1. Introduction
Musculoskeletal pain in the human upper limb constitutes a leading cause of disability in working populations [
1], and wrist pain accounts for a high percentage [
2]. Wrist pain may be the consequence of traumatic, inflammatory, infectious, neoplastic, metabolic, or degenerative conditions [
3,
4]. In addition, wrist pain can be caused by specific disorders of the palmar structures of the carpus, such as carpal tunnel syndrome, a clinical condition secondary to the compression of the
nervus medianus leading to local numbness, weakness, and pain [
5], that may develop independently or be associated and overshadowed by other diseases.
Dogs have been widely used as animal models in musculoskeletal research. However, based on our review of the literature, the use of this species to investigate specific palmar disorders is scarce.
To properly design, plan, and carry out any study on a particular animal model, a correct knowledge of its anatomy is mandatory. Despite the fact that the dog is one of the most studied species in the veterinary field, the information about the anatomy of its carpal region found in the literature is not consistent.
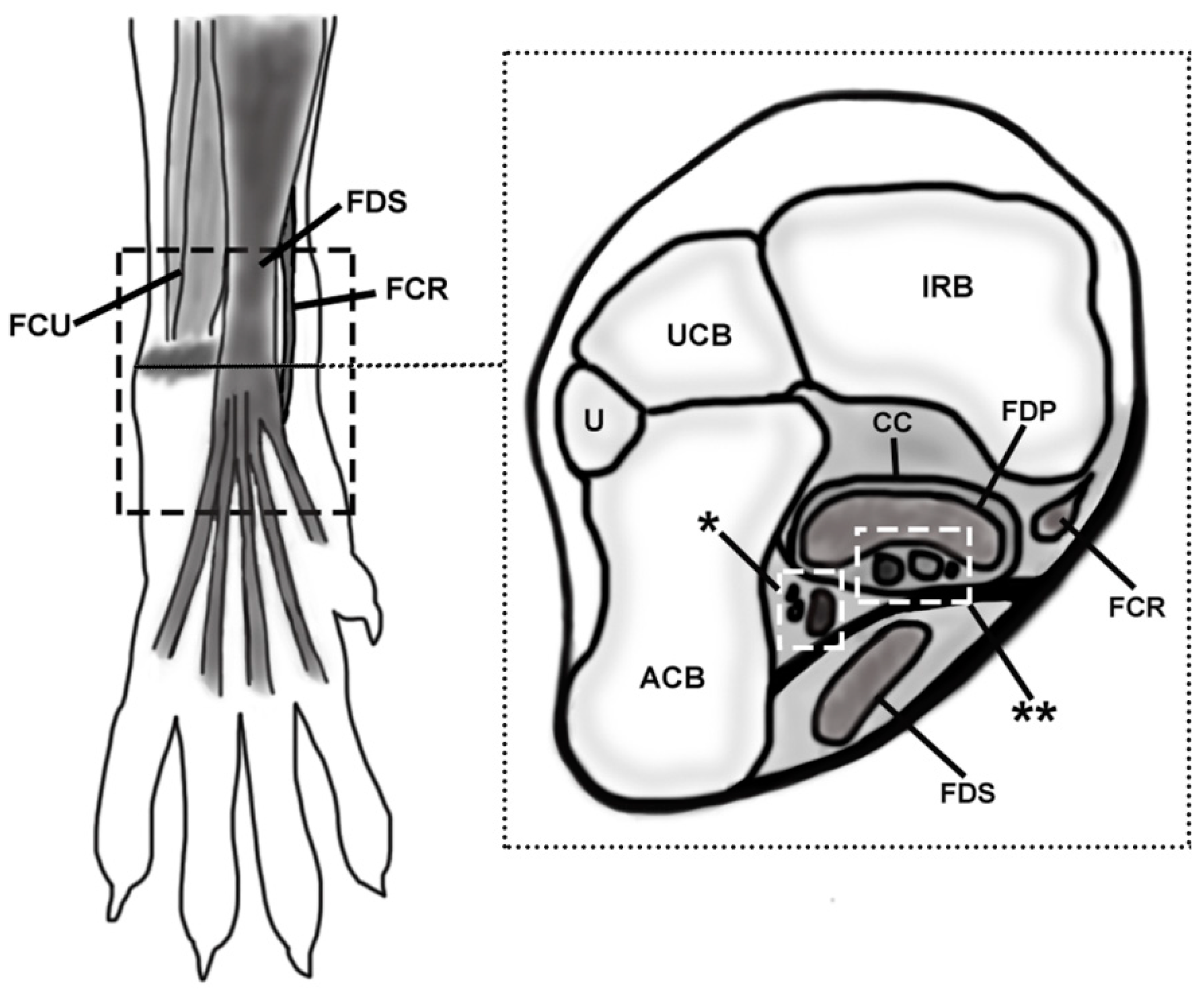
The palmar part of the canine carpus is an anatomically complex region consisting of the tendons of the flexor muscles of the carpus and digits (
flexor carpi radialis,
flexor digitorum superficialis,
flexor digitorum profundus, and
flexor carpi ulnaris), the
flexor retinaculum, the palmar carpal ligaments, and the neurovascular structures (mainly the
arteria and
vena mediana, the
nervus medianus, and the
arteria and
nervus ulnaris (
Figure 1). Barone [
6] described the
canalis carpi as the space located deep in the
flexor retinaculum, containing the
nervus medianus, the
arteria and
vena mediana, and the tendon of the
flexor digitorum profundus muscle. The author stated that the tendon of the
flexor carpi radialis muscle passes inside a synovial sheath through its own fibrous sheath on the medial side of the carpus, independent of the
canalis carpi. Dyce et al. [
7] did not provide a detailed description of the canine
canalis carpi, but they explained that the
flexor digitorum profundus muscle passes through it while the
flexor carpi radialis and the
flexor digitorum superficialis muscles remain apart from the canal. König and Liebich [
