Selective Internal Radiation Combined with Chemotherapy Maintains the Quality of Life in Intrahepatic Cholangiocarcinomas
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Population
3.2. Treatment
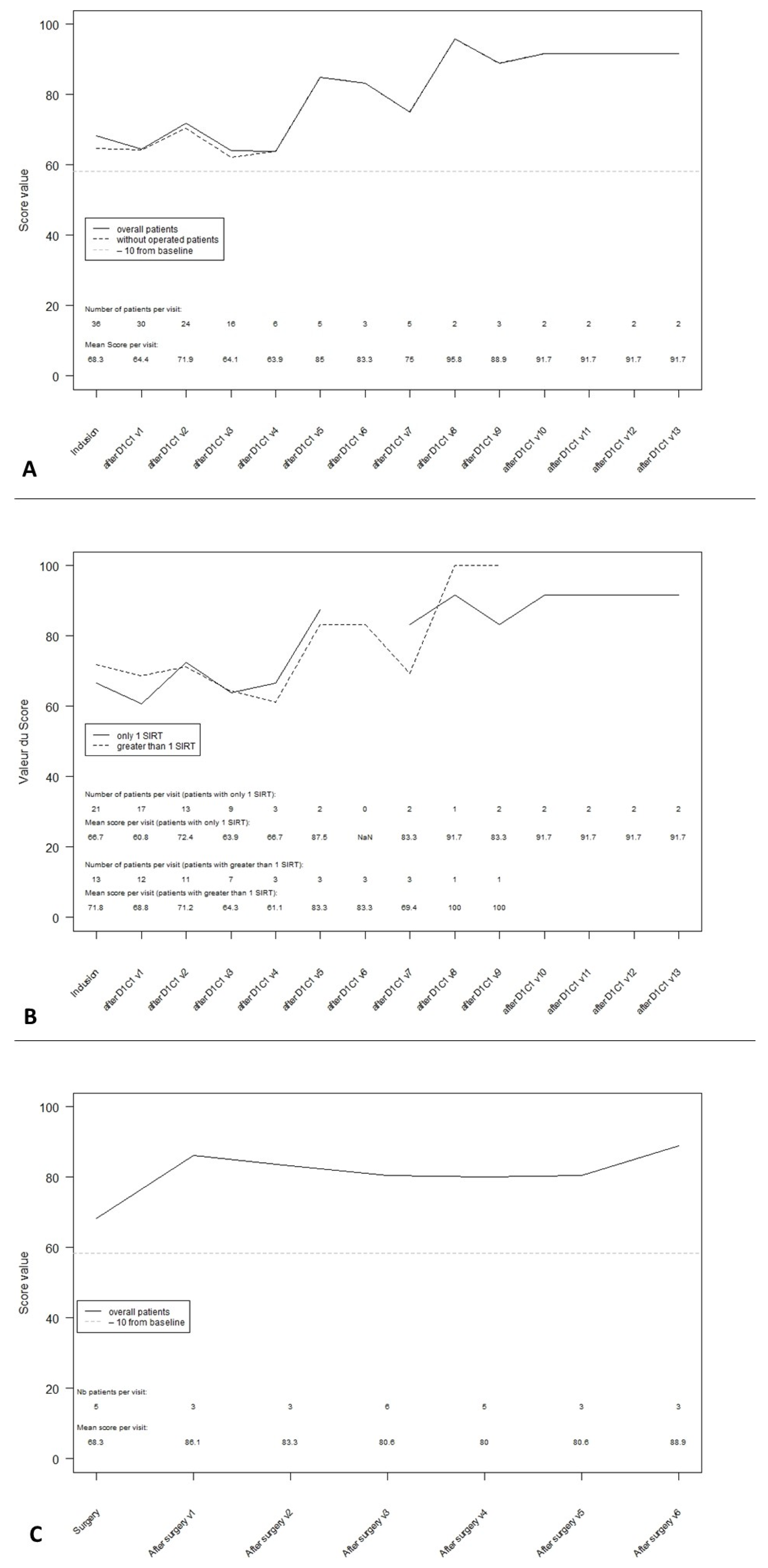
3.3. Global Health Score
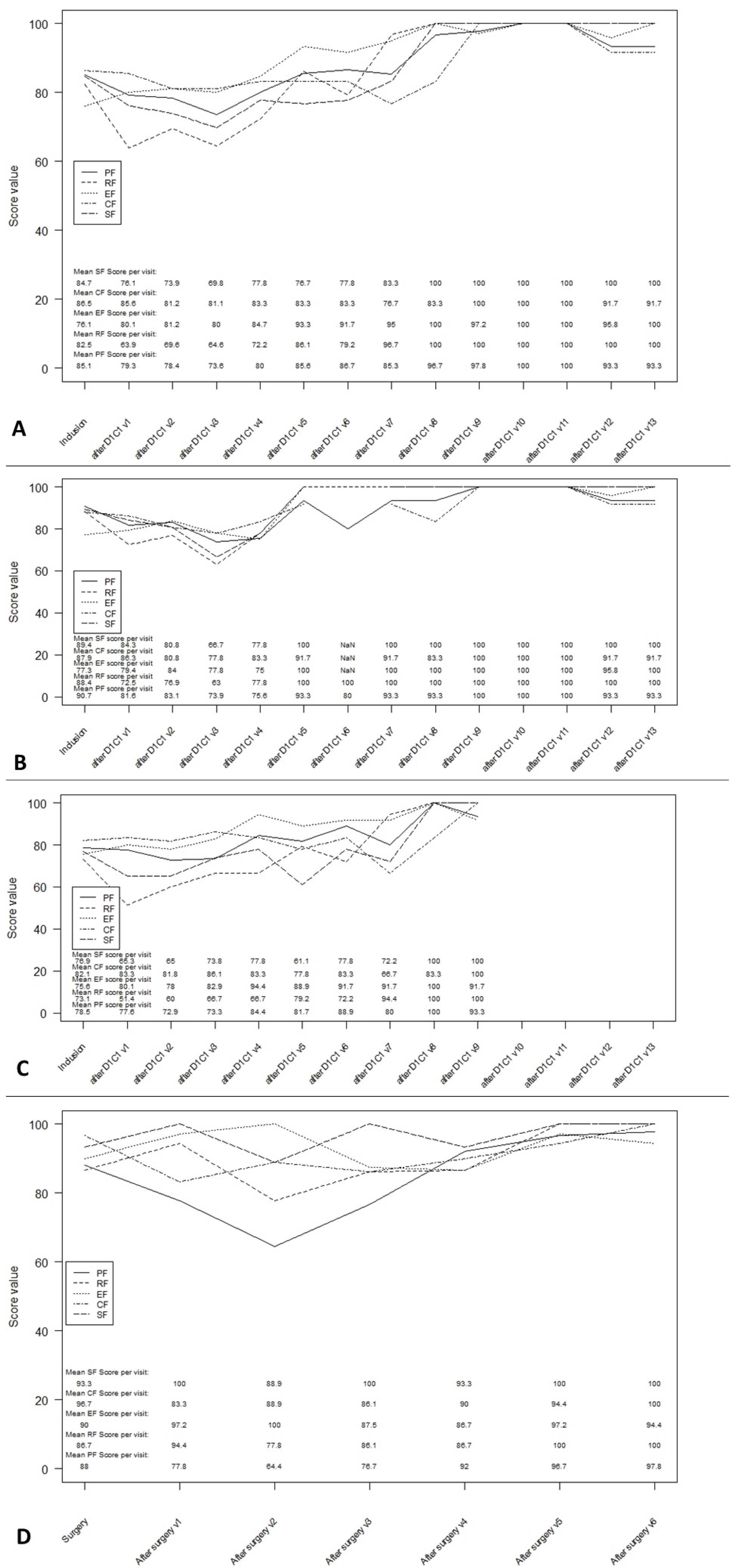
3.4. Functioning Scales
3.5. Symptom Scales
3.6. QLQ-C30 Summary Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- On behalf of the ABC-02 investigators; Bridgewater, J.; Lopes, A.; Palmer, D.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Valle, J.; et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br. J. Cancer 2016, 114, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, D.; Saletti, P.; Borner, M.; Gerber, D.; Dietrich, D.; Caspar, C.B.; Mingrone, W.; Beretta, K.; Strasser, F.; Ruhstaller, T.; et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: A multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J. Clin. Oncol. 2008, 26, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, F.; Tantau, M.; Diaconu, B.; Acalovschi, M. Survival and quality of life of cholangiocarcinoma patients: A prospective study over a 4 year period. J. Gastrointest. Liver Dis. 2010, 19, 285–290. [Google Scholar]
- Woradet, S.; Songserm, N.; Promthet, S.; Parkin, D.M. Health-Related Quality of Life and Survival of Cholangiocarcinoma Patients in Northeastern Region of Thailand. PLoS ONE 2016, 11, e0163448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elberg Dengsø, K.; Hillingsø, J.; Marcussen, A.M.; Thomsen, T. Health-related quality of life and anxiety and depression in patients diagnosed with cholangiocarcinoma: A prospective cohort study. Acta Oncol. 2017, 56, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Gabr, A.; O’Brian, D.P.; Riaz, A.; Desai, K.; Thornburg, B.; Kallini, J.R.; Mouli, S.; Lewandowski, R.J.; Salem, R. Contemporary Systematic Review of Health-Related Quality of Life Outcomes in Locoregional Therapies for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2019, 30, 1924–1933.e2. [Google Scholar] [CrossRef]
