Evolution of Systemic Treatment Uptake and Survival in Advanced Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Data
2.2. Statistical Methods
3. Results
3.1. Baseline Patient Characteristics of Three Time Periods
3.2. Systemic Therapy
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Lung Cancer Statistics. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 May 2020).
- Canadian Cancer Society. Lung Cancer Statistics. 2020. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/?region=pe (accessed on 30 May 2020).
- Lung and Bronchus Cancer. Surveillance, Epidemiology, and End Results Program. 2019. Available online: http://seer.cancer.gov/statfacts/html/lungb.html (accessed on 30 May 2020).
- Brule, S.Y.; Al-Baimani, K.; Jonker, H.; Zhang, T.; Nicholas, G.; Goss, G.; Laurie, S.A.; Wheatley-Price, P. Palliative systemic therapy for advanced non-small cell lung cancer: Investigating disparities between patients who are treated versus those who are not. Lung Cancer 2016, 97, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wheatley-Price, P.; Laurie, K.; Zhang, T.; McKinnon, M. Evolution of Systemic Therapy Uptake in NSCLC Over Time: The Impact of New Therapies [abstract P1.01-16]. J. Thorac. Oncol. 2019, 14 (Suppl. 10), S361–S362. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.C.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995, 311, 899–909. [Google Scholar] [CrossRef]
- Melosky, B.; Blais, N.; Cheema, P.; Couture, C.; Juergens, R.; Kamel-Reid, S.; Tsao, M.-S.; Wheatley-Price, P.; Xu, Z.; Ionescu, D. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr. Oncol. 2018, 25, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.; Faivre-Finn, C.; Mok, T.; Reck, M.; Van Schil, P.; Hellmann, M.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Takano, N.; Ariyasu, R.; Koyama, J.; Sonoda, T.; Saiki, M.; Kawashima, Y.; Oguri, T.; Hisakane, K.; Uchibori, K.; Nishikawa, S.; et al. Improvement in the survival of patients with stage IV non-small-cell lung cancer: Experience in a single institutional 1995-2017. Lung Cancer 2019, 131, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, G.P.; Bootsma, V.C.; Tjan-Heijnen, V.C.G.; van der Wilt, G.J.; Cox, A.L.; Brouwer, M.H.J.; Corstens, F.H.M.; Oyen, W.J.G. FDG-PET in staging lung cancer: How does it change the algorithm? Lung Cancer 2004, 44, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Westeel, V.; Zalcman, G.; Milleron, B. Chemotherapy in elderly patients with advanced non-small cell lung cancer. Lung Cancer 2011, 74, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Shayne, M.; Culakova, E.; Poniewierski, M.S.; Wolff, D.; Dale, D.C.; Crawford, J.; Lyman, G.H. