. Infect. Dis. 2019, 88, 120–126. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.M.; Morin, D.P. Cardiac resynchronization therapy: History, present status, and future directions. Ochsner J. 2014, 14, 596–607. [Google Scholar] [PubMed]
- Thomas, G.; Kim, J.; Lerman, B.B. Improving Cardiac Resynchronisation Therapy. Arrhythm. Electrophysiol. Rev. 2019, 8, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Yaman, B.; Kemal, H.S.; Donmez, Y.; Cerit, L.; Usalp, S.; Yuksek, U.; Gunsel, A.; Duygu, H.; Akpinar, O. Improvement of abnormal systolic motion of the interventricular septum with cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2019, 42, 1213–1218. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Vernooy, K.; Auricchio, A. Cardiac resynchronization therapy: State-of-the-art of current applications, guidelines, ongoing trials, and areas of controversy. Circulation 2013, 128, 2407–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, S.M.; Tardiff, J.C.; Ostap, E.M. Myosin modulators: Emerging approaches for the treatment of cardiomyopathies and heart failure. J. Clin. Investig. 2022, 132, e148557. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Ramchand, J.; Nissen, S.E.; Desai, M.Y. Mavacamten: A novel small molecule modulator of beta-cardiac myosin for treatment of hypertrophic cardiomyopathy. Expert Opin. Investig. Drugs 2020, 29, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Spertus, J.A.; Fine, J.T.; Elliott, P.; Ho, C.Y.; Olivotto, I.; Saberi, S.; Li, W.; Dolan, C.; Reaney, M.; Sehnert, A.J.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): Health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2467–2475. [Google Scholar] [CrossRef]
- Hegde, S.M.; Lester, S.J.; Solomon, S.D.; Michels, M.; Elliott, P.M.; Nagueh, S.F.; Choudhury, L.; Zemanek, D.; Zwas, D.R.; Jacoby, D.; et al. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients With Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2518–2532. [Google Scholar] [CrossRef]
- Kimmelstiel, C.; Zisa, D.C.; Kuttab, J.S.; Wells, S.; Udelson, J.E.; Wessler, B.S.; Rastegar, H.; Kapur, N.K.; Weintraub, A.R.; Maron, B.J.; et al. Guideline-Based Referral for Septal Reduction Therapy in Obstructive Hypertrophic Cardiomyopathy Is Associated With Excellent Clinical Outcomes. Circ. Cardiovasc. Interv. 2019, 12, e007673. [Google Scholar] [CrossRef]
- Batzner, A.; Pfeiffer, B.; Neugebauer, A.; Aicha, D.; Blank, C.; Seggewiss, H. Survival after Alcohol Septal Ablation in Patients with Hypertrophic Obstructive Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 3087–3094. [Google Scholar] [CrossRef]
- Minette, M.S.; Sahn, D.J. Ventricular septal defects. Circulation 2006, 114, 2190–2197. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J. Beyond atrial septal defect closure, it is time to start seriously considering closing ventricular septal defects with devices. Curr. Opin. Cardiol. 2020, 35, 58–62. [Google Scholar] [CrossRef]
- Jiang, D.; Han, B.; Zhao, L.; Yi, Y.; Zhang, J.; Fan, Y.; Lv, J.; Wang, J.; Wang, Y. Transcatheter Device Closure of Perimembranous and Intracristal Ventricular Septal Defects in Children: Medium- and Long-Term Results. J. Am. Heart Assoc. 2021, 10, e020417. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Z.; Wang, Y.; Ding, Y.; Ni, R.; Xiao, P. Transcatheter closure for postinfarction ventricular septal defect: A meta-analysis of the current evidence. J. Card. Surg. 2021, 36, 4625–4633. [Google Scholar] [CrossRef]
- Romero, J.; Shivkumar, K.; Valderrabano, M.; Diaz, J.C.; Alviz, I.; Briceno, D.; Natale, A.; Di Biase, L. Modern mapping and ablation techniques to treat ventricular arrhythmias from the left ventricular summit and interventricular septum. Heart Rhythm 2020, 17, 1609–1620. [Google Scholar] [CrossRef]
- Della Bella, P.; Peretto, G.; Paglino, G.; Bisceglia, C.; Radinovic, A.; Sala, S.; Baratto, F.; Limite, L.R.; Cireddu, M.; Marzi, A.; et al. Bipolar radiofrequency ablation for ventricular tachycardias originating from the interventricular septum: Safety and efficacy in a pilot cohort study. Heart Rhythm 2020, 17, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Rattanawong, P.; Ladia, V.; Minaskeian, N.; Sorajja, D.; Shen, W.K.; Srivathsan, K.S. Empirical Ablation to Prevent Sequential Purkinje System Recruitment: A Novel Therapy for Idiopathic Ventricular Fibrillation. JACC Case Rep. 2021, 3, 517–522. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triposkiadis, F.; Xanthopoulos, A.; Boudoulas, K.D.; Giamouzis, G.; Boudoulas, H.; Skoularigis, J. The Interventricular Septum: Structure, Function, Dysfunction, and Diseases. J. Clin. Med. 2022, 11, 3227. https://doi.org/10.3390/jcm11113227
Triposkiadis F, Xanthopoulos A, Boudoulas KD, Giamouzis G, Boudoulas H, Skoularigis J. The Interventricular Septum: Structure, Function, Dysfunction, and Diseases. Journal of Clinical Medicine. 2022; 11(11):3227. https://doi.org/10.3390/jcm11113227
Chicago/Turabian StyleTriposkiadis, Filippos, Andrew Xanthopoulos, Konstantinos Dean Boudoulas, Grigorios Giamouzis, Harisios Boudoulas, and John Skoularigis. 2022. "The Interventricular Septum: Structure, Function, Dysfunction, and Diseases" Journal of Clinical Medicine 11, no. 11: 3227. https://doi.org/10.3390/jcm11113227
APA StyleTriposkiadis, F., Xanthopoulos, A., Boudoulas, K. D., Giamouzis, G., Boudoulas, H., & Skoularigis, J. (2022). The Interventricular Septum: Structure, Function, Dysfunction, and Diseases. Journal of Clinical Medicine, 11(11), 3227. https://doi.org/10.3390/jcm11113227






