CT-Detected MTA Score Related to Disability and Behavior in Older People with Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Clinical, Cognitive, Neuropsychiatric, and Functional Assessment
2.3. MTA Score Detection
2.4. Quantification of Homocysteine and Other Biochemical Concentrations
2.5. Statistical Analyses
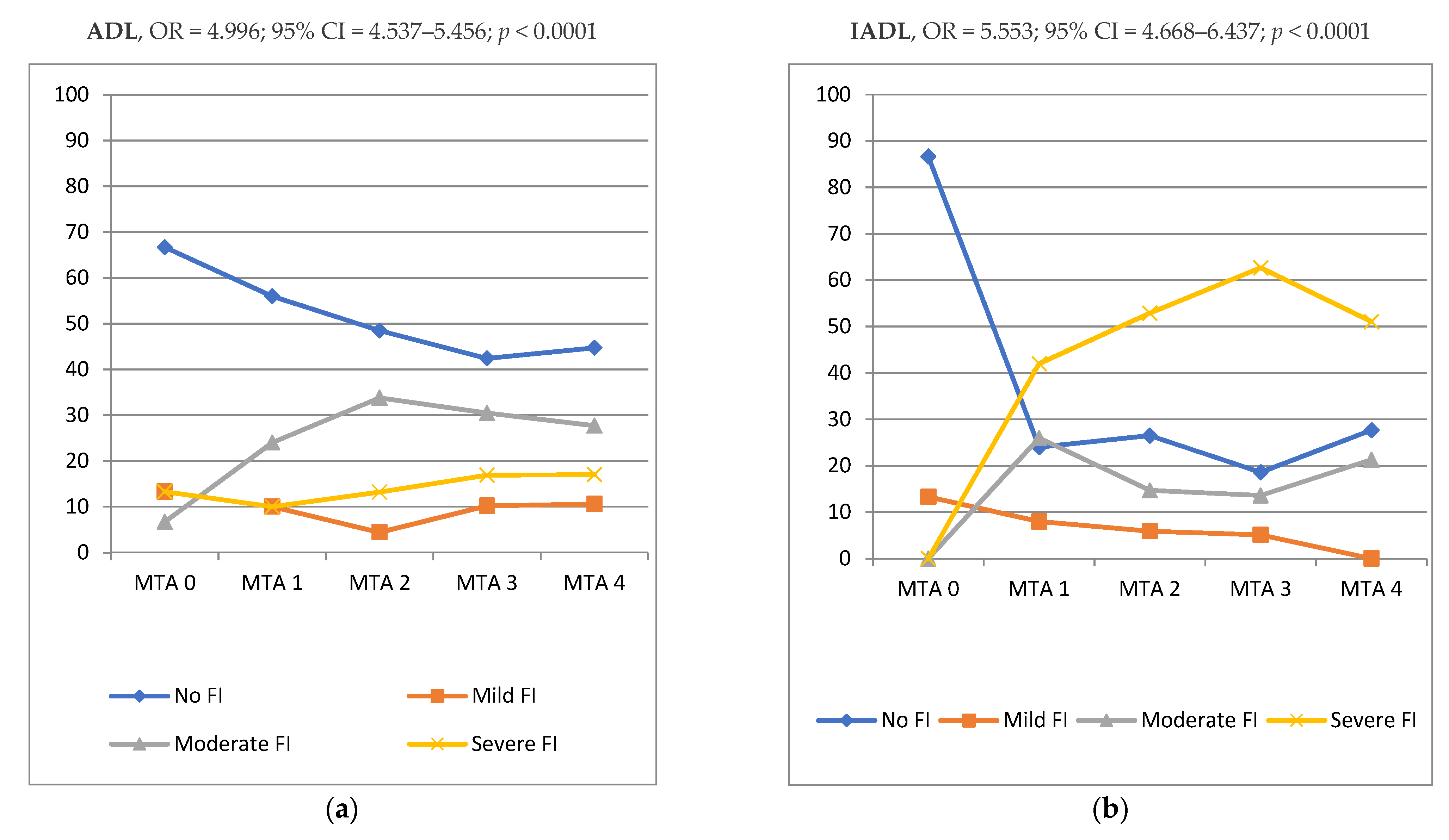
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Dementia, Posted 2 September 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 10 May 2022).