- Salem, R.; Gilbertsen, M.; Butt, Z.; Memon, K.; Vouche, M.; Hickey, R.; Baker, T.; Abecassis, M.M.; Atassi, R.; Riaz, A.; et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin. Gastroenterol. Hepatol. 2013, 11, 1358–1365.e1. [Google Scholar] [CrossRef]
- Kirchner, T.; Marquardt, S.; Werncke, T.; Kirstein, M.M.; Brunkhorst, T.; Wacker, F.; Vogel, A.; Rodt, T. Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom. Radiol. 2019, 44, 1554–1561. [Google Scholar] [CrossRef]
- Xing, M.; Kokabi, N.; Camacho, J.C.; Kim, H.S. Prospective longitudinal quality of life and survival outcomes in patients with advanced infiltrative hepatocellular carcinoma and portal vein thrombosis treated with Yttrium-90 radioembolization. BMC Cancer 2018, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Salem, R.; Hassan, S.; Lewandowski, R.J.; Grace, K.; Martin, R.C.G.; Sichlau, M.J.; Fung, J.; Kim, E.; Chao, S.; Rosner, B.I. Quality of Life after Radioembolization for Hepatocellular Carcinoma Using a Digital Patient-Reported Outcome Tool. J. Vasc. Interv. Radiol. 2020, 31, 311–314.e1. [Google Scholar] [CrossRef]
- Vilgrain, V.; Abdel-Rehim, M.; Sibert, A.; Ronot, M.; Lebtahi, R.; Castéra, L.; Chatellier, G. SARAH Trial Group Radioembolisation with yttrium‒90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): Study protocol for a randomised controlled trial. Trials 2014, 15, 474. [Google Scholar] [CrossRef] [Green Version]
- Loffroy, R.; Ronot, M.; Greget, M.; Bouvier, A.; Mastier, C.; Sengel, C.; Tselikas, L.; Arnold, D.; Maleux, G.; Pelage, J.-P.; et al. Short-term Safety and Quality of Life Outcomes Following Radioembolization in Primary and Secondary Liver Tumours: A Multi-centre Analysis of 200 Patients in France. Cardiovasc. Interv. Radiol. 2021, 44, 36–49. [Google Scholar] [CrossRef]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Kieffer, J.M.; Fayers, P.M.; Groenvold, M.; Petersen, M.A.; Scott, N.W.; Sprangers, M.A.G.; Velikova, G.; Aaronson, N.K. EORTC Quality of Life Group Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016, 69, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Mosconi, C.; Gramenzi, A.; Ascanio, S.; Cappelli, A.; Renzulli, M.; Pettinato, C.; Brandi, G.; Monari, F.; Cucchetti, A.; Trevisani, F.; et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: A survival, efficacy and safety study. Br. J. Cancer 2016, 115, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Soydal, C.; Kucuk, O.N.; Bilgic, S.; Ibis, E. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: Prognostic factors. Ann. Nucl. Med. 2016, 30, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Du, F.L.; Rayar, M.; Rolland, Y.; Beuzit, L.; Boudjema, K.; Rohou, T.; Latournerie, M.; Campillo-Gimenez, B.; Garin, E.; et al. Glass Microspheres 90Y Selective Internal Radiation Therapy and Chemotherapy as First-Line Treatment of Intrahepatic Cholangiocarcinoma. Clin. Nucl. Med. 2015, 40, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Mouli, S.; Memon, K.; Baker, T.; Benson, A.B.; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, R.-T.; Paprottka, P.M.; Schön, A.; Bamberg, F.; Haug, A.; Dürr, E.-M.; Rauch, B.; Trumm, C.T.; Jakobs, T.F.; Helmberger, T.K.; et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: Factors associated with prolonged survival. Cardiovasc. Interv. Radiol. 2012, 35, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Virarkar, M.K.; Binnenhei, M.; Justinger, M.; Schön, M.R.; Tatsch, K. Prognostic Factors in Overall Survival of Patients with Unresectable Intrahepatic Cholangiocarcinoma Treated by Means of Yttrium-90 Radioembolization: Results in Therapy-Naïve Patients. Cardiovasc. Interv. Radiol. 2018, 41, 744–752. [Google Scholar] [CrossRef]
- Swinburne, N.C.; Biederman, D.M.; Besa, C.; Tabori, N.E.; Fischman, A.M.; Patel, R.S.; Nowakowski, F.S.; Gunasekaran, G.; Schwartz, M.E.; Lookstein, R.A.; et al. Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: Review of Safety, Response Evaluation Criteria in Solid Tumors 1.1 Imaging Response and Survival. Cancer Biother. Radiopharm. 2017, 32, 161–168. [Google Scholar] [CrossRef]
- Gangi, A.; Shah, J.; Hatfield, N.; Smith, J.; Sweeney, J.; Choi, J.; El-Haddad, G.; Biebel, B.; Parikh, N.; Arslan, B.; et al. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J. Vasc. Interv. Radiol. 2018, 29, 1101–1108. [Google Scholar] [CrossRef]
