Cancer Testis Antigen, NOL4, Is an Immunogenic Antigen Specifically Expressed in Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biospecimens and Cell Lines
2.2. Total RNA Extraction from Tissues and Cell Lines
2.3. Preparation of cDNA Library and Serum
2.4. Preparation of Human Sera
2.5. Immunoscreening
2.6. Reverse Transcriptase-PCR (RT-PCR) Analysis
2.7. Western Blot Analysis
2.8. Serological Assay
2.9. Immunohistochemistry
3. Results
3.1. Identification and Characterization of SCLC Antigens by SEREX
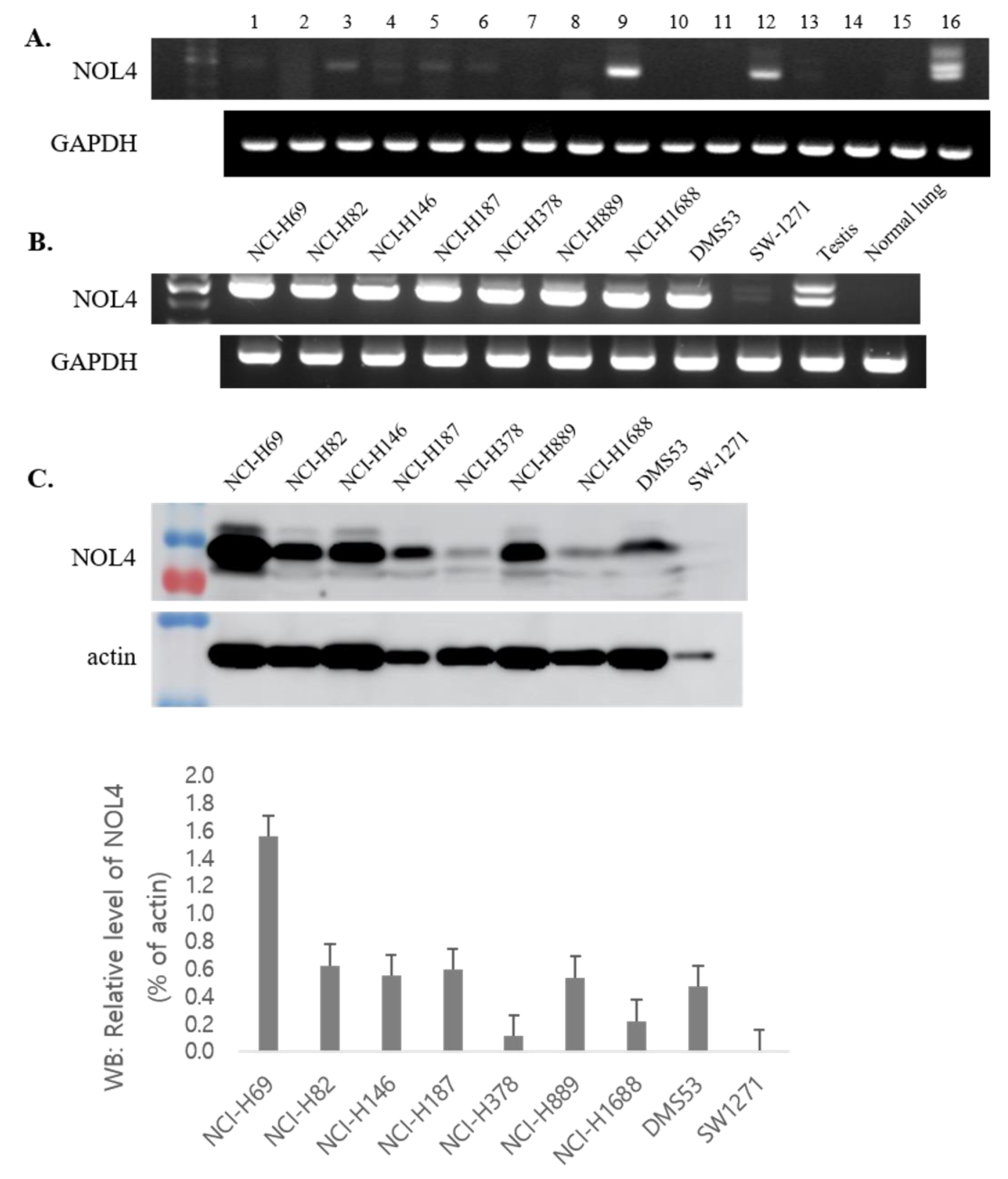
3.2. The mRNA and Protein Expression Profiles of NOL4
3.3. NOL4 Protein Is Specifically Expressed in Tissues of SCLC
3.4. Seroreactivity of NOL4 by Western Blot Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Stag-ing of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Minna, J.D. Small cell lung cancers made from scratch. J. Exp. Med. 2019, 216, 476–478. [Google Scholar] [CrossRef] [Green Version]
- van der Bruggen, P.; Szikora, J.P.; Boel, P.; Wildmann, C.; Somville, M.; Sensi, M.; Boon, T. Autologous cytolytic T lymphocytes recognize a MAGE-1 nonapeptide on melanomas expressing HLA-Cw*1601. Eur. J. Immunol. 1994, 24, 2134–2140. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Zamani, M.R.; Pourvahedi, M.; Golchehre, Z.; Bereshneh, A.H.; Reza, Z.M. Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers. Immunol. Investig. 2016, 45, 619–640. [Google Scholar] [CrossRef] [PubMed]
- Jongeneel, V. Towards a cancer immunome database. Cancer Immun. 2001, 1, 3. [Google Scholar] [PubMed]
- Van den Eynde, B.; Peeters, O.; de Backer, O.; Gaugler, B.; Lucas, S.; Boon, T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J. Exp. Med. 1995, 182, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.; Skipper, J.; Chen, Y.; Henderson, R.; Darrow, T.; Shabanowitz, J.; Engelhard, V.; Hunt, D.; Slingluff, C. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science 1994, 264, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, S.; Schirle, M.; Gückel, B.; Dumrese, T.; Stumm, S.; Kayser, S.; Moris, A.; Wallwiener, D.; Rammensee, H.G.; Stevanovic, S. A MAGE-A1 HLA-A A*0201 epitope identified by mass spectrometry. Cancer Res. 2001, 61, 4072–4077. [Google Scholar]
- Sahin, U.; Tureci, O.; Schmitt, H.; Cochlovius, B.; Johannes, T.; Schmits, R.; Stenner, F.; Luo, G.; Schobert, I.; Pfreundschuh, M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA 1995, 92, 11810–11813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, U.; Tureci, O. Antigen identification using SEREX. Methods Mol. Biol. 2013, 1061, 59–77. [Google Scholar] [PubMed]
