High Doses of Inhaled Nitric Oxide as an Innovative Antimicrobial Strategy for Lung Infections
Abstract
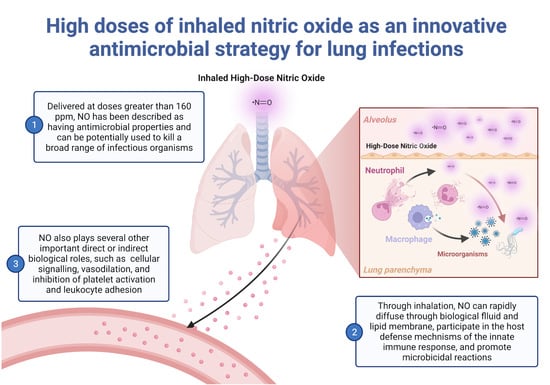
:1. Introduction
2. Biosynthesis of Nitric Oxide
3. Nitric Oxide Concentration-Dependent Mechanisms of Action
3.1. Low-Dose NO as a Molecular Mediator of Cellular Signalling
3.2. High-Dose NO and Its Antimicrobial Effect
4. High-Dose Exogenous NO as an Antimicrobial Treatment
4.1. High-Dose Exogenous Nitric Oxide: In Vitro Studies
4.2. High-Dose Exogenous Nitric Oxide: In Vivo Experimental Studies
4.3. High-Dose Exogenous Nitric Oxide: Clinical Studies
5. Novel In Vivo Strategies to Deliver Continuously Antimicrobial Doses of Inhaled Nitric Oxide
5.1. Continuous Inhaled Nitric Oxide Delivery during Ex-Vivo Lung Perfusion
5.2. Continuous Inhaled Nitric Oxide Delivery in an In Vivo Large Animal Model
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NO | Nitric oxide |
| iNO | Inhaled nitric oxide |
| ppm | Parts per million |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| cGMP | Cyclic guanosine monophosphate |
| cNOS | Constitutive NO synthase |
| NOS | Nitric oxide synthase |
| eNOS/NOS3 | Endothelial NO synthase |
| nNOS/NOS1 | Neuronal NO synthase |
| iNOS/NOS2 | Inducible NO synthase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| O2− | Superoxide |
| H2O2 | Hydrogen peroxide |
| OH− | Hydroxide |
| ONOO− | Peroxynitrite |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| FDA | U.S Food & Drug Administration |
| SNAP | S-nitroso-L-acetylpenicillamine) |
| HSV 1 | Herpes simplex virus type 1 |
| RAW 264.7 | Monocyte/macrophage-like cells |
| H1N1 | Influenza A virus subtype H1N1 |
| H3N2 | Influenza A virus subtype H3N2 |
| MDCK | Mabin Darby canine kidney cells |
| PFU | Plaque forming units |
| CFU | Colony forming units |
| metHb | Methemoglobin |
| NO2 | Nitrogen dioxide |
| CF | Cystic fibrosis |
| NTM | Non-tuberculous mycobacterium |
| COVID-19 | Coronavirus disease 2019 |
| VT | Tidal volume |
| PEEP | Positive end-expiratory pressure |
| bpm | Beats per minute |
| FiO2 | Fraction of inspired oxygen |
| EVLP | Ex vivo lung perfusion |
| PC | Pressure control |
| MB | Methylene blue |
| IV | Intravenous |
References
- Gao, F.; Lucke-Wold, B.P.; Li, X.; Logsdon, A.F.; Xu, L.C.; Xu, S.; LaPenna, K.B.; Wang, H.; Talukder, M.A.H.; Siedlecki, C.A.; et al. Reduction of Endothelial Nitric Oxide Increases the Adhesiveness of Constitutive Endothelial Membrane ICAM-1 through Src-Mediated Phosphorylation. Front. Physiol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Byrns, R.E.; Buga, G.M.; Wood, K.S. Endothelium-Derived Relaxing Factor from Pulmonary Artery and Vein Possesses Pharmacologic and Chemical Properties Identical to Those of Nitric Oxide Radical. Circ. Res. 1987, 61, 866–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Furchgott, R.; Zawadzki, J. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Degiacomi, G.; Egorova, A.; Makarov, V.; Pasca, M.R. Nitric Oxide-Releasing Compounds for the Treatment of Lung Infections. Drug Discov Today 2021, 26, 542–550. [Google Scholar] [CrossRef]
- Cai, Y.M.; Zhang, Y.D.; Yang, L. NO Donors and NO Delivery Methods for Controlling Biofilms in Chronic Lung Infections. Appl. Microbiol. Biotechnol. 2021, 105, 3931–3954. [Google Scholar] [CrossRef]