- Kapasi, A.; DeCarli, C.; Schneider, J.A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017, 134, 71–186. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimersdisease (accessed on 7 July 2021).
- Liu, J.L.; Hlavka, J.P.; Hillestad, R.; Mattke, S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer’s Treatment 2017. Available online: https://www.rand.org/pubs/research_reports/RR2272.html (accessed on 5 May 2018).
- Galvin, J.E.; Sadowsky, C.H. NINCDS-ADRDA. Practical guidelines for the recognition and diagnosis of dementia. J. Am. Board Fam. Med. 2012, 25, 367–382. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Beynon, R.; Sterne, J.A.; Wilcock, G.; Likeman, M.; Harbord, R.M.; Astin, M.; Burke, M.; Bessell, A.; Ben-Shlomo, Y.; Hawkins, J.; et al. Is MRI better than CT for detecting a vascular component to dementia? A systematic review and meta-analysis. BMC Neurol. 2012, 6, 33. [Google Scholar] [CrossRef]
- Bin Zahid, A.; Mikheev, A.; Srivatsa, N.; Babb, J.; Samadani, U.; Rusinek, H. Accelerated Brain Atrophy on Serial Computed Tomography: Potential Marker of the Progression of Alzheimer Disease. J. Comput. Assist. Tomogr. 2016, 40, 827–832. [Google Scholar] [CrossRef]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef]
- Gosche, K.M.; Mortimer, J.A.; Smith, C.D.; Markesbery, W.R.; Snowdon, D.A. Hippocampal volume as an index of Alzheimerneuropathology: Findings from the Nun Study. Neurology 2002, 58, 1476–1482. [Google Scholar] [CrossRef]
- Mortimer, J.A.; Gosche, K.M.; Riley, K.P.; Markesbery, W.R.; Snowdon, D.A. Delayed recall, hippocampal volume and Alzhei-mer neuropathology: Findings from the Nun Study. Neurology 2004, 62, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Janssen, J.C.; Whitwell, J.L.; Watt, H.C.; Jenkins, R.; Frost, C.; Rossor, M.N.; Fox, N.C. Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: Longitudinal MRI study. Lancet 2003, 362, 1121–1122. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Prestia, A.; Rasser, P.E.; Bonetti, M.; Thompson, P.M. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer’s disease. J. Neurol. 2009, 256, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Rodriguez, M.; Barker, W.; Greig-Custo, M.; Rosselli, M.; Loewenstein, D.; Duara, R. Relationship between Cognitive Performance and Measures of Neurodegeneration among Hispanic and White Non-Hispanic Individuals with Normal Cognition, Mild Cognitive Impairment, and Dementia. J. Int. Neuropsychol. Soc. 2018, 24, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Hu, B.; Liang, C.; Zhao, L.; Jackson, M.; the Alzheimer’s Disease Neuroimaging Initiative. A longitudinal study of atrophy in amnestic mild cognitive impairment and normal aging revealed by cortical thickness. PLoS ONE 2012, 7, e48973. [Google Scholar] [CrossRef] [PubMed]
- García-Alberca, J.M.; Florido, M.; Cáceres, M.; Sánchez-Toro, A.; Lara, J.P.; García-Casares, N. Medial temporal lobe atrophy is independently associated with behavioural and psychological symptoms in Alzheimer’s disease. Psychogeriatrics 2019, 19, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef]
