Comparative Analysis of Flavor, Taste, and Volatile Organic Compounds in Opossum Shrimp Paste during Long-Term Natural Fermentation Using E-Nose, E-Tongue, and HS-SPME-GC-MS
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Collection and Preparation
2.2. Reagents and Chemicals
2.3. Appearance Observation and Color Analysis
2.4. Volatile Flavor Analysis by E-Nose
2.5. Taste Analysis by E-Tongue
2.6. High-Throughput Analysis of Characteristic Volatile Compounds
2.6.1. Sample Preparation
2.6.2. Headspace Solid-Phase Microextraction (HS-SPME) Pretreatment
2.6.3. Identification and Relative Quantification of VOCs by GC-MS Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Appearance and Color Change of Shrimp Paste
3.2. Volatile Flavor Composition and Identification of Taste Attributes
3.3. Species and Numbers of VOCs Detected in Shrimp Paste
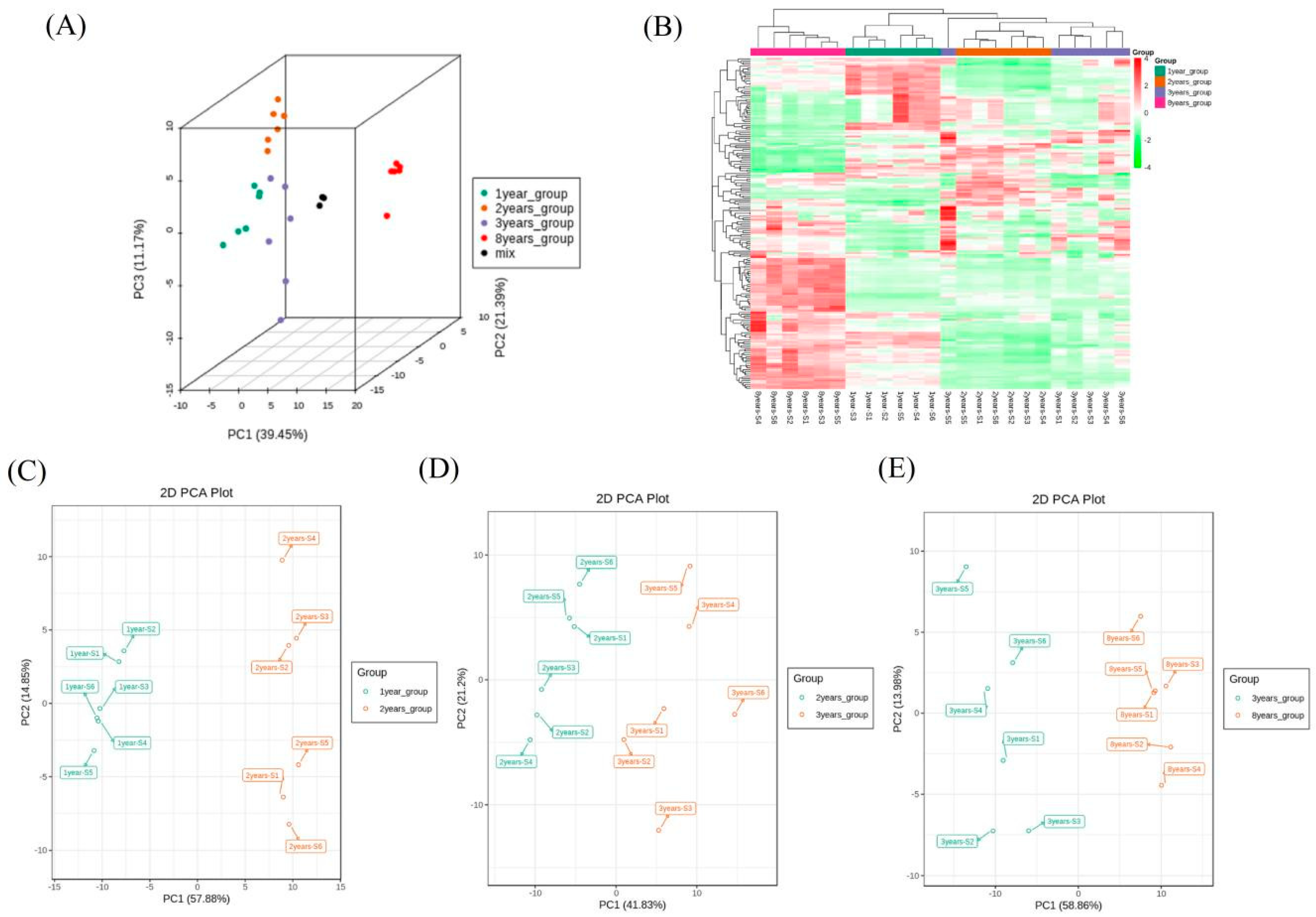
3.4. Multivariate PCA and OPLS-DA Analyses of VOCs in Shrimp Paste
3.5. Screening of Characteristic Flavor Compound Indexes
3.6. Correlation Analysis of Taste Characteristics and Flavor Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Padilah, B.; Bahruddin, S.; Fazilah, A.; Ahmad, M.; Gulam, R.R.A. Biogenic amines analysis in shrimp pastes belacan obtained from the Northern States of Peninsular Malaysia. Int. Food Res. J. 2018, 25, 1893–1899. [Google Scholar]
- Sang, X.; Ma, X.X.; Hao, H.S.; Bi, J.R.; Zhang, G.L.; Hou, H.M. Evaluation of biogenic amines and microbial composition in the Chinese traditional fermented food grasshopper sub shrimp paste. LWT Food Sci. Technol. 2020, 134, 109979. [Google Scholar] [CrossRef]
- Suhartini, W.; Yang, F.; Xia, W.S. Physiochemical properties, volatile compounds and sensory evaluation of chili sauce shrimp paste from different regions in Indonesia. Food Nutr. Sci. 2019, 10, 333–348. [Google Scholar] [CrossRef]
- Li, W.Y.; Lu, H.Q.; He, Z.H.; Sang, Y.X.; Sun, J.L. Quality characteristics and bacterial community of a Chinese salt-fermented shrimp paste. LWT Food Sci. Technol. 2021, 136, 110358. [Google Scholar] [CrossRef]
- Shahrim, K.; Khairunnisak, M.; Tang, S.C.; Jinap, S.; Hajeb, P.; Ilya Nur, A.R. Sensory attributes of dishes containing shrimp paste with different concentrations of glutamate and 5′-nucleotides. Appetite 2010, 55, 238–244. [Google Scholar] [CrossRef]
- Cai, L.Y.; Wang, Q.J.; Dong, Z.J.; Liu, S.C.; Zhang, C.H.; Li, J.R. Biochemical, nutritional, and sensory quality of the low salt fermented shrimp paste. J. Aquat. Food Prod. Technol. 2017, 26, 706–718. [Google Scholar] [CrossRef]
- Lv, X.R.; Li, Y.C.; Tian, Q.; Sun, M.T.; Bai, F.L.; Li, X.P.; Li, J.R. Bacterial community succession and volatile compound changes during fermentation of shrimp paste from Chinese Jinzhou region. LWT Food Sci. Technol. 2020, 122, 108998. [Google Scholar] [CrossRef]