8] defined the
canalis carpi as the space delimited between the
flexor retinaculum and the joint capsule of the carpus, containing the tendon of the
flexor digitorum profundus, the
nervus medianus, and the
nervus ulnaris. On the other hand, Evans and de Lahunta [
9] described that the
canalis carpi has a wider extent, being the space generated deep to the
flexor retinaculum and occupied by the tendons of the
flexor carpi radialis,
flexor digitorum superficialis, and
flexor digitorum profundus muscles, the
nervus medianus, the
arteria and
vena mediana, and the
arteria and
nervus ulnaris. This was also supported by Turan et al. [
10,
11,
12] in later studies about the diagnosis of potential carpal canal syndrome in dogs. Ettema et al. [
13], however, stated that the tendon of the
flexor digitorum superficialis muscle is not located inside the
canalis carpi.
The lack of agreement found in the veterinary literature impairs the understanding of the precise anatomical composition of this region in the canine carpus, complicating the comprehension of the real configuration of the canalis carpi and the anatomic relations of the carpal structures. Therefore, the objective of this study is the detailed description of the anatomy of the palmar region of the carpus of the dog, with special reference to the canalis carpi, in order to provide the basis for further studies of comparative anatomy and clinical research, that result in a better understanding of the carpal problems in both veterinary and human medicine
2. Materials and Methods
This study is a prospective, descriptive, anatomic design, part of a larger project for a doctoral thesis that was approved by the animal bioethics committee of the Rof Codina Veterinary University Hospital (HVU-RC) of the University of Santiago de Compostela.
The study’s methodology is based on similar procedures performed in other species such as humans [
14] and equines [
15]. For the anatomic identification of the carpal structures, the
Nomina Anatomica Veterinaria and veterinary anatomic textbooks [
6,
7,
8,
9] were used as references.
For this study, 92 cadaveric specimens were obtained from 46 dogs that had died for reasons unrelated to this study. The specimens were acquired from the Service of Pathological Anatomy in the HVU-RC.