- Folstein, M.; Folstein, S.; McHugh, P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V.C.; Iliff, L.D.; Zilhka, E.; Du Boulay, G.H.; McAllister, V.L.; Marshall, J.; Russell, R.W.; Symon, L. Cerebral blood flow in dementia. Arch. Neurol. 1975, 32, 632–637. [Google Scholar] [CrossRef]
- Cummings, J.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Downs, T.; Cash, H.; Grotz, R. Progress in the development of an index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Lawton, M.; Brody, E. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Evans, D.A.; Hebert, L.; Langa, K.M.; Heeringa, S.G.; Plassman, B.L.; Kukull, W.A. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011, 7, 61–73. [Google Scholar] [CrossRef]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet 2022, 7, 105–125. [Google Scholar]
- Montine, T.J.; Bukhari, S.A.; White, L.R. Cognitive Impairment in Older Adults and Therapeutic Strategies. Pharm. Rev. 2021, 73, 152–162. [Google Scholar] [CrossRef]
- Corey-Bloom, J. The ABC of Alzheimer’s disease: Cognitive changes and their management in Alzheimer’s disease and related dementias. Int. Psychogeriatr. 2002, 14, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain Imaging in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- Pasi, M.; Poggesi, A.; Pantoni, L. The use of CT in dementia. Int. Psychogeriatr. 2011, 23, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS—ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Boutet, C.; Chupin, M.; Colliot, O.; Sarazin, M.; Mutlu, G.; Drier, A.; Pellot, A.; Dormont, D.; Lehéricy, S. Alzheimer’s Disease Neuroimaging Initiative. Is radiological evaluation as good as computer-based volumetry to assess hippocampal atrophy in Alzheimer’s disease? Neuroradiology 2012, 54, 1321–1330. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Henneman, W.J.; van der Flier, W.M.; de Vries, O.; Träber, F.; Geurts, J.J.; Scheltens, P.; Vrenken, H.; Barkhof, F. Diagnostic imaging of patients in a memory clinic: Comparison of MR imaging and 64-detector row CT. Radiology 2009, 253, 174–183. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, X.; Chang, L.; Song, X.; Li, X.; Wang, J.; Li, J. Influence of MRI on Diagnostic Efficacy and Satisfaction of Patients with Alzheimer’s Disease. Comput. Math. Methods Med. 2021, 2021, 9038784. [Google Scholar] [CrossRef]
- Kaushik, S.; Vani, K.; Chumber, S.; Anand, K.S.; Dhamija, R.K. Evaluation of MR Visual Rating Scales in Major Forms of Dementia. J. Neurosci. Rural Pract. 2021, 12, 16–23. [Google Scholar] [CrossRef]
- Rau, A.; Urbach, H. The MTA score—simple and reliable, the best for now? Eur. Radiol. 2021, 31, 9057–9059. [Google Scholar] [CrossRef]
