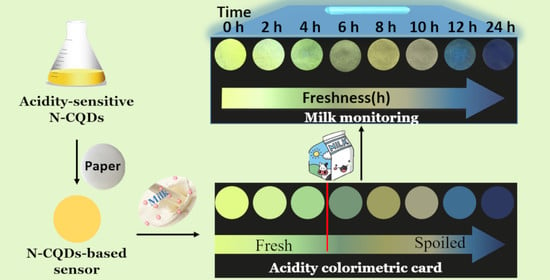
Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
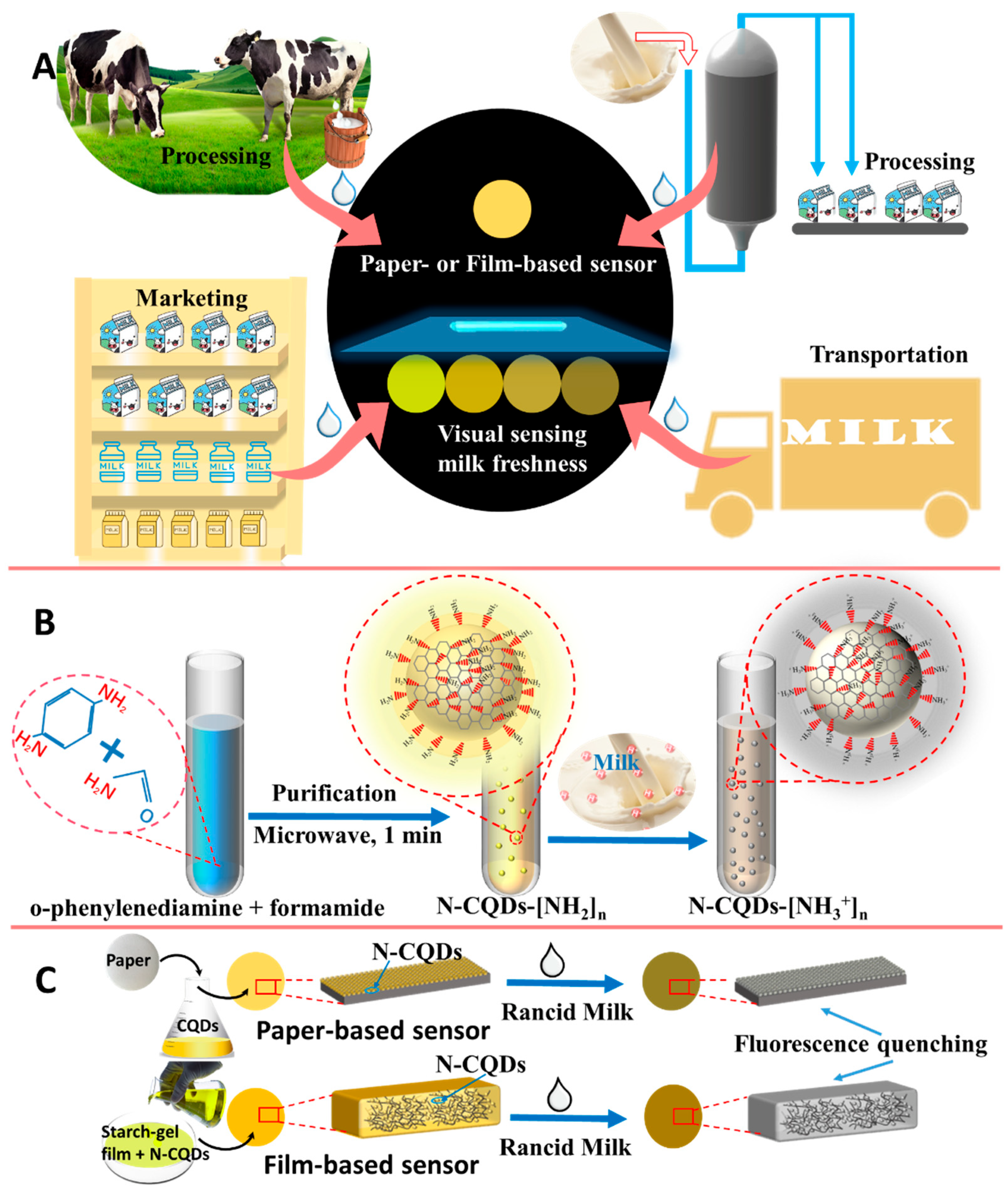
2.2. Preparation of Acidity-Responsive N-CQDs
2.3. Investigation of Fluorescence Change Mechanism
2.4. Establishment of Fluorescence Method
2.5. Fabrication of Visual Fluorescence Method
2.6. Fluorescence Monitoring of Milk Freshness
2.7. Standard Methods for Evaluating Milk Freshness
3. Results and Discussion
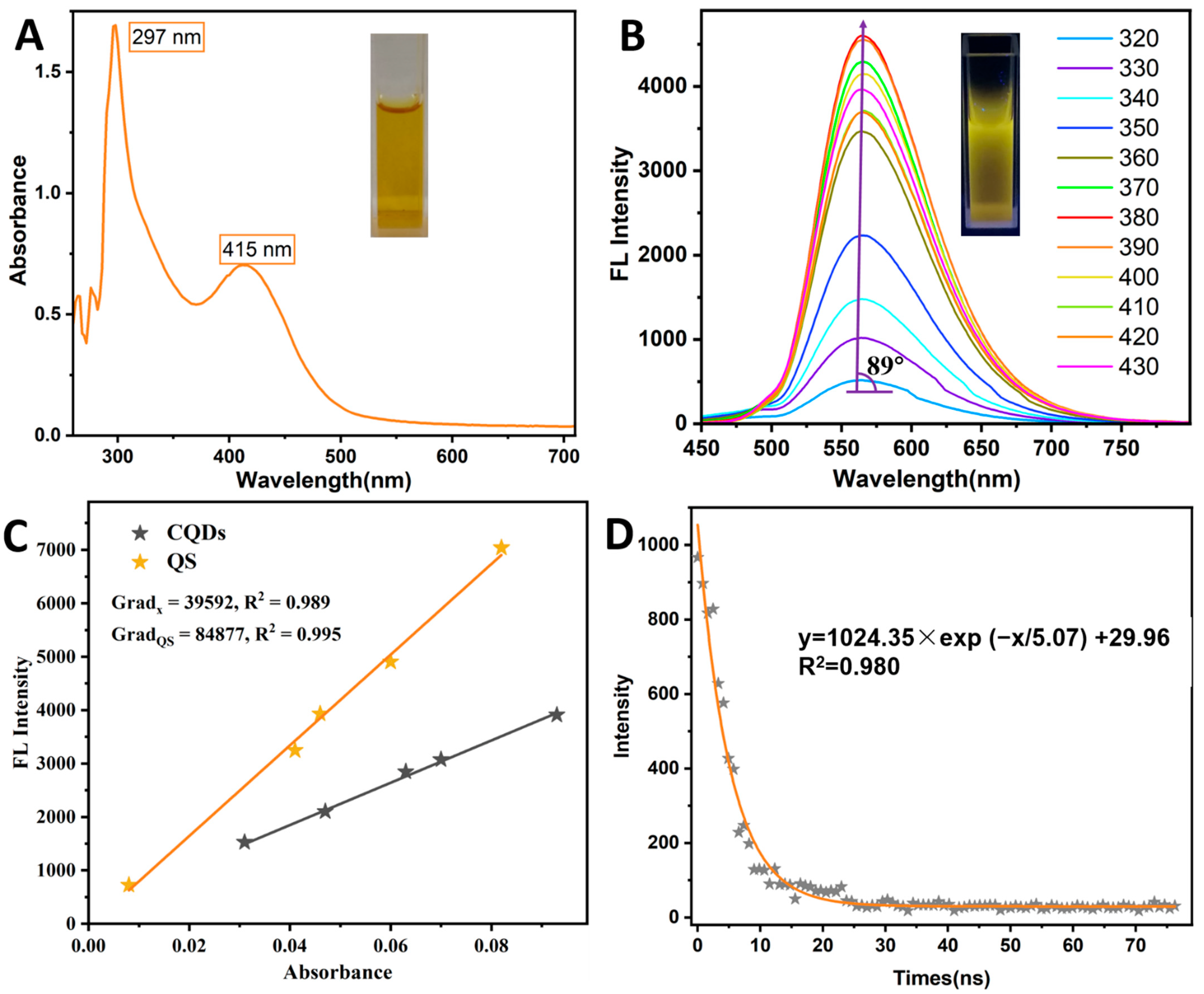
3.1. Structural Characterization of N-CQDs
3.2. Optical Properties of N-CQDs
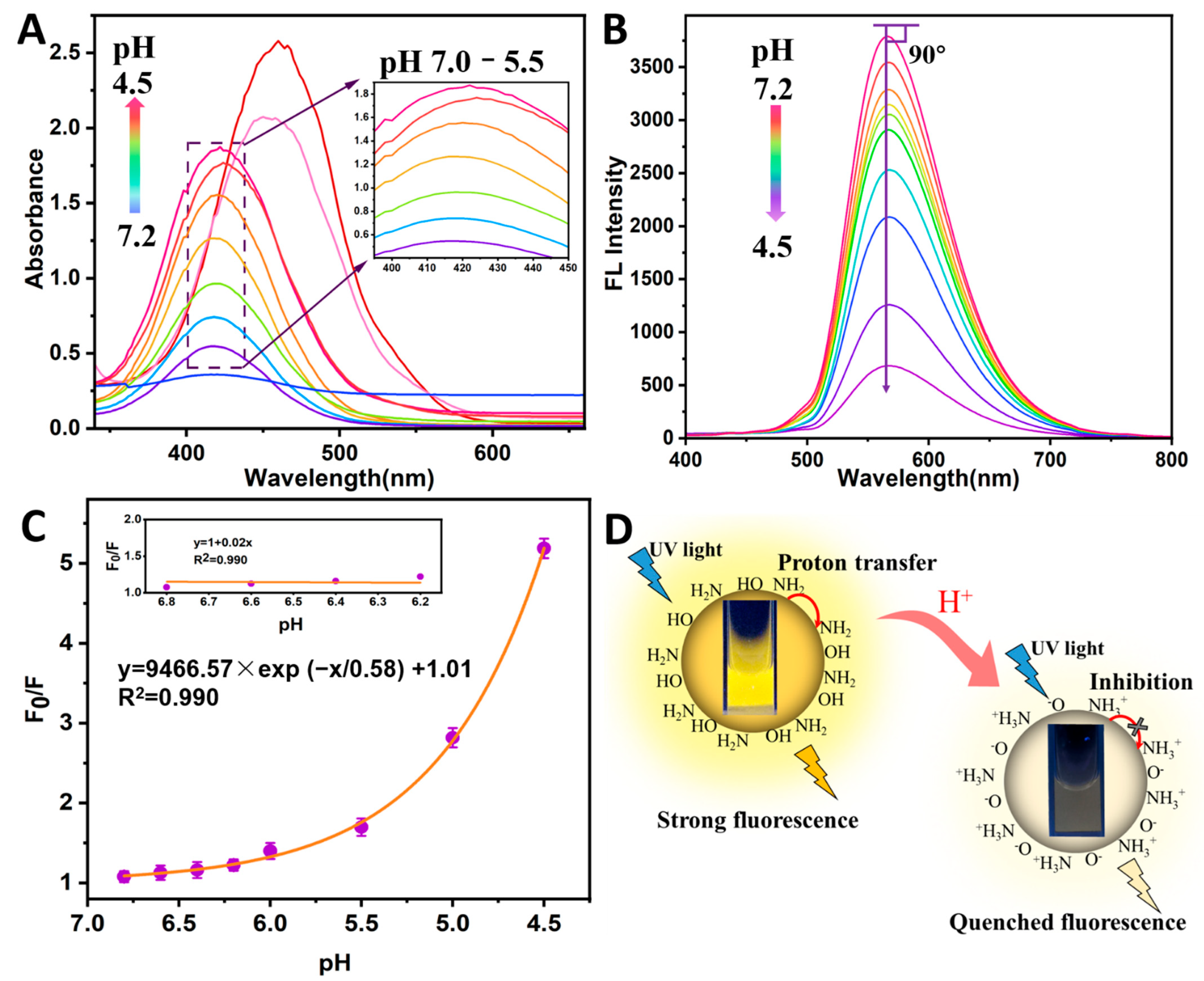
3.3. FL Response to Solutions with Various pH
3.4. N-CQDs-Based Sensitive FL Assay
3.5. FL-Based Visual Assay
3.6. Monitoring of Milk Freshness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, W.; Fu, L. (Eds.) Food Spoilage Microorganisms: Ecology and Control; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Malik, A.; Erginkaya, Z.; Erten, H. Health and Safety Aspects of Food Processing Technologies; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Martin, N.H.; Torres-Frenzel, P.; Wiedmann, M. Invited review: Controlling dairy product spoilage to reduce food loss and waste. J. Dairy Sci. 2020, 104, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Kaushik, G.; Hussain, C.M.; Chel, A. Food safety issues associated with Milk: A review. Saf. Issues Beverage Prod. 2020, 18, 399–427. [Google Scholar]
- Pathot, Y.D. Hygienic Practices and Bacteriological Quality of Milk: A Review. Int. J. Res. Granthaalayah 2019, 7, 341–356. [Google Scholar] [CrossRef]