Of these, 43 medium-to-large-breed dogs were randomly selected for dissection according to inclusion criteria, which were weighing more than 20 kg, the absence of gross musculoskeletal disease in an orthopedic examination, having no history of musculoskeletal or nervous diseases in their medical records, and the lack of radiographic signs of musculoskeletal disorders in the carpal region. Of these 43 canine cadavers, 20 were females (46.5%) and 23 were males (53.5%). The mean age of the specimens was 6.73 years (range 1.5–12 years). Of the 43 carpi in group A, 21 were right and 22 were left. Of the 43 carpi in group B, 22 were right and 22 were left. The breeds of the dogs were Beagle (n = 5), mixed breed (n = 5), German Shepherd (n = 12), Golden Retriever (n = 3), Great Dane (n = 1), Labrador Retriever (n = 3), Mastiff (n = 8), Pointer (n = 2), Rottweiler (n = 1), Spanish Scenthound (n = 1), and St. Bernard (n = 2).
The 86 cadaveric specimens were transected at the level of the elbow and frozen at −18 °C until used. Limbs were thawed at room temperature for 8 h, and radiographs of the carpal region were performed to exclude signs of musculoskeletal disorders. Orthogonal views (dorsopalmar and mediolateral) were obtained. During the design of the protocol, the use of diagnostic techniques such as ultrasound, which is more sensitive to evaluate soft tissues than radiography, was considered. However, given that the normal ultrasonographic anatomy of the components of the palmar region of the canine carpus has not been published, in contrast with the dorsal structures [
16], we decided not to use this technique to avoid potential bias. Both carpi of each dog were randomly assigned to group A or B, which included right and left specimens. The carpi of group A were dissected, and the carpi of group B were frozen again to −18 °C for 48 h in order to obtain cross-sections using a hand saw.
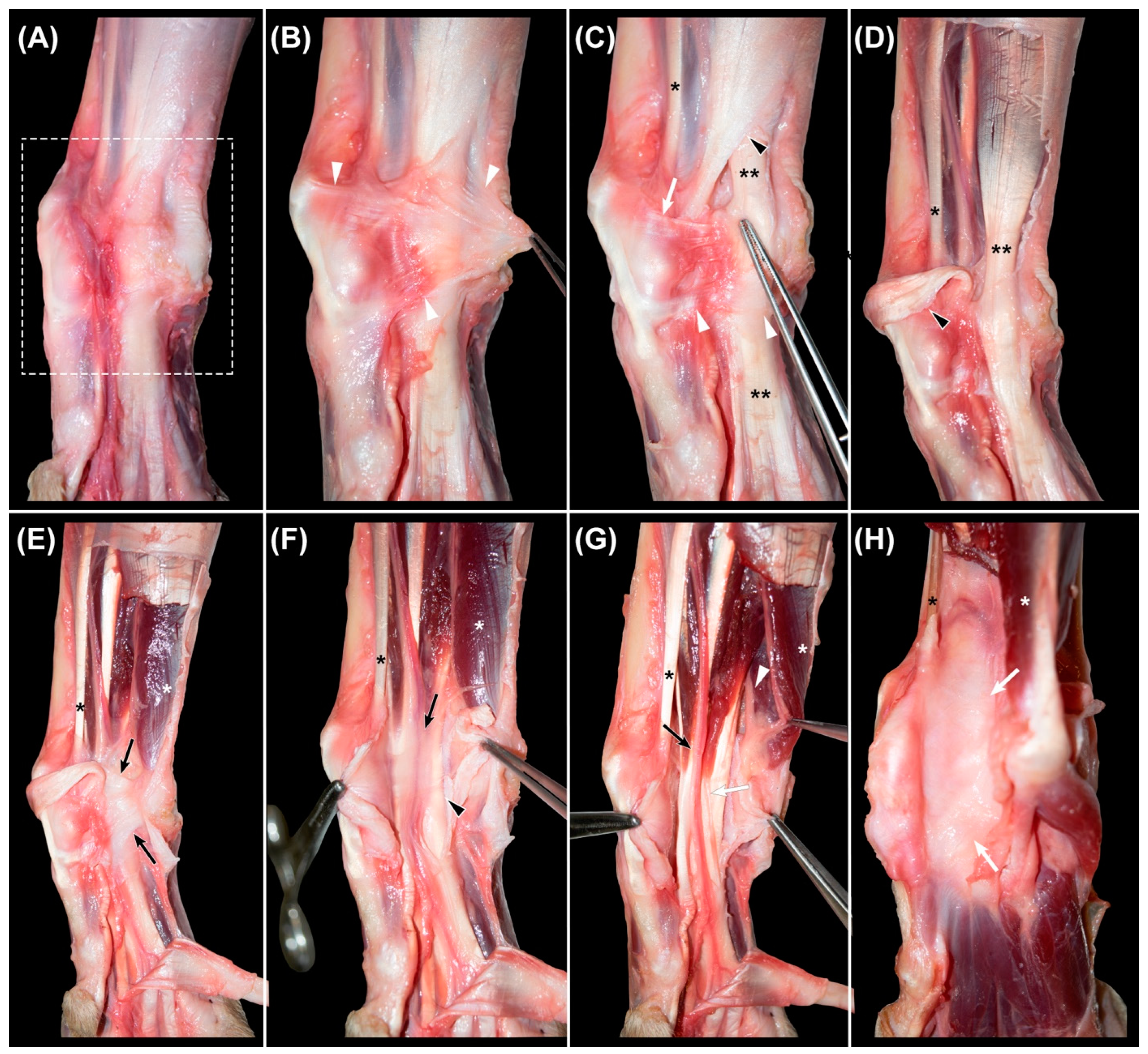
In the specimens in group A, the hair was clipped from the middle antebrachial region to the distal metacarpus. The skin was longitudinally incised at the dorsolateral aspect, then reflected, and the overlying fascia, connective tissue, and fat were removed. The dissection was performed sequentially, in a medial to lateral and superficial to deep fashion. The studied region of the included soft tissue structures was the area between the distal part of the antebrachium and the proximal aspect of the metacarpus (