- Amalia, U.; Sumardianto; Agustini, T.W. Characterization of lactic acid bacteria (LAB) isolated from Indonesian shrimp paste (terasi). IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012049. [Google Scholar] [CrossRef]
- Yao, Y.P.; Zhou, X.Y.; Hadiatullah, H.; Zhang, J.; Zhao, G.Z. Determination of microbial diversities and aroma characteristics of Beitang shrimp paste. Food Chem. 2021, 344, 128695. [Google Scholar] [CrossRef]
- Che, H.X.; Yu, J.; Sun, J.Y.; Lu, K.; Xie, W.C. Bacterial composition changes and volatile compounds during the fermentation of shrimp paste: Dynamic changes of microbial communities and flavor composition. Food Biosci. 2021, 43, 101169. [Google Scholar] [CrossRef]
- Pilapil, A.R.; Neyrinck, E.; Deloof, D.; Bekaert, K.; Robbens, J.; Raes, K. Chemical quality assessment of traditional salt-fermented shrimp paste from Northern Mindanao, Philippines. J. Sci.Food Agri. 2016, 96, 933–938. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Ji, H.; Liu, A. Isolation and identification of beneficial bacteria in Bohai shrimp paste. J. China Inst. Food Sci. Technol. 2020, 20, 258–265. [Google Scholar]
- Kobayashi, T.; Kajiwara, M.; Wahyuni, M.; Kitakado, T.; Hamada-Sato, N.; Imada, C.; Watanabe, E. Isolation and characterization of halophilic lactic acid bacteria isolated from “terasi” shrimp paste: A traditional fermented seafood product in Indonesia. J. Gene. Appl. Microbiol. 2003, 49, 279–286. [Google Scholar] [CrossRef]
- Tan, J.Z.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Art. Intel. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Du, H.Z.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B.H. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef]
- Hong, J.H.; Khan, N.; Jamila, N.; Hong, Y.S.; Nho, E.Y.; Choi, J.Y.; Lee, C.M.; Kim, K.S. Determination of volatile flavour profiles of Citrus spp. fruits by SDE-GC-MS and enantiomeric composition of chiral compounds by MDGC-MS. Phytochem. Anal. 2017, 28, 392–403. [Google Scholar] [CrossRef]
- Zhang, J.H.; Cao, J.; Pei, Z.S.; Wei, P.Y.; Xiang, D.; Cao, X.Y.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.Y.; Qin, Y.Y.; Jiang, L.W.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Nešpor, J.; Karabn, M.; Štulkov, K.; Dostlek, P.; McPhee, D.J. An HS-SPME-GC-MS method for profiling volatile compounds as related to technology used in cider production. Molecules 2019, 24, 2117. [Google Scholar] [CrossRef]
- Guo, R.C.; Yu, F.T.; Wang, C.H.; Jiang, H.R.; Yu, L.; Zhao, M.M.; Liu, X.L. Determination of the volatiles in fermented bamboo shoots by head space–solid-phase micro extraction (HS-SPME) with gas chromatography–olfactory–mass spectrometry (GC-O-MS) and aroma extract dilution analysis (AEDA). Anal. Lett. 2021, 54, 1162–1179. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. General statistics. In Principle and Procedure of Statistics; Steel, R.G.D., Ed.; McGraw-Hill: New York, NY, USA, 1980; pp. 457–490. [Google Scholar]
