Clinical Outcomes of RTOG 9310 Protocol for Primary Central Nervous System Lymphoma: Single-Center Experience with 87 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Treatment Protocol
2.3. Clinical Data Collection
2.4. Follow-Up
2.5. Survival and Statistical Analyses
3. Results
3.1. Patient and Tumor Characteristics
3.2. Follow-Up and Treatment Response
3.3. PFS and OS
3.4. Multivariate Analysis for Predicting Factors of PFS and OS
3.5. Adverse Effects of RTOG 9310 Protocol for PCNSL Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef] [Green Version]
- Dho, Y.S.; Jung, K.W.; Ha, J.; Seo, Y.B.; Park, C.K.; Won, Y.J.; Yoo, H. An Updated Nationwide Epidemiology of Primary Brain Tumors in Republic of Korea, 2013. Brain Tumor Res. Treat. 2017, 5, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Bessell, E.M.; Dickinson, P.; Dickinson, S.; Salmon, J. Increasing age at diagnosis and worsening renal function in patients with primary central nervous system lymphoma. J. Neurooncol. 2011, 104, 191–193. [Google Scholar] [CrossRef]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.P.; Decker, P.A.; Tieu, C.; Cerhan, J.R. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am. J. Hematol. 2013, 88, 997–1000. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, B.P.; O’Fallon, J.R.; Earle, J.D.; Colgan, J.P.; Brown, L.D.; Krigel, R.L. Primary central nervous system non-Hodgkin’s lymphoma: Survival advantages with combined initial therapy? Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 663–673. [Google Scholar] [CrossRef]
- Schultz, C.; Scott, C.; Sherman, W.; Donahue, B.; Fields, J.; Murray, K.; Fisher, B.; Abrams, R.; Meis-Kindblom, J. Pre-irradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone (CHOD) for PCNSL: Initial report of Radiation Therapy Oncology Group (RTOG) protocol 88-06. J. Clin. Oncol. 1996, 14, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.M.; Bleehen, N.M.; Gregor, A.; Bullimore, J.; Shirley, D.; Rampling, R.P.; Trevor, J.; Glaser, M.G.; Lantos, P.; Ironside, J.W.; et al. A Medical Research Council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: Cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 2000, 89, 1359–1370. [Google Scholar] [CrossRef]
- Hiraga, S.; Arita, N.; Ohnishi, T.; Kohmura, E.; Yamamoto, K.; Oku, Y.; Taki, T.; Sato, M.; Aozasa, K.; Yoshimine, T. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into CSF and intensified tumour response in PCNSL. J. Neurosurg. 1999, 91, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.; Gruber, M.L.; Cher, L.; Hochberg, F.H. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: Long-term outcome. J. Neurosurg. 1994, 81, 188–195. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M.; Seiferheld, W.; Schold, S.C.; Fisher, B.; Schultz, C.J. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J. Clin. Oncol. 2002, 20, 4643–4648. [Google Scholar] [CrossRef]
- Fisher, B.; Seiferheld, W.; Schultz, C.; DeAngelis, L.; Nelson, D.; Schold, S.C.; Curran, W.; Mehta, M. Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: An intergroup phase II combined modality treatment of primary central nervous system lymphoma. J. Neuro-Oncol. 2005, 74, 201–205. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group Experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Lauw, M.I.S.; Lucas, C.H.G.; Ohgami, R.S.; Wen, K.W. Primary Central Nervous System Lymphomas: A diagnostic overview of key histomorphologic, immunophenotypic, and genetic features. Diagnostics 2020, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef]
- National Institutes of Health and National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 5 July 2021).