Figure 1). The anatomic characteristics (location, shape, size, and topographic relations) of the structures of the palmar region of the canine carpus were qualitatively described and documented, with special attention to the following structures:
flexor retinaculum,
flexor carpi radialis muscle,
nervus medianus, arteria and
vena mediana,
interflexorius muscle,
flexor digitorum profundus muscle,
canalis carpi and
arteria, and
nervus ulnaris. In group B, 3 mm thick slices were cut with a hand saw, and photographs of both faces were taken and correlated with the findings of the dissection. Complementary histology was performed in samples of the
flexor retinaculum and
flexor carpi radialis tendon and surrounding tissues.
For complementary histology of the palmar structures in their anatomical position, three small breed dogs were randomly selected for obtaining transverse slices. The inclusion criteria were the same as for the other dogs, except for the weight. Therefore, three Yorkshire specimens were included, one male and two females, with a mean age of 12.03 years (range 10.25–13.83 years). The carpi were transected (3 mm thick slices), and the samples were fixed by immersion in 10% buffered formaldehyde for a minimum of 48 h. After decalcification in Shandon TDB-1TM, given the difficulty of cutting the calcified tissue, the samples were embedded in paraffin according to standard laboratory procedures, and sections of 5 μm thickness were mounted onto silanized slides and dried overnight at 37 °C. The sections were stained with H and E for routine histological analyses, Azan trichrome stain, OMSB (combined orcein and Martius scarlet blue) for elastic fibers, and Picro Sirius Red for collagen fibers. Samples were evaluated in terms of the general structure and anatomical relations with an Olympus AX70 microscope and a coupled digital camera Olympus DP74.
4. Discussion
The anatomy of the main structures of the palmar region of the carpus of the dog is described in this study.