- Blasco, H.; Błaszczyński, J.; Billaut, J.C.; Nadal-Desbarats, L.; Pradat, P.F.; Devos, D.; Moreau, C.C.; Andres, C.R.; Emond, P.; Corcia, P.; et al. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K.; Faithong, N. Changes in volatile compounds, ATP-related compounds and antioxidative properties of Kapi, produced from Acetes vulgaris, during processing and fermentation. Food Biosci. 2017, 19, 49–56. [Google Scholar] [CrossRef]
- Schiedt, K.; Bischof, S.; Glinz, E. Metabolism of carotenoids and in vivo racemization of (3S,3′S)-astaxanthin in the crustacean Penaeus. Methods Enzymol. 1993, 214, 148–168. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S. Distribution and characteristics of polyphenoloxidase from Pacific white shrimp (Litopenaeus vannamei). J. Food Sci. 2019, 84, 1078–1086. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Han, Z.H.; Gao, J.X.; Wang, X.M.; Wang, W.X.; Dong, J.; Zhang, Y.; Wang, S. Formation and alterations of the potentially garmful Maillard reaction products during the production and storage of brown fermented milk. Molecules 2019, 24, 24272. [Google Scholar] [CrossRef] [PubMed]
- Peralta, E.; Hatate, H.; Kawabe, D.; Kuwahara, R.; Wakamatsu, S.; Murata, H. Improving antioxidant activity and nutritional components of Philippine salt-fermented shrimp paste through prolonged fermentation. Food Chem. 2008, 11, 72–77. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.; Fusella, G.C.; Paparella, A. Spoilage potential of H2S producing bacteria in seafood. Ital. J. Food Sci. 2011, 23, 201–203. [Google Scholar]
- Kuuliala, L.; Sader, M.; Solimeo, A.; Pérez-Fernández, R.; Vanderroost, M.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; Devlieghere, F. Spoilage evaluation of raw Atlantic salmon (Salmo salar) stored under modified atmospheres by multivariate statistics and augmented ordinal regression. Int. J. Food Microbiol. 2019, 303, 46–57. [Google Scholar] [CrossRef]
- Qi, H.; Xu, Z.; Li, Y.B.; Ji, X.L.; Dong, X.F.; Yu, C.X. Seafood flavourings characterization as prepared from the enzymatic hydrolysis of Undaria pinnatifida sporophyll by-product. Int. J. Food Prop. 2017, 20, 2867–2876. [Google Scholar] [CrossRef]
- Fuke, S.; Ueda, Y. Interactions between umami and other flavor characteristics. Trends Food Sci. Technol. 1996, 7, 407–411. [Google Scholar] [CrossRef]
- Serkan, S.; Carole, P.; Thierry, S. Odour-active and off-odour components in rainbow trout (Oncorhynchus mykiss) extracts obtained by microwave assisted distillation-solvent extraction. Food Chem. 2009, 114, 317–322. [Google Scholar] [CrossRef]
- Thi, V.; Jong-Hyun, P. Characteristics of Potential Gamma-Aminobutyric Acid-Producing Bacteria Isolated from Korean and Vietnamese Fermented Fish Products. J. Microb. Biot. 2019, 29, 209–221. [Google Scholar] [CrossRef]