- Petersen, R.C.; Jack, C.R.; Xu, Y.C.; Waring, S.C.; O’brien, P.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Boeve, B.F.; Kokmen, E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000, 54, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.J.; Cvoro, V.; MacLullich, A.M.J.; Shenkin, S.D.; Sandercock, P.A.G.; Sakka, E.; Wardlaw, J.M. Visual rating scales of white matter hyperintensities and atrophy: Comparison of computed tomography and magnetic resonance imaging. J. Stroke Cereb. Dis. 2018, 7, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Claus, J.J.; Staekenborg, S.S.; Holl, D.C.; Roorda, J.J.; Schuur, J.; Koster, P.; Tielkes, C.E.M.; Scheltens, P. Practical use of visual medial temporal lobe atrophy cut-off scores in Alzheimer’s disease: Validation in a large memory clinic population. Eur. Radiol. 2017, 27, 3147–3155. [Google Scholar] [CrossRef]
- Zhang, Y.; Londos, E.; Minthon, L.; Wattmo, C.; Liu, H.; Aspelin, P.; Wahlund, L.O. Usefulness of computed tomography linear measurements in diagnosing Alzheimer’s disease. Acta Radiol. 2008, 49, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bosco, P.; Redolfi, A.; Bocchetta, M.; Ferrari, C.; Mega, A.; Galluzzi, S.; Austin, M.; Chincarini, A.; Collins, D.L.; Duchesne, S.; et al. The impact of automated hippocampal volumetry on diagnostic confidence in patients with suspected Alzheimer’s disease: A European Alzheimer’s Disease Consortium study. Alzheimers Dement. 2017, 13, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Du, A.T.; Schuff, N.; Amend, D.; Laakso, M.P.; Hsu, Y.Y.; Jagust, W.J.; Yaffe, K.; Kramer, J.H.; Reed, B.; Norman, D.; et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 71, 441–447. [Google Scholar] [CrossRef]
- Molinder, A.; Ziegelitz, D.; Maier, S.E.; Eckerström, C. Validity and reliability of the medial temporal lobe atrophy scale in a memory clinic population. BMC Neurol. 2021, 21, 289. [Google Scholar] [CrossRef]
- Visser, P.J.; Verhey, F.R.; Hofman, P.A.; Scheltens, P.; Jolles, J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2002, 72, 491–497. [Google Scholar]
- Du, A.T.; Schuff, N.; Amend, D.; Laakso, M.P.; Hsu, Y.Y.; Jagust, W.J.; Yaffe, K.; Kramer, J.H.; Reed, B.; Norman, D.; et al. Visual assessment of medial temporal atrophy on MR films in Alzheimer’s disease: Comparison with volumetry. Aging Clin. Exp. Res. 2005, 17, 8–13. [Google Scholar]
- Schoonenboom, N.S.; van der Flier, W.M.; Blankenstein, M.A.; Bouwman, F.H.; Van Kamp, G.J.; Barkhof, F.; Scheltens, P. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol. Aging 2008, 29, 669–675. [Google Scholar] [CrossRef]
- Westman, E.; Cavallin, L.; Muehlboeck, J.S.; Zhang, Y.; Mecocci, P.; Vellas, B.; Tsolaki, M.; Kłoszewska, I.; Soininen, H.; Spenger, C.; et al. Sensitivity and specificity of medial temporal lobe visual ratings and multivariate regional MRI classification in Alzheimer’s disease. PLoS ONE 2011, 6, e22506. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yang, H.; Choi, Y.J.; Kang, H.J.; Choi, K.G.; Jeong, J.H. Predictive Factors for Decline in Activities of Daily Living in Alzheimer’s Disease Dementia with More than 2 Follow-up. Dement. Neurocogn. Disord. 2013, 12, 100–106. [Google Scholar] [CrossRef]