- Wang, K.; Xu, X.; Nie, Y.; Dai, L.; Wang, P.; Zhang, J. Identification of tumor-associated antigens by using SEREX in hepatocellular carcinoma. Cancer Lett. 2009, 281, 144–150. [Google Scholar] [CrossRef]
- Zhou, S.F.; Xie, X.X.; Bin, Y.H.; Lan, L.; Chen, F.; Luo, G.R. Identification of HCC-22-5 tumor-associated antigen and antibody response in patients. Clin. Chim. Acta 2006, 366, 274–280. [Google Scholar] [CrossRef]
- Caballero, O.L.; Chen, Y.-T. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009, 100, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Gati, A.; Lajmi, N.; Derouiche, A.; Marrakchi, R.; Chebil, M.; Benammar-Elgaaied, A. NY-ESO-1 expression and immunogenicity in prostate cancer patients. La Tunisie Medicale 2011, 89, 779–783. [Google Scholar]
- Dong, Z.; Hong, Z.; Quanxing, L.; Xiao, L.; XuFeng, D.; Li, J.; Bing, H.; Yong, F.; Feng, Z.; Yan, D.; et al. Attenuated plasmodium sporozoite expressing MAGE-A3 induces antigen-specific CD8+ T cell response against lung cancer in mice. Cancer Biol. Med. 2019, 16, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Pol, J.G.; Acuna, S.A.; Yadollahi, B.; Tang, N.; Stephenson, K.B.; Atherton, M.J.; Hanwell, D.; El-Warrak, A.; Goldstein, A.; Moloo, B.; et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. OncoImmunology 2019, 8, e1512329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Ren, B.; Zou, G.; Liu, J.; Chen, W.; Huang, Y.; Chen, X.; Fu, Y. SPAG9/MKK3/p38 axis is a novel therapeutic target for liver cancer. Oncol. Rep. 2019, 41, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wei, X.; Zou, G.; He, J.; Xu, G.; Xu, F.; Huang, Y.; Zhu, H.; Li, Y.; Ma, G.; et al. Cancer testis antigen SPAG9 is a promising marker for the diagnosis and treatment of lung cancer. Oncol. Rep. 2016, 35, 2599–2605. [Google Scholar] [CrossRef] [Green Version]
- Song, M.-H.; Kim, Y.-R.; Bae, J.-H.; Shin, N.-H.; Lee, S.-Y. A cancer/testis antigen, NY-SAR-35, induces EpCAM, CD44, and CD133, and activates ERK in HEK293 cells. Biochem. Biophys. Res. Commun. 2017, 484, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Gao, A.; Pan, Y.; Zhang, G.; Tu, J.; Zhou, Y.; Yang, P.; Cao, Z.; Wei, Q.; Ding, Y.; et al. CT45A1 acts as a new proto-oncogene to trigger tumorigenesis and cancer metastasis. Cell Death Dis. 2014, 5, e1285. [Google Scholar] [CrossRef]