- Scott, J.A.; Maarsingh, H.; Holguin, F.; Grasemann, H. Arginine Therapy for Lung Diseases. Front. Pharmacol. 2021, 12, 627503. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Zimmerman, J.L.; Taylor, R.W.; Sträube, R.C.; Hauser, D.L.; Criner, G.J.; Davis, K.; Hyers, T.M.; Papadakos, P. Effects of Inhaled Nitric Oxide in Patients with Acute Respiratory Distress Syndrome: Results of a Randomized Phase II Trial. Inhaled Nitric Oxide in ARDS Study Group. Crit. Care Med. 1998, 26, 15–23. [Google Scholar] [CrossRef]
- Ventetuolo, C.E.; Klinger, J.R. Management of Acute Right Ventricular Failure in the Intensive Care Unit. Ann. Am. Thorac. Soc. 2014, 11, 811–822. [Google Scholar] [CrossRef] [Green Version]
- Zapol, W.M. Nitric Oxide Story. Anesthesiology 2019, 130, 435–440. [Google Scholar] [CrossRef]
- Ma, G.G.; Hao, G.W.; Lai, H.; Yang, X.M.; Liu, L.; Wang, C.S.; Tu, G.W.; Luo, Z. Initial Clinical Impact of Inhaled Nitric Oxide Therapy for Refractory Hypoxemia Following Type A Acute Aortic Dissection Surgery. J. Thorac. Dis. 2019, 11, 495–504. [Google Scholar] [CrossRef]
- Ghaffari, A.; Neil, D.H.; Ardakani, A.; Road, J.; Ghahary, A.; Miller, C.C. A Direct Nitric Oxide Gas Delivery System for Bacterial and Mammalian Cell Cultures. Nitric Oxide—Biol. Chem. 2005, 12, 129–140. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The Role of Nitric Oxide in Innate Immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial Reactive Oxygen and Nitrogen Species: Concepts and Controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Järhult, J.D.; Lennerstrand, J.; Lundkvist, Å. Mitigation of the Replication of SARS-CoV-2 by Nitric Oxide in Vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef]
- Wiegand, S.B.; Fakhr, B.S.; Carroll, R.W.; Zapol, W.M.; Kacmarek, R.M.; Berra, L. Rescue Treatment With High-Dose Gaseous Nitric Oxide in Spontaneously Breathing Patients With Severe Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0277. [Google Scholar] [CrossRef]
- Ferrari, M.; Santini, A.; Protti, A.; Andreis, D.T.; Iapichino, G.; Castellani, G.; Rendiniello, V.; Costantini, E.; Cecconi, M. Inhaled Nitric Oxide in Mechanically Ventilated Patients with COVID-19. J. Crit. Care 2020, 60, 159–160. [Google Scholar] [CrossRef]
- Safaee Fakhr, B.; Wiegand, S.B.; Pinciroli, R.; Gianni, S.; Morais, C.C.A.; Ikeda, T.; Miyazaki, Y.; Marutani, E.; di Fenza, R.; Larson, G.M.; et al. High Concentrations of Nitric Oxide Inhalation Therapy in Pregnant Patients With Severe Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 1109–1113. [Google Scholar] [CrossRef]
- Longobardo, A.; Montanari, C.; Shulman, R.; Benhalim, S.; Singer, M.; Arulkumaran, N. Inhaled Nitric Oxide Minimally Improves Oxygenation in COVID-19 Related Acute Respiratory Distress Syndrome. BJA Br. J. Anaesth. 2021, 126, e44. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Rubilar, O.; Weller, R.B.; Tortella, G.R.; Seabra, A.B. Nitric Oxide (NO) and Nanoparticles—Potential Small Tools for the War against COVID-19 and Other Human Coronavirus Infections. Virus Res. 2021, 291, 198202. [Google Scholar] [CrossRef]
- Feng, W.X.; Yang, Y.; Wen, J.; Liu, Y.X.; Liu, L.; Feng, C. Implication of Inhaled Nitric Oxide for the Treatment of Critically Ill COVID-19 Patients with Pulmonary Hypertension. ESC Heart Fail. 2021, 8, 714–718. [Google Scholar] [CrossRef]