- Yoon, B.; Shim, Y.S.; Cheong, H.K.; Hong, Y.J.; Lee, K.S.; Park, K.H.; Ahn, K.J.; Kim, D.J.; Kim, Y.D.; Choi, S.H.; et al. White matter hyperintensities in mild cognitive impairment: Clinical impact of location and interaction with lacunes and medial temporal atrophy. J Stroke Cereb. Dis. 2014, 23, 365–372. [Google Scholar] [CrossRef]
- An, M.h.; Kim, H.; Lee, K.J. Association between Medial Temporal Atrophy, White Matter Hyperintensities, Neurocognitive Functions and Activities of Daily Living in Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Korean J. Psychosom. Med. 2021, 29, 67–76. [Google Scholar]
- Tae, W.S.; Kim, S.S.; Lee, K.U.; Nam, E.C.; Kim, K.W. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology 2008, 50, 569–581. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef]
- Dhikav, V.; Sethi, M.; Anand, K.S. Medial temporal lobe atrophy in Alzheimer’s disease/mild cognitive impairment with depression. Br. J. Radiol. 2014, 87, 20140150. [Google Scholar] [CrossRef]
- Vanhoenacker, A.S.; Sneyers, B.; De Keyzer, F.; Heye, S.; Demaerel, P. Evaluation and clinical correlation of practical cut-offs for visual rating scales of atrophy: Normal aging versus mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Belg. 2017, 117, 661–669. [Google Scholar] [CrossRef]
- Van de Pol, L.A.; Hensel, A.; Barkhof, F.; Gertz, H.J.; Scheltens, P.; van der Flier, W.M. Hippocampal atrophy in Alzheimer disease: Age matters. Neurology 2006, 66, 236–238. [Google Scholar] [CrossRef]
- Raji, C.A.; Lopez, O.L.; Kuller, L.H.; Carmichael, O.T.; Becker, J.T. Age, Alzheimer disease, and brain structure. Neurology 2009, 73, 1899–1905. [Google Scholar] [CrossRef]
- Guaita, A.; Brunelli, L.; Davin, A.; Poloni, T.E.; Vaccaro, R.; Gagliardi, S.; Pansarasa, O.; Cereda, C. Homocysteine, Folic Acid, Cyanocobalamin, and Frailty in Older People: Findings From the “Invece. Ab” Study. Front. Physiol. 2021, 15, 775803. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; D’Onofrio, G.; Ciccone, F.; Germano, C.; Cascavilla, L.; Paris, F.; Greco, A. Relationship of Homocysteine Plasma Levels with Mild Cognitive Impairment, Alzheimer’s Disease, Vascular Dementia, Psychobehavioral, and Functional Complications. J. Alzheimers Dis. 2021, 82, 235–248. [Google Scholar] [CrossRef] [PubMed]


| MTA 0 n = 14 | MTA 1 n = 44 | MTA 2 n = 63 | MTA 3 n = 79 | MTA 4 n = 39 | p-Value | |
|---|---|---|---|---|---|---|
| Sex | 0.025 | |||||
| Males/Females | 7/7 | 22/22 | 22/41 | 25/54 | 16/23 | |
| Males (%) | 50.0 | 50.00 | 34.90 | 31.6 | 41.0 | |
| Age (years) | <0.0001 | |||||
| Mean ± SD | 72.60 ± 9.30 | 78.10 ± 6.57 | 79.12 ± 6.21 | 81.08 ± 6.19 | 80.70 ± 6.55 | |
| Range | 55.00–87.00 | 64.00–91.00 | 53.00–90.00 | 69.00–97.00 | 62.00–95.00 | |
| MMSE | <0.0001 | |||||