- D’Arcy, P.; Maruwge, W.; Wolahan, B.; Ma, L.; Brodin, B. Oncogenic Functions of the Cancer-Testis Antigen SSX on the Proliferation, Survival, and Signaling Pathways of Cancer Cells. PLoS ONE 2014, 9, e95136. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.; Kim, Y.; Jeoung, O. miR-30a Regulates the Expression of CAGE and p53 and Regulates the Response to Anti-Cancer Drugs. Mol. Cells 2016, 39, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Gjerstorff, M.F.; Andersen, M.H.; Ditzel, H.J. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget 2015, 6, 15772–15787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-Y.; Obata, Y.; Yoshida, M.; Stockert, E.; Williamson, B.; Jungbluth, A.A.; Chen, Y.-T.; Old, L.J.; Scanlan, M.J. Immunomic analysis of human sarcoma. Proc. Natl. Acad. Sci. USA 2003, 100, 2651–2656. [Google Scholar] [CrossRef] [Green Version]
- Song, M.H.; Ha, J.M.; Shin, D.H.; Lee, C.H.; Old, L.; Lee, S.Y. KP-CoT-23 (CCDC83) is a novel immunogenic cancer/testis antigen in colon cancer. Int. J. Oncol. 2012, 41, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Ueki, N.; Kondo, M.; Seki, N.; Yano, K.; Oda, T.; Masuho, Y.; Muramatsu, M.A. NOLP: Identification of a novel human nucleolar protein and determination of sequence requirements for its nucleolar localization. Biochem. Biophys. Res. Commun. 1998, 252, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Demokan, S.; Chuang, A.Y.; Pattani, K.M.; Sidransky, D.; Koch, W.; Califano, J.A. Validation of nucleolar protein 4 as a novel methylated tumor suppressor gene in head and neck cancer. Oncol. Rep. 2014, 31, 1014–1020. [Google Scholar] [CrossRef]
- Kanehira, M.; Katagiri, T.; Shimo, A.; Takata, R.; Shuin, T.; Miki, T.; Fujioka, T.; Nakamura, Y. Oncogenic Role of MPHOSPH1, a Cancer-Testis Antigen Specific to Human Bladder Cancer. Cancer Res. 2007, 67, 3276–3285. [Google Scholar] [CrossRef] [Green Version]
- Güre, A.O.; Stockert, E.; Scanlan, M.J.; Keresztes, R.S.; Jäger, D.; Altorki, N.K.; Old, L.J.; Chen, Y.-T. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 4198–4203. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.; Smiraglia, D.J.; Wu, Y.-Z.; Ghosh, S.; Rader, J.S.; Cho, K.R.; Bonfiglio, T.A.; Nayar, R.; Plass, C.; Sherman, M.E. Identification of Novel Methylation Markers in Cervical Cancer Using Restriction Landmark Genomic Scanning. Cancer Res. 2008, 68, 2489–2497. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Shiraishi, T.; Miles, N.; Trock, B.J.; Kulkarni, P.; Getzenberg, R.H. Nanowire Analysis of Cancer-Testis Antigens as Biomarkers of Aggressive Prostate Cancer. Urology 2015, 85, 704.e1–704.e7. [Google Scholar] [CrossRef] [Green Version]