- Bourien, H.; Palard, X.; Rolland, Y.; Le Du, F.; Beuzit, L.; Uguen, T.; Le Sourd, S.; Pracht, M.; Manceau, V.; Lièvre, A.; et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: A large single-center experience. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 669–676. [Google Scholar] [CrossRef]
- Al-Adra, D.P.; Gill, R.S.; Axford, S.J.; Shi, X.; Kneteman, N.; Liau, S.-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: A systematic review and pooled analysis. Eur. J. Surg. Oncol. 2015, 41, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Cucchetti, A.; Cappelli, A.; Mosconi, C.; Zhong, J.-H.; Cescon, M.; Pinna, A.D.; Golfieri, R. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: A meta-regression study. Liver Int. 2017, 37, 1056–1064. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Loth, F.L.C.; Aaronson, N.K.; Arraras, J.I.; Caocci, G.; Efficace, F.; Groenvold, M.; van Leeuwen, M.; Petersen, M.A.; Ramage, J.; et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J. Clin. Epidemiol. 2020, 118, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kaupp-Roberts, S.D.; Yadegarfar, G.; Friend, E.; O’Donnell, C.M.; Valle, J.W.; Byrne, C.; Bahar, I.; Finch-Jones, M.; Gillmore, R.; Johnson, C.D.; et al. Validation of the EORTC QLQ-BIL21 questionnaire for measuring quality of life in patients with cholangiocarcinoma and cancer of the gallbladder. Br. J. Cancer 2016, 115, 1032–1038. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | All Included Patients (n = 41) | Down-Staged Patients (n = 9) | |
|---|---|---|---|
| Median age at inclusion | 67.3 (36.7–82.2) | 71.2 (46.5–74.9) | |
| Gender | Male | 26 (63%) | 4 (44%) |
| Cirrhosis | 12 (29%) | 2 (22%) | |
| Child Pugh score at inclusion in patients with cirrhosis | A5 | 9 (75%) | 2 (100%) |
| A6 | 2 (16%) | 0 (0%) | |
| B7 | 1 (8%) | 0 (0%) | |
| Performance status at inclusion (n = 40) | PS0 | 26 (65%) | 7 (78%) |
| Albumin (g/L) (n = 39) | 40 (24–47) | 41 (39–44) | |
| Prothrombin time (% relative to control) | 89 (32–117) | 90 (73–117) | |