We found a strong similarity between the canine and human
flexor retinaculum, given that both are composed of a superficial part, a specialized thickening of the fascia, and a deep one, a wider fibrous band, resembling a ligament. In humans, the superficial part is also constituted by proximal and distal sections [
17], as we have seen in the dog. The superficial part showed the characteristics of a typical
retinaculum, being a specialized thickening of the fascia that covered flexor tendons. However, the deep part was a strong fibrous band that resembled more a ligament and, despite being a deep continuation of the superficial part, it additionally showed attachment to bones. In human wrists, this deep structure has been named the “transverse carpal ligament” [
18], but the use of this term is controversial in the literature. Despite its anatomic resemblance to a ligament, its main function appears to be the reinforcement of the underlying tendons, which corresponds to that of a
retinaculum. Stecco et al. [
19] reviewed the terminology of these structures in humans, concluding that naming both parts as the “
flexor retinaculum” may induce confusion about which part is alluded to, so the term “transverse carpal ligament” should be used to specifically refer to the deep part that acts as the roof of the carpal canal (considering the carpus in dorsal recumbency).
In the veterinary literature, there is no specific terminology for this deep part. As previously explained, the
flexor retinaculum in dogs is briefly mentioned among the main anatomic reference sources and in the NAV, and a detailed description is lacking. Nevertheless, Nickel et al. [
20] state that the
flexor retinaculum corresponds to the transverse carpal ligament that forms the
canalis carpi. Therefore, considering our results, we propose to term this structure in the canine carpus as the “deep part of the
flexor retinaculum”, understanding that it is more accurate given its function and location.
In this study, the
canalis carpi in dogs was described as a non-distensible and well-defined oval space delimitated palmarly by the deep part of the
flexor retinaculum, laterally by a thin fibrous septum, dorsally by the common palmar ligament that covers the palmar region of the carpal bones, and medially by the fixations of the fused antebrachial fascia to the deep part of the
flexor retinaculum and the common palmar ligament. It contained the tendons of the
flexor digitorum profundus and the
interflexorius muscle, as well as the
nervus medianus and the
arteria and
vena mediana. The inconsistency found in the literature is probably related to the non-differentiation between the superficial and deep parts of the
flexor retinaculum. Dyce et al. [
7], Evans and de Lahunta [
9], and Turan et al. [
10,
11,
12] made their descriptions assuming that the
flexor retinaculum was only constituted by the superficial part, which led to the inclusion of other tendons and neurovascular structures inside the hypothetical
canalis carpi. However, the superficial part of the
retinaculum did not generate a true canal below because, by definition, a canal is a tubular duct or channel. The presence of the superficial part of the
retinaculum did not create a canal itself, and there is no defined canal beneath the
extensor retinaculum of the carpus. Barone [
6] and Ettema et al. [
13] did describe the
canalis carpi only as the space underlying the deep part of the
flexor retinaculum. Our results support this and correlate with human anatomy, where the
canalis carpi is a space in the palmar region of the carpus formed between the deep part of the
flexor retinaculum—the transverse carpal ligament—and the carpal bones, which contains the tendons of the
flexor digitorum superficialis,
flexor digitorum profundus, and
flexor pollicis longus muscles, as well as the
nervus medianus [
21]. In humans, the entrapment of this nerve originates a well-known clinical condition, carpal tunnel syndrome, which is the most common disorder of the hand [
19] and the most common peripheral mononeuropathy [
22]. In veterinary medicine, to the best of our knowledge, the clinical significance of this condition in dogs has not been established. However, its potential occurrence has been proposed [
11].