- Garfield, B.; McFadyen, C.; Briar, C.; Bleakley, C.; Vlachou, A.; Baldwin, M.; Lees, N.; Price, S.; Ledot, S.; McCabe, C.; et al. Potential for Personalised Application of Inhaled Nitric Oxide in COVID-19 Pneumonia. BJA: Br. J. Anaesth. 2021, 126, e72. [Google Scholar] [CrossRef]
- Frostell, C.G.; Hedenstierna, G. Nitric Oxide and COVID-19: Dose, Timing and How to Administer It Might Be Crucial. Acta Anaesthesiol. Scand. 2021, 65, 576–577. [Google Scholar] [CrossRef]
- Srivastava, S.; Garg, I.; Hembrom, A.A.; Kumar, B. Assessment of Nitric Oxide (NO) Potential to Mitigate COVID-19 Severity. VirusDisease 2021, 32, 589–594. [Google Scholar] [CrossRef]
- Safaee Fakhr, B.; di Fenza, R.; Gianni, S.; Wiegand, S.B.; Miyazaki, Y.; Araujo Morais, C.C.; Gibson, L.E.; Chang, M.G.; Mueller, A.L.; Rodriguez-Lopez, J.M.; et al. Inhaled High Dose Nitric Oxide Is a Safe and Effective Respiratory Treatment in Spontaneous Breathing Hospitalized Patients with COVID-19 Pneumonia. Nitric Oxide 2021, 116, 7–13. [Google Scholar] [CrossRef]
- Gianni, S.; di Fenza, R.; Morais, C.C.A.; Fakhr, B.S.; Mueller, A.L.; Yu, B.; Carroll, R.W.; Ichinose, F.; Zapol, W.M.; Berra, L. High-Dose Nitric Oxide From Pressurized Cylinders and Nitric Oxide Produced by an Electric Generator From Air. Respir. Care 2022, 67, 201–208. [Google Scholar] [CrossRef]
- Abou-Arab, O.; Huette, P.; Debouvries, F.; Dupont, H.; Jounieaux, V.; Mahjoub, Y. Inhaled Nitric Oxide for Critically Ill COVID-19 Patients: A Prospective Study. Crit. Care 2020, 24, 645. [Google Scholar] [CrossRef]
- Yamasaki, H. Blood Nitrate and Nitrite Modulating Nitric Oxide Bioavailability: Potential Therapeutic Functions in COVID-19. Nitric Oxide 2020, 103, 29–30. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Chen, L.; Hedenstierna, M.; Lieberman, R.; Fine, D.H. Nitric Oxide Dosed in Short Bursts at High Concentrations May Protect against Covid 19. Nitric Oxide 2020, 103, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Marco, P.; Mongodi, S.; Dammassa, V.; Romito, G.; Mojoli, F. Inhaled Nitric Oxide in Patients Admitted to Intensive Care Unit with COVID-19 Pneumonia. Crit. Care 2020, 24, 508. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Esnault, P.; Cotte, J.; Cungi, P.J.; Goutorbe, P. Effect of Almitrine Bismesylate and Inhaled Nitric Oxide on Oxygenation in COVID-19 Acute Respiratory Distress Syndrome. Anaesth. Crit. Care Pain Med. 2020, 39, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, R.T.; Pollack, C.V., Jr.; Gentile, M.A.; Rashid, M.; Fox, J.C.; Mahaffey, K.W.; de Jesus Perez, V. Outpatient Inhaled Nitric Oxide in a Patient with Vasoreactive Idiopathic Pulmonary Arterial Hypertension and COVID-19 Infection. Am. J. Respir. Crit. Care Med. 2020, 202, 130–132. [Google Scholar] [CrossRef]
- Kobayashi, J.; Murata, I. Nitric Oxide Inhalation as an Interventional Rescue Therapy for COVID-19-Induced Acute Respiratory Distress Syndrome. Ann. Intensive Care 2020, 10, 61. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Gerlach, H.; Rossaint, R.; Pappert, D.; Knorr, M.; Falke, K. Autoinhalation of Nitric Oxide after Endogenous Synthesis in Nasopharynx. Lancet 1994, 343, 518–519. [Google Scholar] [CrossRef]