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 2007, 110, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Repetto, L.; Audisio, R.A. Elderly patients have become the leading drug consumers: It’s high time to properly evaluate new drugs within the real targeted population. J. Clin. Oncol. 2006, 24, e62–e63. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; O’Neil, B.; Price, T.J.; Falchook, G.S.; Desai, J.; Kuo, J.; Govindan, R.; Rasmussen, E.; Morrow, P.K.H.; Ngang, J.; et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]


| Characteristics | Cohort A | Cohort B | Cohort C |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Number of Patients (total) | 528 | 463 | 93 |
| Age at Diagnosis Median Years and (Range) | 68 (35–90) | 69 (23–94) | 69 (39–89) |
| Male/female | 292 (55)/236 (45) | 256 (55)/207 (45) | 52 (56)/41 (44) |
| ECOG 0 | 46 (9) | 41 (9) | 9 (10) |
| ECOG 1 | 220 (42) | 177 (38) | 33 (35) |
| ECOG 2 | 111 (21) | 101 (22) | 16 (17) |
| ECOG 3 | 92 (17) | 56 (12) | 21 (23) |
| ECOG 4 | 19 (4) | 14 (3) | 3 (3) |
| Unknown | 40 (8) | 74 (16) | 11 (12) |
| Current Smoker | 228 (43) | 196 (42) | 43 (46) |
| Ex-Smoker | 257 (49) | 207 (45) | 40 (43) |
| Never Smoker | 37 (7) | 58 (13) | 9 (10) |
| Unknown | 6 (1) | 2 (0.4) | 1 (1) |
| Adenocarcinoma | 308 (58) | 344 (74) | 70 (75) |
| Squamous Cell | 118 (22) | 82 (18) | 16 (17) |
| Large Cell | 27 (5) | 9 (2) | 0 |
| Other NSCLC/NOS | 30 (6)/0 | 9 (2)/19 (4) | 3 (3)/4 (4) |
| Unknown | 45 (9) | 0 | 0 |
| Stage IIIB/IIIC/IV | 35 (7)/0/493 (93) | 36 (8)/0/427 (92) | 5 (5)/1 (1)/83 (89) |
| Unknown | 0 | 0 | 4 (4) |
| PDL1 tested * | N/A | 26 (6) | 90 (97) |
| Not Tested | N/A | 434 (94) | 2 (2) |
| Unknown | N/A | 3 (0.7) | 1 (1) |
| 50% and Higher | 10 (38) | 37 (41) | |
| 1–49% | 2 (8) | 19 (21) | |
| <1% | N/A | 13 (50) | 24 (27) |
| Insufficient Sample | 0 | 10 (11) | |
| Not Tested | 1 (4) | 0 | |
| Non-Squamous | 365 (69) | 381 (82) | 77 (83) |
| EGFR Tested ** | 77 (21) | 316 (83) | 73 (95) |
| Negative | 59 (77) | 247 (78) | 60 (82) |
| Positive | 18 (23) | 42 (13) | 9 (12) |
| Inconclusive | 0 | 27 (9) | 4 (5) |
| Not Tested | 288 (79) | 65 (17) | 4 (5) |
| L858R | N/A | 18 (43) | 3 (33) |
| Exon 19 Deletion | N/A | 17 (40) | 4 (44) |
| Other | N/A | 7 (17) | 2 (22) |
| Unstated *** | 18 (100) | 0 | 0 |
| Non-Squamous | 365 (69) | 381 (82) | 77 (83) |
| ALK tested ** | 24 (7) | 338 (89) | 74 (96) |
| Negative | 20 (83) | 313 (93) | 71 (96) |
| Positive | 4 (17) | 12 (4) | 2 (3) |