In this study, the tendon of the
flexor carpi radialis muscle was described as passing through its own fibrocartilaginous tunnel, independent of the
canalis carpi. Dyce et al. [
7] and Evans and de Lahunta [
9] included the course of this tendon inside the
canalis carpi, not mentioning this specific tunnel. König and Liebich [
8] and Nickel et al. [
20] described this tendon outside the
canalis carpi, passing into a synovial and tendinous sheath, respectively. Our results support the description of Barone [
6] and also correlate with human anatomy. In the radial side of the palmar region of the human wrist, the tendon of the
flexor carpi radialis muscle passes through its own osteofibrous tunnel independent of the
canalis carpi [
23], to insert in the base of the second, and often third, metacarpal bones [
24]. In humans, this tunnel also does not completely surround the tendon through its entire course; therefore, the
flexor carpi radialis muscle lies in close contact with the shared joint capsule of the scaphotrapeziotrapezoid (STT) or triscaphe articulation, which may lead to the extension of disease from one compartment to the other [
25]. Additionally, the anatomic characteristics of the tendon and the non-distensible nature of the tunnel are related to tendinitis [
23] and stenotic tendinopathy [
26], also known as the
flexor carpi radialis tunnel syndrome [
27], clinical conditions characterized by inflammation and pain. To our knowledge, there is no published information about these injuries in dogs.
Evans and de Lahunta [
9] and König and Liebich [
8] described the path of the
nervus ulnaris inside the
canalis carpi. Our results, however, place the
nervus ulnaris lateral to it, separated by a septum of connective tissue. These findings also correlate with human anatomy. In the ulnar side of the palmar region of the wrist, the
nervus ulnaris also passes outside the
canalis carpi, running through a tunnel termed the Guyon’s canal [
28,
29]. This is clinically important given that the nerve may suffer entrapment, which leads to a condition known as the ulnar tunnel syndrome; though less prevalent than the carpal tunnel syndrome, it constitutes the fourth most common entrapment neuropathy in the literature [
30], and the second most frequent in the human upper extremity after carpal tunnel syndrome [
31]. In the canine carpus, the nerve passes between the accessory carpal bone and the lateral septum of the
canalis carpi. Then, it continues deep to the medial accessory-metacarpal ligament, where it is surrounded by fat tissue. To the best of our knowledge, there is no published information about the entrapment of this nerve in canines.
Dogs have been widely selected as animal models in musculoskeletal research and have been used in multiple studies about osteoarthritis, both naturally and induced-occurring [
32,
33]. This species has been considered the closest to a gold standard as an experimental model for osteoarthritis because of its similarities with humans in anatomy, disease progression, and outcome [
32]. Furthermore, the size of these animals has shown other advantages as an experimental model in comparison with smaller species such as mice and rabbits, such as the possibility of using antemortem diagnostic and monitoring assessments (i.e., diagnostic imaging techniques). However, given that dogs are quadrupeds, their biomechanics differ from humans; in the resting position, the flexor compartment of the carpus is tensioned, which is not the case in bipedal subjects. This concept should be considered when canines are selected as animal models in musculoskeletal research. Although they have been used in studies centered on the carpus [
34,
35,
36] and the flexor tendons [
13], based on our review of the literature, the use of this animal to investigate the above-described specific palmar disorders is scarce.
The confusing anatomic descriptions published so far in the veterinary literature may have prevented the proper application and interpretation of diagnostic techniques. Further research is needed about all these carpal conditions in dogs in veterinary medicine, and the use of this species as animal models to investigate the aforementioned diseases in human wrists merits promotion.
The limitations of the present study are, first, the heterogeneity of the included dogs, which did not permit obtaining measurements of the structures transferable to the whole canine population. Additional studies may be interested in providing these data on particular breeds. Second, our results were mainly obtained from a gross anatomy approach, presenting a complete macroscopic perspective of the location, extensions, and anatomic relations of the main structures of the palmar side of the canine carpus. However, more histologically specific studies may be needed to determine the precise microscopic composition of these elements. Third, radiography is a sensitive technique to evaluate bones, but its sensitivity for the examination of soft tissue is poor. The use of more sensitive techniques such as ultrasound to rule out disease in these structures would have been optimal during the selection process of the cadaveric specimens. However, despite the fact that the normal ultrasonographic anatomy of the dorsal region of the carpus of the dog has recently been published [
16], the same information about the palmar structures is lacking. For that reason, this technique has not been included in the protocol of this study, but we consider that once a correct description of the anatomy of this region is published, further studies about the normal and pathological ultrasonographic appearance of the palmar structures of the canine carpus will be highly interesting.