- Förstermann, U.; Schmidt, H.H.H.W.; Pollock, J.S.; Sheng, H.; Mitchell, J.A.; Warner, T.D.; Nakane, M.; Murad, F. Isoforms of Nitric Oxide Synthase Characterization and Purification from Different Cell Types. Biochem. Pharmacol. 1991, 42, 1849–1857. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Adnot, S.; Raffestin, B.; Eddahibi, S. NO in the Lung. Respir. Physiol. 1995, 101, 109–120. [Google Scholar] [CrossRef]
- Murad, F. Cyclic Guanosine Monophosphate as a Mediator of Vasodilation. J. Clin. Investig. 1986, 78, 1–5. [Google Scholar] [CrossRef]
- Roberts, J.D.; Polaner, D.M.; Zapol, W.M.; Lang, P. Inhaled Nitric Oxide in Persistent Pulmonary Hypertension of the Newborn. Lancet 1992, 340, 818–819. [Google Scholar] [CrossRef]
- Liew, F.Y.; Cox, F.E.G. Nonspecific Defence Mechanism: The Role of Nitric Oxide. Immunol. Today 1991, 12, A17–A21. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The Potential of Nitric Oxide Releasing Therapies as Antimicrobial Agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Shiloh, M.U. Reactive Oxygen and Nitrogen Intermediates in the Relationship between Mammalian Hosts and Microbial Pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef] [Green Version]
- De Groote, M.A.; Fang, F.C. NO Inhibitions: Antimicrobial Properties of Nitric Oxide. Clin. Infect. Dis. 1995, 21 (Suppl. S2), S162–S165. [Google Scholar] [CrossRef]
- Lisi, F.; Zelikin, A.N.; Chandrawati, R. Nitric Oxide to Fight Viral Infections. Adv. Sci. 2021, 8, 2003895. [Google Scholar] [CrossRef]
- Macmicking, J.D.; North, R.J.; Lacourse, R.; Mudgett, J.S.; Shah, S.K.; Nathan, C.F. Identification of Nitric Oxide Synthase as a Protective Locus against Tuberculosis. Proc. Natl. Acad. Sci. USA 1997, 94, 5243–5248. [Google Scholar] [CrossRef] [Green Version]
- Darling, K.E.A.; Evans, T.J. Effects of Nitric Oxide on Pseudomonas Aeruginosa Infection of Epithelial Cells from a Human Respiratory Cell Line Derived from a Patient with Cystic Fibrosis. Infect. Immun. 2003, 71, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Stenger, S.; Donhauser, N.; Thüring, H.; Röllinghoff, M.; Bogdan, C. Reactivation of Latent Leishmaniasis by Inhibition of Inducible Nitric Oxide Synthase. J. Exp. Med. 1996, 183, 1501–1514. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, A.; Miller, C.C.; McMullin, B.; Ghahary, A. Potential Application of Gaseous Nitric Oxide as a Topical Antimicrobial Agent. Nitric Oxide—Biol. Chem. 2006, 14, 21–29. [Google Scholar] [CrossRef]
- Ghaffari, A.; Jalili, R.; Ghaffari, M.; Miller, C.; Ghahary, A. Efficacy of Gaseous Nitric Oxide in the Treatment of Skin and Soft Tissue Infections. Wound Repair Regen. 2007, 15, 368–377. [Google Scholar] [CrossRef]
- Miller, C.C.; Miller, M.K.; Ghaffari, A.; Kunimoto, B. Treatment of Chronic Nonhealing Leg Ulceration with Gaseous Nitric Oxide: A Case Study. J. Cutan. Med. Surg. 2004, 8, 233–238. [Google Scholar] [CrossRef]
- McMullin, B.B.; Chittock, D.R.; Roscoe, D.L.; Garcha, H.; Wang, L.; Miller, C.C. The Antimicrobial Effect of Nitric Oxide on the Bacteria That Cause Nosocomial Pneumonia in Mechanically Ventilated Patients in the Intensive Care Unit. Respir. Care 2005, 50, 1451–1456. [Google Scholar]
- Miller, C.; McMullin, B.; Ghaffari, A.; Stenzler, A.; Pick, N.; Roscoe, D.; Ghahary, A.; Road, J.; Av-Gay, Y. Gaseous Nitric Oxide Bactericidal Activity Retained during Intermittent High-Dose Short Duration Exposure. Nitric Oxide—Biol. Chem. 2009, 20, 16–23. [Google Scholar] [CrossRef]