| Total bilirubin at inclusion (µmol/L) | 13.3 (4–38) | 13.6 (4–20.1) | |
| ALT (UI/L) | 28 (10–346) | 20 (10–346) | |
| AST (UI/L) | 36 (12–138) | 27 (12–115) | |
| Alkaline phosphatase (UI/L) | 111 (49–366) | 106 (52–300) | |
| Gamma GT rate (UI/L) (n = 40) | 136.5 (25–613) | 166 (61–597) | |
| CA19.9 (n = 40) | 52 (0.6–32099) | 36.5 (1–499) | |
| CEA (n = 40) | 3.1 (0.4–51) | 2.4 (1–5.1) | |
| Previous resection | 5 (12%) | 0 (0%) | |
| Days between diagnosis and enrollment | 48 (13–728) | 63 (14–77) | |
| Unifocal tumor | 14 (34%) | 7 (78%) | |
| Unilobar disease | 27 (66%) | 8 (89%) | |
| Liver hilar lymph nodes ≤ 3 cm | 12 (29%) | 2 (22%) | |
| Abdominal lymph nodes | 14 (34%) | 2 (22%) | |
| Lung metastasis ≤ 1 cm | 7 (17%) | 0 (0%) | |
| Patient with locally advanced disease only (including hilar nodules) without abdominal lymph nodes or lung metastasis | 24 (58%) | 7 (78%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goislard de Monsabert, C.; Touchefeu, Y.; Guiu, B.; Campillo-Gimenez, B.; Farges, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Beuzit, L.; Pracht, M.; et al. Selective Internal Radiation Combined with Chemotherapy Maintains the Quality of Life in Intrahepatic Cholangiocarcinomas. Curr. Oncol. 2021, 28, 4530-4541. https://doi.org/10.3390/curroncol28060384
Goislard de Monsabert C, Touchefeu Y, Guiu B, Campillo-Gimenez B, Farges O, Tougeron D, Baumgaertner I, Ayav A, Beuzit L, Pracht M, et al. Selective Internal Radiation Combined with Chemotherapy Maintains the Quality of Life in Intrahepatic Cholangiocarcinomas. Current Oncology. 2021; 28(6):4530-4541. https://doi.org/10.3390/curroncol28060384
Chicago/Turabian StyleGoislard de Monsabert, Camille, Yann Touchefeu, Boris Guiu, Boris Campillo-Gimenez, Olivier Farges, David Tougeron, Isabelle Baumgaertner, Ahmet Ayav, Luc Beuzit, Marc Pracht, and et al. 2021. "Selective Internal Radiation Combined with Chemotherapy Maintains the Quality of Life in Intrahepatic Cholangiocarcinomas" Current Oncology 28, no. 6: 4530-4541. https://doi.org/10.3390/curroncol28060384