- Li, J.L.; Wan, L.; Chen, C.Y.; Hu, X.F.; Li, X.N.; Huang, L.; Tu, Z.C. Studies on formation of flavor compounds in fish meat during heat process based on oxidation models of ω-3 LCPUFAs. J. Chin. Ins. Food Sci. Technol. 2020, 20, 95–105. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Seo, S.H.; Park, S.E.; Yoo, S.A.; Lee, K.I.; Na, C.S.; Son, H.S. Metabolite profiling of Makgeolli for the understanding of yeast fermentation characteristics during fermentation and aging. Process Biochem. 2016, 51, 1363–1373. [Google Scholar] [CrossRef]
- Ditrych, M.; Filipowska, W.; De Rouck, G.; Jaskula-Goiris, B.; Aerts, G.; De Cooman, L.; Andersen, M.L. Investigating the evolution of free staling aldehydes throughout the wort production process. Brew Sci. 2019, 72, 10–17. [Google Scholar] [CrossRef]
- Wei, G.M.; Yang, Z.H.; Regensteinc, J.M.; Liua, X.M.; Liu, D.S. Characterizing aroma profiles of fermented soybean curd with ageing solutions during fermentation. Food Biosci. 2020, 33, 100508. [Google Scholar] [CrossRef]
- Wang, H.J.; Shen, S.; Wang, J.J.; Jiang, Y.W.; Li, J.; Yang, Y.Q.; Hua, J.J.; Yuan, H.B. Novel insight into the effect of fermentation time on quality of Yunnan Congou black tea. LWT Food Sci. Technol. 2022, 155, 112939. [Google Scholar] [CrossRef]
- Cai, R.K.; Wu, J.J.; Zhu, J.L.; Qian, P.; Dai, Z.Y. Analysis of volatile compounds and odor-active compounds in fermented large yellow croaker. J. China Inst. Food Sci. Technol. 2017, 17, 264–273. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Zi, J.W.; Yang, X.H.; Xie, W.C. Characterization of aroma profiles and aroma-active compounds in high-salt and low-salt shrimp paste by molecular sensory science. Food Biosci. 2022, 45, 101470. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.T.; Resende, M.F.R.; Lin, T.; Taylor, M.; Zhang, B.; Ikeda, H.; Liu, Z.Y.; Fisher, J.; Zemach, I. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Amárita, F.; Martínez-Cuesta, C.M.; Taborda, G.; Soto-Yárritu, P.L.; Requena, T.; Peláez, C. Formation of methional by Lactococcus lactis IFPL730 under cheese model conditions. Eur. Food Res. Technol. A 2002, 214, 58–62. [Google Scholar] [CrossRef][Green Version]
- Song, X.B.; Wang, G.G.; Zhu, L.; Zheng, F.P.; Ji, J.; Sun, J.Y.; Li, H.H.; Huang, M.Q.; Zhao, Q.Z.; Zhao, M.M.; et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT Food Sci. Technol. 2021, 146, 111427. [Google Scholar] [CrossRef]
- Escudero, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Clues about the role of methional as character impact odorant of some oxidized wines. J. Agri. Food Chem. 2000, 48, 4268–4272. [Google Scholar] [CrossRef]
- Chalannavar, R.K.; Venugopala, K.N.; Baijnath, H.; Odhav, B. The Chemical Composition of Leaf Essential Oils of Psidium guajava L. (White and Pink fruit forms) from South Africa. J. Essent. Oil Bear. Pl. 2014, 17, 1293–1302. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of the sesquiterpene fraction from Annona reticulata L. bark. Nat. Prod. Res. 2012, 26, 1515–1518. [Google Scholar] [CrossRef]