- Akaike, T.; Maeda, H. Nitric Oxide and Virus Infection. Immunology 2000, 101, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Reiss, C.S.; Komatsu, T. Does Nitric Oxide Play a Critical Role in Viral Infections? J. Virol. 1998, 72, 4547–4551. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; van Ranst, M. Growth Kinetics of SARS-Coronavirus in Vero E6 Cells. Biochem. Biophys. Res. Commun. 2005, 329, 1147–1151. [Google Scholar] [CrossRef]
- Åkerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, Å.; Mirazimi, A. Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual Effect of Nitric Oxide on SARS-CoV Replication: Viral RNA Production and Palmitoylation of the S Protein Are Affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; van Ranst, M. Inhibition of SARS-Coronavirus Infection in Vitro by S-Nitroso-N-Acetylpenicillamine, a Nitric Oxide Donor Compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croen, K.D. Evidence for Antiviral Effect of Nitric Oxide. Inhibition of Herpes Simplex Virus Type 1 Replication. J. Clin. Investig. 1993, 91, 2446–2452. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; Baars, M.M.J.W.; de Lijster, P.; Fouchier, R.A.M.; Osterhaus, A.D.M.E. Inhibition of Influenza Virus Replication by Nitric Oxide. J. Virol. 1999, 73, 8880–8883. [Google Scholar] [CrossRef] [Green Version]
- Regev-Shoshani, G.; Vimalanathan, S.; McMullin, B.; Road, J.; Av-Gay, Y.; Miller, C. Gaseous Nitric Oxide Reduces Influenza Infectivity in Vitro. Nitric Oxide 2013, 31, 48–53. [Google Scholar] [CrossRef]
- Miller, C.C.; Hergott, C.A.; Rohan, M.; Arsenault-Mehta, K.; Döring, G.; Mehta, S. Inhaled Nitric Oxide Decreases the Bacterial Load in a Rat Model of Pseudomonas Aeruginosa Pneumonia. J. Cyst. Fibros. 2013, 12, 817–820. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, S.B.; Traeger, L.; Nguyen, H.K.; Rouillard, K.R.; Fischbach, A.; Zadek, F.; Ichinose, F.; Schoenfisch, M.H.; Carroll, R.W.; Bloch, D.B.; et al. Antimicrobial Effects of Nitric Oxide in Murine Models of Klebsiella Pneumonia. Redox Biol. 2021, 39, 101826. [Google Scholar] [CrossRef]
- Darwish, I.; Miller, C.; Kain, K.C.; Liles, W.C. Inhaled Nitric Oxide Therapy Fails to Improve Outcome in Experimental Severe Influenza. Int. J. Med. Sci. 2012, 9, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.; Miller, M.; McMullin, B.; Regev, G.; Serghides, L.; Kain, K.; Road, J.; Av-Gay, Y. A Phase I Clinical Study of Inhaled Nitric Oxide in Healthy Adults. J. Cyst. Fibros. 2012, 11, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Deppisch, C.; Herrmann, G.; Graepler-Mainka, U.; Wirtz, H.; Heyder, S.; Engel, C.; Marschal, M.; Miller, C.C.; Riethmüller, J. Gaseous Nitric Oxide to Treat Antibiotic Resistant Bacterial and Fungal Lung Infections in Patients with Cystic Fibrosis: A Phase I Clinical Study. Infection 2016, 44, 513–520. [Google Scholar] [CrossRef]
- Bentur, L.; Gur, M.; Ashkenazi, M.; Livnat-Levanon, G.; Mizrahi, M.; Tal, A.; Ghaffari, A.; Geffen, Y.; Aviram, M.; Efrati, O. Pilot Study to Test Inhaled Nitric Oxide in Cystic Fibrosis Patients with Refractory Mycobacterium Abscessus Lung Infection. J. Cyst. Fibros. 2020, 19, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovski, K.; Chau, T.; Robinson, C.J.; MacDonald, S.D.; Peterson, A.M.; Mashek, C.M.; Wallin, W.A.; Rimkus, M.; Montgomery, F.; Lucas da Silva, J.; et al. Antibacterial Activity of High-Dose Nitric Oxide against Pulmonary Mycobacterium Abscessus Disease. Access Microbiol. 2020, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.; Gatt, D.; Golan Tripto, I. Non-Nuberculous Mycobacteria Infection Treated with Intermittently Inhaled High-Dose Nitric Oxide. BMJ Case. Rep. 2021, 14, e243979. [Google Scholar] [CrossRef] [PubMed]