- Ziyaina, M.; Govindan, B.N.; Rasco, B.; Coffey, T.; Sablani, S.S. Monitoring Shelf Life of Pasteurized Whole Milk under Refrigerated Storage Conditions: Predictive Models for Quality Loss. J. Food Sci. 2018, 83, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Vieco, D.; Vázquez-Román, S.; Sánchez-Pallás, J.; Ureta-Velasco, N.; Mosqueda-Peña, R.; Pallás-Alonso, C.R. Determination of Acidity in Donor Milk. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2016, 32, NP73–NP75. [Google Scholar] [CrossRef]
- Choudhary, S.; Joshi, B.; Pandey, G.; Joshi, A. Application of single and dual fluorophore-based pH sensors for determination of milk quality and shelf life using a fibre optic spectrophotometer. Sens. Actuators B Chem. 2019, 298, 126925. [Google Scholar] [CrossRef]
- Lu, M.; Shiau, Y.; Wong, J.; Lin, R.; Kravis, H.; Blackmon, T.; Pakzad, T.; Jen, T.; Cheng, A.; Chang, J. Milk spoilage: Methods and practices of detecting milk quality. Food Nutr. Sci. 2013, 4, 113. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Ping, T.; Wu, W.; Yang, Q. Sandwich Fluorescence Detection of Foodborne Pathogen Staphylococcus aureus with CD Fluorescence Signal Amplification in Food Samples. Foods 2022, 11, 945. [Google Scholar] [CrossRef]
- Kondee, S.; Arayawut, O.; Pon-On, W.; Wongchoosuk, C. Nitrogen-doped carbon oxide quantum dots for flexible humidity sensor: Experimental and SCC-DFTB study. Vacuum 2022, 195, 110648. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Magnaghi, L.R.; Capone, F.; Alberti, G.; Zanoni, C.; Mannucci, B.; Quadrelli, P.; Biesuz, R. EVOH-Based pH-Sensitive Optode Array and Chemometrics: From Naked-Eye Analysis to Predictive Modeling to Detect Milk Freshness. ACS Food Sci. Technol. 2021, 1, 819–828. [Google Scholar] [CrossRef]