- Stangeland, B.; Mughal, A.A.; Grieg, Z.; Sandberg, C.J.; Joel, M.; Nygård, S.; Meling, T.; Murrell, W.; Mo, E.O.V.; Langmoen, I.A. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget 2015, 6, 26192–26215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Y.; Zhang, X.; Liu, X.-Y.; Peng, A.; Chen, Y.; Meng, L.; Chen, H.; Zhang, Y.; Miao, X.; et al. Inhibition of kinesin family member 20B sensitizes hepatocellular carcinoma cell to microtubule-targeting agents by blocking cytokinesis. Cancer Sci. 2018, 109, 3450–3460. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, M.; Ueda, S.; Yew, P.Y.; Fukuda, I.; Yoshimura, S.; Kishi, H.; Hamana, H.; Hirayama, M.; Yatsuda, J.; Irie, A. Bladder cancer-associated cancer-testis antigen-derived long peptides encompassing both CTL and promiscuous HLA class II-restricted Th cell epitopes induced CD4(+) T cells expressing converged T-cell receptor genes in vitro. Oncoimmunology 2018, 7, e1415687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obara, W.; Eto, M.; Mimata, H.; Kohri, K.; Mitsuhata, N.; Miura, I.; Shuin, T.; Miki, T.; Koie, T.; Fujimoto, H.; et al. A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann. Oncol. 2017, 28, 798–803. [Google Scholar] [CrossRef]
- Jäger, E.; Nagata, Y.; Gnjatic, S.; Wada, H.; Stockert, E.; Karbach, J.; Dunbar, P.R.; Lee, S.Y.; Jungbluth, A.; Jäger, D.; et al. Monitoring CD8 T cell responses to NY-ESO-1: Correlation of humoral and cellular immune responses. Proc. Natl. Acad. Sci. USA 2000, 97, 4760–4765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeper, C.; Gambazzi, F.; Zajac, P.; Bubendorf, L.; Adamina, M.; Rosenthal, R.; Zerkowski, H.-R.; Heberer, M.; Spagnoli, G.C. Cancer/testis antigen expression and specific cytotoxic T lymphocyte responses in non small cell lung cancer. Int. J. Cancer 2006, 120, 337–343. [Google Scholar] [CrossRef]


| KP-SCLC-Antigen a | Gene Name/REFSEQ mRNAs | Cancers-Related Published Papers b | KP-SCLC-Antigen | Gene Name/REFSEQ mRNAs | Cancers-Related Published Papers |
|---|---|---|---|---|---|
| 1 | EIF5B/NM_015904.4 | Yes | 38 | CTBP2/NM_001329.4 | Yes |
| 2 | GNB2L1/NM_006098.5 | Yes | 39 | RPL36/NM_033643.3 | Yes |
| 3 | PSMD3/NM_002809.4 | Yes | 40 | CYC1/NM_001916.5 | Yes |
| 4 | TAF-I/NM_001122821.2 | Yes | 41 | RPS27A/NM_002954.6 | Yes |