- Kleekayai, T.; Pinitklang, S.; Laohakunjit, N.; Suntornsuk, W. Volatile components and sensory characteristics of Thai traditional fermented shrimp pastes during fermentation periods. J. Food Sci. Technol. 2016, 53, 1399–1410. [Google Scholar] [CrossRef]
- Cheng, L.L.; Luo, J.F.; Li, P.; Yu, H.; Huang, J.F.; Luo, L.X. Microbial diversity and flavor formation in onion fermentation. Food Funct. 2014, 5, 2338–2347. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, T.; Wang, Y.; Xie, J. Roles of different amino-containing precursors in enzymatically hydrolyzed chicken in meat flavor formation. J. China Inst. Food Sci. Technol. 2020, 20, 221–232. [Google Scholar] [CrossRef]
- Hernandez-Valdes, J.A.; Dalglish, M.M.; Hermans, J.; Kuipers, O.P. Development of Lactococcus lactis biosensors for detection of sulfur-containing amino acids. Front. Microbiol. 2020, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Maalouf, J.H.; Lazouski, N.; Corbin, N.; Yang, D.T.; Manthiram, K. Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts. J. Am. Chem. Soc. 2019, 141, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yin, L.; Xue, Y.; Li, Z.J.; Hou, H.; Xue, C.H. Analyzing the flavor compounds in Chinese traditional fermented shrimp pastes by HS-SPME-GC/MS and electronic nose. J. Ocean Univ. China 2017, 16, 311–318. [Google Scholar] [CrossRef]
- Dacho, V.; Szolcsányi, P. Synthesis and olfactory properties of seco-analogues of lilac aldehydes. Molecules 2021, 26, 7086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Battesti, M.J.; Costa, J.; Dupuy, N.; Paolini, J. Volatile components as chemical markers of the botanical origin of Corsican honeys. Flavour Frag. J. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey volatiles as a fingerprint for botanical origin—A review on their occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Walton, W.C.; Wang, Y. Volatile organic compounds of Eastern oyster (Crassostrea virginica) cultured by two treatments and their changes during cold storage. Aquac. Res. 2021, 52, 1442–1452. [Google Scholar] [CrossRef]
- Lai, B. Research progress of 5H-5-methyl-6, 7-dihydrocyclopentanopyrazine. Guangdong Chem. Ind. 2014, 12, 118. [Google Scholar]
- Zhou, B.; Wang, Y.; Kang, J.J.; Zhong, H.Y.; Prenzler, P.D. The quality and volatile-profile changes of Longwangmo apricot (Prunus armeniaca L.) kernel oil prepared by different oil-producing processes. Eur. J. Lipid Sci. Technol. 2016, 118, 236–243. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, L.; Xu, C.P. Chemical and volatile composition of jujube wines fermented by Saccharomyces cerevisiae with and without pulp contact and protease treatment. Food Sci. Technol. 2016, 36, 204–209. [Google Scholar] [CrossRef]