- Yaacoby-Bianu, K.; Gur, M.; Toukan, Y.; Nir, V.; Hakim, F.; Geffen, Y.; Bentur, L. Compassionate Nitric Oxide Adjuvant Treatment of Persistent Mycobacterium Infection in Cystic Fibrosis Patients. Pediatric Infect. Dis. J. 2018, 37, 336–338. [Google Scholar] [CrossRef]
- Bartley, B.L.; Gardner, K.J.; Spina, S.; Hurley, B.P.; Campeau, D.; Berra, L.; Yonker, L.M.; Carroll, R.W. High-Dose Inhaled Nitric Oxide as Adjunct Therapy in Cystic Fibrosis Targeting Burkholderia Multivorans. Case Rep. Pediatr. 2020, 2020, 1536714. [Google Scholar] [CrossRef]
- Goldbart, A.; Golan-Tripto, I.; Pillar, G.; Livnat-Levanon, G.; Efrati, O.; Spiegel, R.; Lubetzky, R.; Lavie, M.; Carmon, L.; Nahum, A. Inhaled Nitric Oxide Therapy in Acute Bronchiolitis: A Multicenter Randomized Clinical Trial. Sci. Rep. 2020, 10, 2–9. [Google Scholar] [CrossRef]
- Kamenshchikov, N.O.; Berra, L.; Carroll, R.W. Therapeutic Effects of Inhaled Nitric Oxide Therapy in COVID-19 Patients. Biomedicines 2022, 10, 369. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The Toxicology of Inhaled Nitric Oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Cypel, M.; Keshavjee, S. Extending the Donor Pool. Thorac. Surg. Clin. 2015, 25, 27–33. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Divithotawela, C.; Cypel, M.; Martinu, T.; Singer, L.G.; Binnie, M.; Chow, C.W.; Chaparro, C.; Waddell, T.K.; De Perrot, M.; Pierre, A.; et al. Long-Term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg. 2019, 154, 1143–1150. [Google Scholar] [CrossRef]
- Watanabe, T.; Cypel, M.; Keshavjee, S. Ex Vivo Lung Perfusion. J. Thorac. Dis. 2021, 13, 6602–6617. [Google Scholar] [CrossRef]
- Wang, A.; Ribeiro, R.V.P.; Ali, A.; Brambate, E.; Abdelnour-Berchtold, E.; Michaelsen, V.; Zhang, Y.; Rahfeld, P.; Moon, H.; Gokhale, H.; et al. Ex Vivo Enzymatic Treatment Converts Blood Type A Donor Lungs into Universal Blood Type Lungs. Sci. Transl. Med. 2022, 14, eabm7190. [Google Scholar] [CrossRef]
- Michaelsen, V.S.; Ribeiro, R.V.P.; Ali, A.; Wang, A.; Gazzalle, A.; Keshavjee, S.; Cypel, M. Safety of Continuous 12-Hour Delivery of Antimicrobial Doses of Inhaled Nitric Oxide during Ex Vivo Lung Perfusion. J. Thorac. Cardiovasc. Surg. 2021, 3, 841–849. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Hirayama, S.; Rubacha, M.; Fischer, S.; Anraku, M.; Sato, M.; Harwood, S.; Pierre, A.; Waddell, T.K.; et al. Technique for Prolonged Normothermic Ex Vivo Lung Perfusion. J. Heart Lung Transplant. 2008, 27, 1319–1325. [Google Scholar] [CrossRef]
- Michaelsen, V.S.; Ribeiro, R.V.P.; Brambate, E.; Ali, A.; Wang, A.; Pires, L.; Kawashimaid, M.; Zhang, Y.; Gazzalle, A.; Keshavjee, S.; et al. A Novel Pre-Clinical Strategy to Deliver Antimicrobial Doses of Inhaled Nitric Oxide. PLoS ONE 2021, 10, e0258368. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin—It’s Not Just Blue: A Concise Review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- Dötsch, J.; Demirakça, S.; Kratz, M.; Repp, R.; Knerr, I.; Rascher, W. Comparison of Methylene Blue, Riboflavin, and N-Acetylcysteine for the Reduction of Nitric Oxide-Induced Methemoglobinemia. Crit. Care Med. 2000, 4, 958–961. [Google Scholar] [CrossRef]