| 5 | SFRS1/NM_006924.5 | Yes | 42 | ZNF358/NM_018083.5 | No |
| 6 | FAU/NM_001997.5 | NO | 43 | UBTF/NM_014233.4 | Yes |
| 7 | SSX2IP/NM_001166417.2 | Yes | 44 | ANKRD11/NM_001256182.2 | Yes |
| 8 | PYCR1/NM_001330523.1 | Yes | 45 | ASS1/NM_000050.4 | Yes |
| 9 | HARS2/NM_012208.4 | No | 46 | TOP1MT/NM_052963.3 | Yes |
| 10 | CHD7/NM_017780.4 | Yes | 47 | DDX39B/NM_004640.7 | Yes |
| 11 | COASY/NM_025233.7 | Yes | 48 | SMARCA4/NM_001128849.3 | Yes |
| 12 | HMBS/NM_000190.4 | Yes | 49 | NREP/NM_004772.4 | Yes |
| 13 | JSRP1/NM_144616.4 | No | 50 | PTMA/NM_001099285.2 | Yes |
| 14 | RAN/NM_006325.5 | Yes | 51 | HLA-A/NM_002116.8 | Yes |
| 15 | SSSCA1/NM_006396.3 | No | 52 | CPSF3L/NM_001256456.2 | No |
| 16 | HMGB2/NM_002129.4 | Yes | 53 | CCDC124/NM_138442.4 | No |
| 17 | BRD7/NM_001173984.3 | Yes | 54 | HDLBP/NM_005336.6 | Yes |
| 18 | SUV420H2/NM_032701.4 | Yes | 55 | RPL10/NM_006013.5 | Yes |
| 19 | CDKN2A/NM_000077.5 | Yes | 56 | RPL7A/NM_000972.3 | Yes |
| 20 | RIOK1/NM_031480.3 | Yes | 57 | HNRNPA1/NM_002136.4 | Yes |
| 21 | TTC5/NM_138376.3 | Yes | 58 | GNL2/NM_013285.3 | Yes |
| 22 | GDI2/NM_001494.4 | Yes | 59 | CCDC83/NM_173556.5 | Yes |
| 23 | NAPRT1/NM_145201.6 | Yes | 60 | GPBP1L1/NM_021639.5 | No |
| 24 | MRPL12/NM_002949.4 | No | 61 | GCSH/NM_004483.5 | No |
| 25 | EIF3F/NM_003754.3 | No | 62 | CCDC158/NM_001042784.1 | No |
| 26 | MCM3/NM_002388.6 | Yes | 63 | ATP5O/NM_001697.3 | No |
| 27 | STUB1/NM_005861.4 | Yes | 64 | GDI2/NM_001494.4 | Yes |
| 28 | HSPA4/NM_002154.4 | Yes | 65 | IQSEC1/NM_001134382.3 | Yes |
| 29 | NOL4/NM_003787.5 | Yes | 66 | TUBB2/NM_001069.3 | Yes |
| 30 | PUF60/NM_078480.3 | Yes | 67 | CCDC17/NM_001114938.3 | No |
| 31 | RPL9/NM_000661.5 | Yes | 68 | ZNF532/NM_018181.6 | Yes |
| 32 | CDK11A/NM_024011.4 | Yes | 69 | KIF20B/NM_001284259.2 | Yes |
| 33 | GOSR1/NM_004871.3 | Yes | 70 | TMEM9/NM_016456.5 | Yes |
| 34 | NME1/NM_198175.1 | Yes | 71 | KIF27/NM_017576.4 | No |
| 35 | XLS/NM_001329678.2 | Yes | 72 | CLIC4/NM_013943.3 | Yes |
| 36 | HMGN3/NM_004242.4 | No | 73 | ODC1/NM_002539.3 | Yes |
| 37 | ACTG1/NM_001199954.3 | Yes | 74 | ST13/NM_003932.5 | Yes |
| Serum Number | Serum Source a | SeroreacTivity b | Serum Number | Serum Source | Seroreactivity |
|---|---|---|---|---|---|
| N1 | Healthy donor | N | S13 | ED-SCLC | P |
| N2 | Healthy donor | N | S14 | ED-SCLC | P |
| N3 | Healthy donor | P | S15 | ED-SCLC | P |
| N4 | Healthy donor | P | S16 | ED-SCLC | P |
| N5 | Healthy donor | P | S17 | ED-SCLC | P |
| N6 | Healthy donor | N | S18 | ED-SCLC | N |