| Mean ± SD | 22.80 ± 7.39 | 19.91 ± 6.23 | 17.52 ± 6.48 | 14.94 ± 8.34 | 14.90 ± 8.80 | |
| Range | 9.00–30.00 | 5.00–30.00 | 0–30.00 | 0–28.00 | 0–27.00 | |
| FAB | 0.017 | |||||
| Mean ± SD | 11.80 ± 6.03 | 10.68 ± 5.69 | 8.71 ± 5.74 | 7.38 ± 6.01 | 9.00 ± 5.76 | |
| Range | 0 – 18.00 | 0 – 18.00 | 0 – 18.00 | 0 – 18.00 | 0–18.00 | |
| ADL | <0.0001 | |||||
| Mean ± SD | 5.13 ± 1.51 | 4.88 ± 1.49 | 4.52 ± 1.65 | 4.39 ± 1.67 | 4.38 ± 1.84 | |
| Range | 2.00–6.00 | 1.00–6.00 | 1.00–6.00 | 1.00–6.00 | 0–6.00 | |
| IADL | <0.0001 | |||||
| Mean ± SD | 5.60 ± 3.27 | 4.14 ± 3.04 | 3.68 ± 3.25 | 2.92 ± 3.10 | 2.87 ± 3.43 | |
| Range | 0–8.00 | 0–8.00 | 0–8.00 | 0–8.00 | 0–8.00 | |
| Diagnosis | <0.0001 | |||||
| No Cognitive Impairment | 4 (28.6) | 7 (15.9) | 0 | 0 | 0 | |
| Mild Cognitive Impairment | 2 (14.3) | 9 (20.5) | 5 (7.9) | 0 | 0 | |
| Alzheimer’s disease | 0 | 2 (4.5) | 22 (34.9) | 36 (45.6) | 26 (66.7) | |
| Vascular Dementia | 1 (7.1) | 10 (22.7) | 20 (31.7) | 23 (29.1) | 8 (20.5) | |
| Psycho-behavioral symptoms | 7 (50.0) | 16 (36.4) | 16 (25.4) | 20 (25.3) | 5 (12.8) | |
| Homocysteine, μmol/L | 0.032 | |||||
| Mean ± SD | 9.61 ± 2.95 | 11.95 ± 3.65 | 13.05 ± 7.39 | 14.19 ± 7.37 | 14.44 ± 5.07 | |
| Range | 5.90–15.00 | 64.20–23.00 | 6.00–47.00 | 6.30–49.88 | 8.10–31.00 | |
| Hypertension | ||||||
| Yes–n (%) | 9 (64.3) | 28 (63.6) | 38 (60.3) | 48 (60.8) | 24 (61.5) | 0.996 |
| No–n (%) | 5 (35.7) | 16 (36.4) | 25 (39.7) | 31 (39.2) | 15 (38.5) | |
| Diabetes | ||||||
| Yes–n (%) | 2 (14.3) | 9 (20.5) | 17 (27.0) | 26 (32.9) | 12 (30.8) | 0.450 |
| No–n (%) | 12 (85.7) | 35 (79.5) | 46 (73.0) | 53 (67.1) | 27 (69.2) | |
| Atrial fibrillation | ||||||
| Yes–n (%) | 2 (14.3) | 3 (6.8) | 8 (12.7) | 6 (7.6) | 3 (7.7) | 0.735 |
| No–n (%) | 12 (85.7) | 41 (93.2) | 55 (87.3) | 73 (92.4) | 36 (92.3) | |
| Dyslipidemia | ||||||
| Yes–n (%) | 7 (50.0) | 19 (43.2) | 19 (30.2) | 25 (31.6) | 12 (30.8) | 0.404 |
| No–n (%) | 7 (50.0) | 25 (56.8) | 44 (69.8) | 54 (68.4) | 27 (69.2) |
| MTA 0 n = 15 | MTA 1 n = 50 | MTA 2 n = 68 | MTA 3 n = 59 | MTA 4 n = 47 | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| NPI Total score | ||||||||
| Mean ± SD | 19.33 ± 19.52 | 18.44 ± 15.45 | 19.30 ± 18.32 | 21.83 ± 17.17 | 22.44 ± 16.56 | 0.002 | 11.564 | 4.452–18.676 |
| Range | 0–58.00 | 0–54.00 | 0–61.00 | 0–54.00 | 0–58.00 | |||
| Delusion | ||||||||
| Mean ± SD | 0.87 ± 2.47 | 0.44 ± 1.85 | 0.31 ± 1.38 | 0.53 ± 2.05 | 0.60 ± 2.07 | 0.879 | 0.068 | −0.808–0.944 |
| Range | 0–9.00 | 0–9.00 | 0–9.00 | 0–9.00 | 0–9.00 | |||
| Hallucination | ||||||||
| Mean ± SD | 0 | 0.76 ± 2.20 | 0.87 ± 2.46 | 0.90 ± 2.56 | 0.53 ± 2.01 | 0.839 | 0.107 | −0.931–1.145 |
| Range | 0 | 0–9.00 | 0–9.00 | 0–9.00 | 0–9.00 | |||
| Agitation/Aggression | ||||||||
| Mean ± SD | 1.93 ± 3.17 | 1.72 ± 2.70 | 2.50 ± 3.33 | 3.80 ± 4.11 | 3.21 ± 4.06 | 0.296 | 0.836 | −0.735–2.407 |
| Range | 0–9.00 | 0–9.00 | 0–12.00 | 0–12.00 | 0–12.00 | |||
| Depression | ||||||||