| Inconclusive | 0 | 13 (4) | 1 (1) |
| Not Tested | 341 (93) | 43 (11) | 3 (4) |
| Treatment as First Line | Cohort A N (%) | Cohort B N (%) | Cohort C N (%) |
|---|---|---|---|
| Number of Patients (Total) | 528 | 463 | 93 |
| No Treatment Received | 237 (45) | 176 (38) | 31 (33) |
| Treatment Unknown | 0 | 0 | 1 (1) |
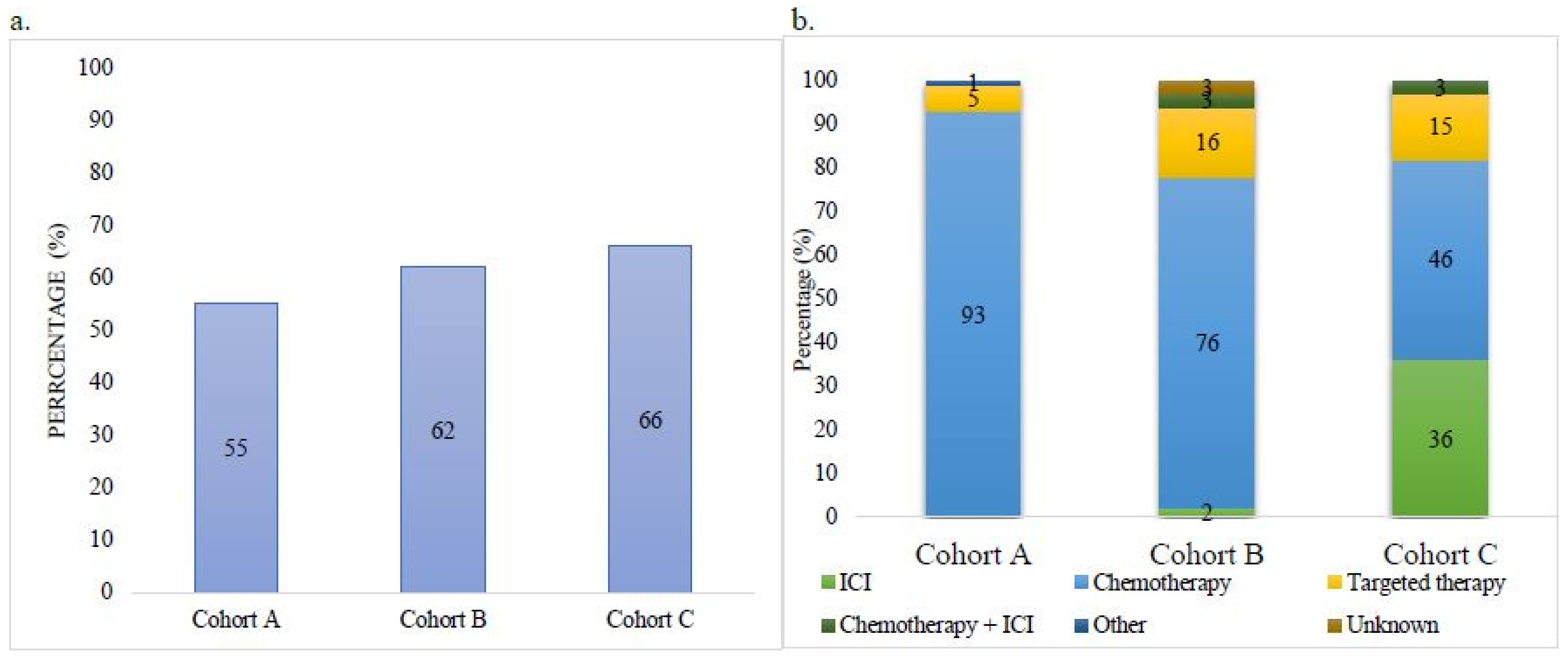
| Received Treatment | 291 (55) | 287 (62) | 61 (66) |
| ICI | 0 | 5 (2) | 22 (36) |
| Chemotherapy | 272 (93) | 219 (76) | 28 (46) |
| Targeted Therapy | 16 (5) | 46 (16) | 9 (15) |
| Chemotherapy + ICI | 0 | 8 (3) | 2 (3) |
| Other (Study Drug) | 3 (1) | 0 | 0 |
| Unknown | 0 | 9 (3) | 0 |
| Patients ≥70 Years Old | 212 (40) | 224 (48) | 44 (47) |
| Received Treatment /No Treatment | 78 (37)/134 (63) | 114 (51)/110 (49) | 23 (52)/20 (48) 1 treatment unknown |
| Patients <70 Years Old | 316 (60) | 239 (52) | 49 (53) |
| Received Treatment /No Treatment | 208 (66)/108 (34) | 173 (72)/66 (28) | 38 (78)/11 (22) |
| Treatment | Cohort A | Cohort B | Cohort C |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Number of Patients EGFR-Positive | 20 (24) | 42 (13) | 9 (12) |
| 1st Line | 16 (80) | 38 (90) | 7 (78) |
| Targeted Therapy | 10 (63) | 36 (94) | 7 (100) |
| Chemotherapy | 6 (38) | 1 (3) | 0 |
| Unknown | 0 | 1 (3) | 0 |
| Number of Patients ALK-Positive | 4 (17) | 12 (4) | 2 (3) |
| 1st Line | 4 (100) | 11 (92) | 2 (100) |
| Targeted Therapy | 0 | 10 (91) | 2 (100) |
| Chemotherapy | 4 (100) | 1 (9) | 0 |
| Unknown | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stock-Martineau, S.; Laurie, K.; McKinnon, M.; Zhang, T.; Wheatley-Price, P. Evolution of Systemic Treatment Uptake and Survival in Advanced Non-Small Cell Lung Cancer. Curr. Oncol. 2021, 28, 60-68. https://doi.org/10.3390/curroncol28010008
Stock-Martineau S, Laurie K, McKinnon M, Zhang T, Wheatley-Price P. Evolution of Systemic Treatment Uptake and Survival in Advanced Non-Small Cell Lung Cancer. Current Oncology. 2021; 28(1):60-68. https://doi.org/10.3390/curroncol28010008
Chicago/Turabian StyleStock-Martineau, Sophie, Katie Laurie, Mathieu McKinnon, Tinghua Zhang, and Paul Wheatley-Price. 2021. "Evolution of Systemic Treatment Uptake and Survival in Advanced Non-Small Cell Lung Cancer" Current Oncology 28, no. 1: 60-68. https://doi.org/10.3390/curroncol28010008
APA StyleStock-Martineau, S., Laurie, K., McKinnon, M., Zhang, T., & Wheatley-Price, P. (2021). Evolution of Systemic Treatment Uptake and Survival in Advanced Non-Small Cell Lung Cancer. Current Oncology, 28(1), 60-68. https://doi.org/10.3390/curroncol28010008