- Yan, X.; Chen, H.; Du, G.; Guo, Q.; Yuan, Y.; Yue, T. Recent trends in fluorescent aptasensors for mycotoxin detection in food: Principles, constituted elements, types, and applications. Food Front. 2022. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Han, Y.; Wang, Z.; Li, H.; Sun, S.; Xu, Y. A pH-sensitive ESIPT molecule with aggregation-induced emission and tunable solid-state fluorescence multicolor for anti-counterfeiting and food freshness detection. Chem. Eng. J. 2022, 428, 130986. [Google Scholar] [CrossRef]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Li, H.; Li, F. pH-Response Quantum Dots with Orange-Red Emission for Monitoring the Residue, Distribution, and Variation of an Organophosphorus Pesticide in an Agricultural Crop. J. Agric. Food Chem. 2021, 69, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Ehtesabi, H.; Hallaji, Z.; Nobar, S.N.; Bagheri, Z. Carbon dots with pH-responsive fluorescence: A review on synthesis and cell biological applications. Mikrochim. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Sun, X.; Pan, W.; Wang, J. Carbon-dot-based ratiometric fluorescent pH sensor for the detections of very weak acids assisted by auxiliary reagents that contribute to the release of protons. Sens. Actuators B Chem. 2017, 244, 441–449. [Google Scholar] [CrossRef]
- Huo, F.; Li, W.; Liu, Y.; Liu, X.; Lee, C.-Y.; Zhang, W. Review of long wavelength luminescent carbon-based nanomaterials: Preparation, biomedical application and future challenges. J. Mater. Sci. 2021, 56, 2814–2837. [Google Scholar] [CrossRef]