| Mean ± SD | 2.51 ± 3.39 | 3.25 ± 3.49 | 4.20 ± 4.65 | 4.24 ± 3.93 | 5.03 ± 4.12 | <0.0001 | 4.869 | 3.053–6.686 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | 0–12.00 | 0–12.00 | |||
| Anxiety | ||||||||
| Mean ± SD | 1.62 ± 3.11 | 1.75 ± 3.24 | 2.08 ± 3.37 | 2.93 ± 3.58 | 3.19 ± 4.02 | 0.001 | 2.792 | 1.120–4.464 |
| Range | 0–9.00 | 0–12.00 | 0–9.00 | 0–9.00 | 0–12.00 | |||
| Euphoria | ||||||||
| Mean ± SD | 0 | 0 | 0.07 ± 0.50 | 0.17 ± 1.18 | 0.09 ± 0.58 | 0.824 | −0.037 | −0.363–0.289 |
| Range | 0 | 0 | 0–4.00 | 0–9.00 | 0–4.00 | |||
| Apathy/Indifference | ||||||||
| Mean ± SD | 3.47 ± 4.66 | 2.88 ± 4.22 | 3.01 ± 3.88 | 4.12 ± 4.37 | 3.45 ± 4.00 | 0.366 | 0.815 | −0.955–2.585 |
| Range | 0–12.00 | 0–12.00 | 0–12.00 | 0–12.00 | 0–12.00 | |||
| Disinhibition | ||||||||
| Mean ± SD | 0 | 0.16 ± 0.79 | 0.65 ± 2.04 | 0.32 ± 1.65 | 0.19 ± 1.31 | 0.932 | 0.031 | −0.692–0.754 |
| Range | 0 | 0–4.00 | 0–9.00 | 0–9.00 | 0–9.00 | |||
| Irritability/Lability | ||||||||
| Mean ± SD | 2.13 ± 3.70 | 1.22 ± 2.79 | 2.47 ± 3.54 | 3.14 ± 3.78 | 2.85 ± 3.95 | 0.898 | 0.103 | −1.470–1.675 |
| Range | 0–9.00 | 0–9.00 | 0–9.00 | 0–9.00 | 0–12.00 | |||
| Aberrant Motor Behavior | ||||||||
| Mean ± SD | 0.27 ± 1.03 | 0.56 ± 2.01 | 0.50 ± 1.76 | 0.53 ± 2.05 | 0.09 ± 0.58 | 0.305 | 0.413 | −0.378–1.204 |
| Range | 0–4.00 | 0–9.00 | 0–9.00 | 0–9.00 | 0–4.00 | |||
| Sleep/night-time behavior | ||||||||
| Mean ± SD | 2.67 ± 4.08 | 3.10 ± 3.92 | 2.94 ± 3.94 | 2.59 ± 3.87 | 3.23 ± 3.93 | 0.333 | 0.858 | −0.884–2.600 |
| Range | 0–9.00 | 0–12.00 | 0–12.00 | 0–12.00 | 0–12.00 | |||
| Appetite/eating change | ||||||||
| Mean ± SD | 0.87 ± 2.48 | 1.20 ± 2.63 | 0.68 ± 1.89 | 0.75 ± 2.53 | 0.85 ± 2.39 | 0.209 | 0.704 | −0.398–1.805 |
| Range | 0–9.00 | 0–9.00 | 0–9.00 | 0–12.00 | 0–12.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauriola, M.; D’Onofrio, G.; la Torre, A.; Ciccone, F.; Germano, C.; Cascavilla, L.; Greco, A. CT-Detected MTA Score Related to Disability and Behavior in Older People with Cognitive Impairment. Biomedicines 2022, 10, 1381. https://doi.org/10.3390/biomedicines10061381
Lauriola M, D’Onofrio G, la Torre A, Ciccone F, Germano C, Cascavilla L, Greco A. CT-Detected MTA Score Related to Disability and Behavior in Older People with Cognitive Impairment. Biomedicines. 2022; 10(6):1381. https://doi.org/10.3390/biomedicines10061381
Chicago/Turabian StyleLauriola, Michele, Grazia D’Onofrio, Annamaria la Torre, Filomena Ciccone, Carmela Germano, Leandro Cascavilla, and Antonio Greco. 2022. "CT-Detected MTA Score Related to Disability and Behavior in Older People with Cognitive Impairment" Biomedicines 10, no. 6: 1381. https://doi.org/10.3390/biomedicines10061381
APA StyleLauriola, M., D’Onofrio, G., la Torre, A., Ciccone, F., Germano, C., Cascavilla, L., & Greco, A. (2022). CT-Detected MTA Score Related to Disability and Behavior in Older People with Cognitive Impairment. Biomedicines, 10(6), 1381. https://doi.org/10.3390/biomedicines10061381