| N7 | Healthy donor | N | S19 | ED-SCLC | P |
| N8 | Healthy donor | P | S20 | ED-SCLC | P |
| N9 | Healthy donor | P | S21 | ED-SCLC | P |
| N10 | Healthy donor | P | S22 | ED-SCLC | P |
| N11 | Healthy donor | N | S23 | ED-SCLC | P |
| N12 | Healthy donor | P | S24 | ED-SCLC | P |
| N13 | Healthy donor | N | S25 | ED-SCLC | P |
| N14 | Healthy donor | P | S26 | ED-SCLC | P |
| N15 | Healthy donor | P | S27 | ED-SCLC | P |
| N16 | Healthy donor | P | S28 | ED-SCLC | P |
| N17 | Healthy donor | N | S29 | ED-SCLC | P |
| N18 | Healthy donor | P | S30 | ED-SCLC | P |
| N19 | Healthy donor | P | S31 | ED-SCLC | P |
| N20 | Healthy donor | P | S32 | LD-SCLC | P |
| S1 | ED-SCLC | N | S33 | LD-SCLC | P |
| S2 | ED-SCLC | N | S34 | LD-SCLC | P |
| S3 | ED-SCLC | N | S35 | LD-SCLC | P |
| S4 | ED-SCLC | N | S36 | LD-SCLC | P |
| S5 | ED-SCLC | P | S37 | LD-SCLC | N |
| S6 | ED-SCLC | P | S38 | LD-SCLC | P |
| S7 | ED-SCLC | P | S39 | LD-SCLC | P |
| S8 | ED-SCLC | N | S40 | LD-SCLC | N |
| S9 | ED-SCLC | P | S41 | LD-SCLC | N |
| S10 | ED-SCLC | N | S42 | LD-SCLC | P |
| S11 | ED-SCLC | P | S43 | LD-SCLC | P |
| S12 | ED-SCLC | P | S44 | LD-SCLC | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-R.; Kim, K.-U.; Lee, J.-H.; Kim, D.-W.; Chung, J.-H.; Kim, Y.-D.; Shin, D.-H.; Lee, M.-K.; Shin, Y.-I.; Lee, S.-Y. Cancer Testis Antigen, NOL4, Is an Immunogenic Antigen Specifically Expressed in Small-Cell Lung Cancer. Curr. Oncol. 2021, 28, 1927-1937. https://doi.org/10.3390/curroncol28030179
Kim Y-R, Kim K-U, Lee J-H, Kim D-W, Chung J-H, Kim Y-D, Shin D-H, Lee M-K, Shin Y-I, Lee S-Y. Cancer Testis Antigen, NOL4, Is an Immunogenic Antigen Specifically Expressed in Small-Cell Lung Cancer. Current Oncology. 2021; 28(3):1927-1937. https://doi.org/10.3390/curroncol28030179
Chicago/Turabian StyleKim, Ye-Rin, Ki-Uk Kim, Jung-Hee Lee, Deok-Won Kim, Jae-Heun Chung, Yeong-Dae Kim, Dong-Hoon Shin, Min-Ki Lee, Yong-Il Shin, and Sang-Yull Lee. 2021. "Cancer Testis Antigen, NOL4, Is an Immunogenic Antigen Specifically Expressed in Small-Cell Lung Cancer" Current Oncology 28, no. 3: 1927-1937. https://doi.org/10.3390/curroncol28030179
APA StyleKim, Y.-R., Kim, K.-U., Lee, J.-H., Kim, D.-W., Chung, J.-H., Kim, Y.-D., Shin, D.-H., Lee, M.-K., Shin, Y.-I., & Lee, S.-Y. (2021). Cancer Testis Antigen, NOL4, Is an Immunogenic Antigen Specifically Expressed in Small-Cell Lung Cancer. Current Oncology, 28(3), 1927-1937. https://doi.org/10.3390/curroncol28030179