- Fan, H.P.; Zheng, X.L.; Ai, Z.L.; Liu, C.; Li, R.; Bian, K. Analysis of volatile aroma components from Mantou fermented by different starters. J. Food Processing Preserv. 2018, 42, e13627. [Google Scholar] [CrossRef]
- Buescher, R.H.; Buescher, R.W. Production and stability of (E,Z)-2,6-nonadienal, the major flavor volatile of cucumbers. J. Food Sci. 2001, 66, 357–361. [Google Scholar] [CrossRef]
- Cho, M.J.; Buescher, R.W.; Johnson, M.; Janes, M. Inactivation of pathogenic bacteria by cucumber volatiles (E,Z)-2,6-Nonadienal and (E)-2-Nonenal. J. Food Prot. 2004, 67, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Feizabadi, M.M. Antimicrobial activity of ducrosia anethifolia essential oil and main component, decanal against methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus. J. Essent. Oil Bear. Pl. 2009, 12, 574–579. [Google Scholar] [CrossRef]
- Ghavidel, F.; Zarshenas, M.M.; Ghasemi, Y.; Gholami, A.; Sakhteman, A.; Faridi, P. Impact of two different extraction methods on chemical composition and antimicrobial activities of multi-ingredients essential oils and hydrosols. Trends Pharm. Sci. 2019, 3, 161–176. [Google Scholar]
- Siek, T.J.; Albin., I.A.; Sather., L.A.; Lindsay., R.C. Comparison of flavor thresholds of aliphatic lactones with those of fatty acids, esters, aldehydes, alcohols, and ketones. J. Dairy Sci. 1971, 54, 1–4. [Google Scholar] [CrossRef]







| Sensor Number | Sensor Name | Performance Description (Sensitivity to) |
|---|---|---|
| R1 | W1S | Aromatic compounds |
| R2 | W5S | Nitrogen oxides, very sensitive to negative signal |
| R3 | W3C | Aromatic amines |
| R4 | W6S | Hydride, mainly selective for hydrogen |
| R5 | W5C | Short-chain alkanes |
| R6 | W1C | Methyl compounds |
| R7 | W1W | Inorganic sulfides |
| R8 | W2S | Alcohol |
| R9 | W2W | Aromatic ingredients, sensitive to organic sulfides |
| R10 | W3S | Long-chain alkanes |
| Groups (Year) | L* | a* | b* | △E |
|---|---|---|---|---|
| 1 | 36.68 ± 0.39 | 4.38 ± 0.16 | 6.28 ± 0.07 | 57.12 ± 0.41 |
| 2 | 30.27 ± 1.94 * | 3.99 ± 0.12 * | 6.43 ± 0.14 * | 62.99 ± 1.94 * |
| 3 | 29.83 ± 1.20 * | 3.12 ± 0.08 ** | 5.85 ± 0.04 * | 66.68 ± 1.24 * |
| 8 | 26.77 ± 1.04 ** | 3.98 ± 0.24 * | 5.19 ± 0.43 ** | 66.42 ± 1.04 * |
| Flavor Types | Fermentation Duration (Years) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 8 | |
| Sourness | −19.07 ± 0.12 | −20.26 ± 0.35 * | −21.21 ± 0.10 * | −21.47 ± 0.23 * |
| Bitterness | −2.28 ± 0.16 | −2.65 ± 0.08 * | −2.92 ± 0.11 * | −3.69 ± 0.07 * |
| Astringency | 7.145 ± 0.25 | 6.33 ± 0.32 * | 5.33 ± 0.12 ** | 5.21 ± 0.15 ** |
| aftertaste-B | 4.06 ± 0.06 | 4.52 ± 0.13 * | 4.84 ± 0.10 * | 3.22 ± 0.11 * |
| aftertaste-A | 4.12 ± 0.17 | 3.56 ± 0.14 * | 3.31 ± 0.05 * | 3.3 ± 0.17 * |
| Umami | 12.68 ± 0.26 | 13.25 ± 0.15 * | 14.08 ± 0.10 ** | 13.27 ± 0.05 * |
| Richness | 4.01 ± 0.20 | 5.63 ± 0.11 ** | 6.06 ± 0.08 ** | 3.61 ± 0.14 * |
| Saltiness | 7.69 ± 0.32 | 8.63 ± 0.12 * | 9.31 ± 0.27 ** | 10.06 ± 0.11 *** |