- Mondal, T.K.; Saha, S.K. Facile approach to synthesize nitrogen-and oxygen-rich carbon quantum dots for pH sensor, fluorescent indicator, and invisible ink applications. ACS Sustain. Chem. Eng. 2019, 7, 19669–19678. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/orange dual-emissive carbon dots for pH sensing and cell imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, R.; Wang, B.; Deng, Z.; Jin, Y.; Kang, Y.; Chen, J. Orange, yellow and blue luminescent carbon dots controlled by surface state for multicolor cellular imaging, light emission and illumination. Mikrochim. Acta 2018, 185, 539. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wen, S.; Li, W.; Li, M.; Wang, L.; Chang, Q.; Zhang, J.; Lai, J.; Vajtai, R.; Ajayan, P.M. A universal strategy to separate hydrophilic hybrid-light carbon quantum dots using pure water as eluent. Appl. Mater. Today 2020, 18, 100528. [Google Scholar] [CrossRef]
- GB 19301-2010; Raw Milk. Ministry of Health of the People’s Republic of China: Beijing, China, 2010.
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem. Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef]
- Ren, S.; Giusti, M.M. Monitoring the Interaction between Thermally Induced Whey Protein and Anthocyanin by Fluorescence Quenching Spectroscopy. Foods 2021, 10, 310. [Google Scholar] [CrossRef]
- Chen, J.-K.; Yang, S.-M.; Li, B.-H.; Lin, C.-H.; Lee, S. Fluorescence Quenching Investigation of Methyl Red Adsorption on Aluminum-Based Metal-Organic Frameworks. Langmuir ACS J. Surf. Colloids 2018, 34, 1441–1446. [Google Scholar] [CrossRef]
- Ramos-Lledó, P.; Vera, S.; Andrés, M.P.S. Determination of vitamins A and E in milk samples by fluorescence in micellar media. Fresenius J. Anal. Chem. 2001, 369, 91. [Google Scholar] [CrossRef]