- Jang, D.H.; Nelson, L.S.; Hoffman, R.S. Methylene Blue for Distributive Shock: A Potential New Use of an Old Antidote. J. Med. Toxicol. 2013, 3, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Gianni, S.; Morais, C.C.A.; Larson, G.; Pinciroli, R.; Carroll, R.; Yu, B.; Zapol, W.M.; Berra, L. Ideation and Assessment of a Nitric Oxide Delivery System for Spontaneously Breathing Subjects. Nitric Oxide—Biol. Chem. 2020, 104–105, 29–35. [Google Scholar] [CrossRef]






| Study | Model | Dose | Treatment Protocol | Year |
|---|---|---|---|---|
| Ghaffari et al. [13] | in vitro | 160–200 ppm | Continuous 48 h | 2005 |
| McMullin et al. [55] | in vitro | 200 ppm | Continuous 5 h | 2005 |
| Miller et al. [56] | in vitro | 160–200 ppm | Intermittently 30 min every 3.5 h and Continuous for up to 24 h | 2009 |
| Miller et al. [66] | in vivo animal model | 160 ppm | Intermittently 30 min every 4 h | 2013 |
| Wiegand S. et al. [67] | in vivo animal model | 160/200 ppm 300 ppm | Continuous (48 h) Intermittent 12 min, every 3 h for 48 h | 2021 |
| Miller et al. [69] | Clinical Study | 160 ppm | Intermittently 30 min every 3.5 h, 5 times daily | 2012 |
| Deppish et al. [70] | Clinical Study | 160 ppm | Intermittently 30 min, 3 times daily of 5 days | 2016 |
| Yaacoby-Bianu et al. [74] | Compassionate | 160 ppm | Intermittently Minimal time interval 3.5 h (max 21 days) | 2018 |
| Bentur et al. [71] | Pilot Clinical Study | 160 ppm | Intermittently 30 min, 5 times daily for 14 days and 3 times daily for 7 days | 2020 |
| Bartley et al. [75] | Clinical Study | 160 ppm | Intermittent over a 28-days | 2020 |
| Bogdanovski et al. [72] | Compassionate | up to 240 ppm | Intermittently two courses 5 times daily for 5 days and 3 times daily for 8 days | 2020 |
| Goldbart et al. [76] | RCT | 160 ppm | Intermittently 30 min, 5 times daily of 5 days | 2020 |
| Goldbart et al. [73] | Compassionate | 150–250 ppm | Intermittent 4 times a day for 2 weeks, 2 times a day 2 weeks and one in the last day of treatment. (29-day treatment course) | 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorbo, L.D.; Michaelsen, V.S.; Ali, A.; Wang, A.; Ribeiro, R.V.P.; Cypel, M. High Doses of Inhaled Nitric Oxide as an Innovative Antimicrobial Strategy for Lung Infections. Biomedicines 2022, 10, 1525. https://doi.org/10.3390/biomedicines10071525
Sorbo LD, Michaelsen VS, Ali A, Wang A, Ribeiro RVP, Cypel M. High Doses of Inhaled Nitric Oxide as an Innovative Antimicrobial Strategy for Lung Infections. Biomedicines. 2022; 10(7):1525. https://doi.org/10.3390/biomedicines10071525
Chicago/Turabian StyleSorbo, Lorenzo Del, Vinicius S. Michaelsen, Aadil Ali, Aizhou Wang, Rafaela V. P. Ribeiro, and Marcelo Cypel. 2022. "High Doses of Inhaled Nitric Oxide as an Innovative Antimicrobial Strategy for Lung Infections" Biomedicines 10, no. 7: 1525. https://doi.org/10.3390/biomedicines10071525
APA StyleSorbo, L. D., Michaelsen, V. S., Ali, A., Wang, A., Ribeiro, R. V. P., & Cypel, M. (2022). High Doses of Inhaled Nitric Oxide as an Innovative Antimicrobial Strategy for Lung Infections. Biomedicines, 10(7), 1525. https://doi.org/10.3390/biomedicines10071525