| Index | Compounds | Retention Time (min) | CAS | Formula | Peak Area (Log10) | |||
|---|---|---|---|---|---|---|---|---|
| 1-Year Group | 2-Years Group | 3-Years Group | 8-Years Group | |||||
| MW0001 | Dimethyl disulfide | 4.36 | 624-92-0 | C2H6S2 | 5.59 | 5.89 | 6.10 | 5.84 |
| MW0005 | Methional | 7.75 | 3268-49-3 | C4H8OS | 4.54 | 5.11 | 5.29 | 5.49 |
| MW0012 | Cis-2-(2-pentenyl)furan | 9.48 | 70424-13-4 | C9H12O | 5.67 | 4.97 | 5.41 | 6.02 |
| MW0016 | Trimethyl-pyrazine | 9.59 | 14667-55-1 | C7H10N2 | 4.71 | 4.75 | 4.87 | 5.45 |
| MW0017 | (E,E)-2,4-heptadienal | 9.75 | 4313-03-5 | C7H10O | 5.75 | 4.91 | 5.62 | 6.19 |
| MW0020 | Benzeneacetaldehyde | 10.32 | 122-78-1 | C8H8O | 6.15 | 6.64 | 6.61 | 6.58 |
| MW0025 | 3-Ethyl-2,5-dimethyl-pyrazine | 10.91 | 13360-65-1 | C8H12N2 | 5.05 | 5.24 | 4.76 | 6.25 |
| MW0034 | (E,Z)-2,6-nonadienal | 12.33 | 557-48-2 | C9H14O | 5.26 | 5.17 | 5.11 | 5.72 |
| MW0042 | Decanal | 13.31 | 112-31-2 | C10H20O | 4.39 | 4.42 | 4.66 | 5.03 |
| MW0070 | Lilac aldehyde | 15.78 | 53447-47-5 | C10H16O2 | 4.35 | 4.23 | 4.60 | 4.80 |
| MW0081 | Dodecanal | 17.02 | 112-54-9 | C12H24O | 4.88 | 4.61 | 4.63 | 4.96 |
| MW0103 | 1-Undecanol | 18.47 | 112-42-5 | C11H24O | 5.17 | 5.05 | 5.59 | 5.52 |
| MW0104 | 2-Tridecanone | 18.51 | 593-08-8 | C13H26O | 5.64 | 5.58 | 5.79 | 6.02 |
| MW0146 | Tetradecanoic acid ethyl ester | 22.64 | 124-06-1 | C16H32O2 | 4.44 | 4.63 | 4.68 | 5.20 |
| MW0161 | 2-Heptadecanone | 23.43 | 2922-51-2 | C17H34O | 5.03 | 5.04 | 5.12 | 5.56 |
| MW0169 | Hexadecanoic acid ethyl ester | 23.97 | 628-97-7 | C18H36O2 | 4.02 | 4.29 | 4.49 | 5.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Wang, R.; Zhang, Y.; Li, X.; Gooneratne, R.; Li, J. Comparative Analysis of Flavor, Taste, and Volatile Organic Compounds in Opossum Shrimp Paste during Long-Term Natural Fermentation Using E-Nose, E-Tongue, and HS-SPME-GC-MS. Foods 2022, 11, 1938. https://doi.org/10.3390/foods11131938
Deng Y, Wang R, Zhang Y, Li X, Gooneratne R, Li J. Comparative Analysis of Flavor, Taste, and Volatile Organic Compounds in Opossum Shrimp Paste during Long-Term Natural Fermentation Using E-Nose, E-Tongue, and HS-SPME-GC-MS. Foods. 2022; 11(13):1938. https://doi.org/10.3390/foods11131938
Chicago/Turabian StyleDeng, Yijia, Rundong Wang, Yuhao Zhang, Xuepeng Li, Ravi Gooneratne, and Jianrong Li. 2022. "Comparative Analysis of Flavor, Taste, and Volatile Organic Compounds in Opossum Shrimp Paste during Long-Term Natural Fermentation Using E-Nose, E-Tongue, and HS-SPME-GC-MS" Foods 11, no. 13: 1938. https://doi.org/10.3390/foods11131938
APA StyleDeng, Y., Wang, R., Zhang, Y., Li, X., Gooneratne, R., & Li, J. (2022). Comparative Analysis of Flavor, Taste, and Volatile Organic Compounds in Opossum Shrimp Paste during Long-Term Natural Fermentation Using E-Nose, E-Tongue, and HS-SPME-GC-MS. Foods, 11(13), 1938. https://doi.org/10.3390/foods11131938