- Fabro, M.A.; Milanesio, H.V.; Robert, L.M.; Speranza, J.L.; Murphy, M.; Rodríguez, G.; Castañeda, R. Technical Note: Determination of Acidity in Whole Raw Milk: Comparison of Results Obtained by Two Different Analytical Methods. J. Dairy Sci. 2006, 89, 859–861. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Arslan, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. Use of a smartphone for visual detection of melamine in milk based on Au@Carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef]
- Lai, X.; Liu, C.; He, H.; Li, J.; Wang, L.; Long, Q.; Zhang, P.; Huang, Y. Hydrothermal synthesis and characterization of nitrogen-doped fluorescent carbon quantum dots from citric acid and urea. Ferroelectrics 2020, 566, 116–123. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence carbon dots with excitation independent emission for pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Zhang, X.; Li, Y.; Shi, J.; Huang, X.; Li, Z.; Zhang, J.; Li, W.; Xu, Y.; Zou, X. Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots. Foods 2022, 11, 1855. https://doi.org/10.3390/foods11131855
Hu X, Zhang X, Li Y, Shi J, Huang X, Li Z, Zhang J, Li W, Xu Y, Zou X. Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots. Foods. 2022; 11(13):1855. https://doi.org/10.3390/foods11131855
Chicago/Turabian StyleHu, Xuetao, Xinai Zhang, Yanxiao Li, Jiyong Shi, Xiaowei Huang, Zhihua Li, Junjun Zhang, Wenting Li, Yiwei Xu, and Xiaobo Zou. 2022. "Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots" Foods 11, no. 13: 1855. https://doi.org/10.3390/foods11131855
APA StyleHu, X., Zhang, X., Li, Y., Shi, J., Huang, X., Li, Z., Zhang, J., Li, W., Xu, Y., & Zou, X. (2022). Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots. Foods, 11(13), 1855. https://doi.org/10.3390/foods11131855