- Eng, J. Receiver operating characteristic analysis: A primer. Acad. Radiol. 2005, 12, 909–916. [Google Scholar] [CrossRef]
- Glass, J.; Won, M.H.; Schultz, C.J.; Brat, D.; Bartlett, N.L.; Suh, J.H.; Werner-Wasik, M.; Fisher, B.J.; Liepman, M.K.; Augspurger, M.; et al. Phase I and II study of induction chemotherapy with methotrexate, rituximab, and temozolomide, followed by whole-brain radiotherapy and postirradiation temozolomide for primary CNS lymphoma: NRG Oncology RTOG 0227. J. Clin. Oncol. 2016, 34, 1620–1625. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, A.; Monabati, A.; Sadeghipour, A.; Radmanesh, F.; Safaei, A.; Movahedinia, S. MYC, BCL2, and BCL6 rearrangements in primary central nervous system lymphoma of large B cell type. Ann. Hematol. 2019, 98, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kreher, S.; Johrens, K.; Strehlow, F.; Martus, P.; Borowiec, K.; Radke, J.; Heppner, F.; Roth, P.; Thiel, E.; Pietsch, T.; et al. Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro. Oncol. 2015, 17, 1016–1021. [Google Scholar] [CrossRef]
- Löw, S.; Han, C.H.; Batchelor, T.T. Primary central nervous system lymphoma. Ther. Adv. Neurol. Disord. 2018, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Makino, K.; Nakamura, H.; Shinojima, N.; Kuroda, J.-I.; Yano, S.; Mikami, Y.; Mukasa, A. BCL2 expression is associated with a poor prognosis independent of cellular origin in primary central nervous system diffuse large B-cell lymphoma. J. Neuro-Oncol. 2018, 140, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-Y.; Feng, X.; Bao, W.; Ma, J.; Lv, J.-H.; Wang, X.; Rao, Q.; Shi, Q.-L. MYC/BCL2 co-expression is a stronger prognostic factor compared with the cell-of-origin classification in primary CNS DLBCL. J. Neuropathol. Exp. Neurol. 2017, 76, 942–948. [Google Scholar] [CrossRef]
- Kim, S.; Nam, S.J.; Kwon, D.; Kim, H.; Lee, E.; Kim, T.M.; Heo, D.S.; Park, S.H.; Kim, C.W.; Jeon, Y.K. MYC and BCL2 overexpression is associated with a higher class of Memorial Sloan-Kettering Cancer Center prognostic model and poor clinical outcome in primary diffuse large B-cell lymphoma of the central nervous system. BMC. Cancer 2016, 16, 363. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Liu, Y.; Liu, Z.; Cui, Q.; Ji, N.; Sun, S.; Wang, B.; Wang, Y.; Sun, X.; et al. Immunohistochemical profile and prognostic significance in primary central nervous system lymphoma: Analysis of 89 cases. Oncol. Lett. 2017, 14, 5505–5512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia, G.; Baptista, M.-J.; Muñoz-Marmol, A.-M.; Gaafar, A.; Puente-Pomposo, M.; Garcia, O.; Marginet-Flinch, R.; Sanz, C.; Navarro, J.-T.; Sancho, J.-M.; et al. MYC protein expression is associated with poor prognosis in primary diffuse large B-cell lymphoma of the central nervous system. APMIS 2015, 123, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.Z.; Iwamoto, F.; Allen, A.; Hoehn, D.; Murty, V.V.; Alobeid, B.; Bhagat, G. MYC Protein Expression in Primary Diffuse Large B-Cell Lymphoma of the Central Nervous System. PLoS ONE 2014, 9, e114398. [Google Scholar]
- Cady, F.M.; O’Neill, B.P.; Law, M.E.; Decker, P.A.; Kurtz, D.M.; Giannini, C.; Porter, A.B.; Kurtin, P.J.; Johnston, P.B.; Dogan, A.; et al. Del(6)(q22) and BCL6 Rearrangements in Primary CNS Lymphoma Are Indicators of an Aggressive Clinical Course. J. Clin. Oncol. 2008, 26, 4814–4819. [Google Scholar] [CrossRef] [Green Version]
- Brunn, A.; Nagel, I.; Montesinos-Rongen, M.; Klapper, W.; Vater, I.; Paulus, W.; Hans, V.; Blümcke, I.; Weis, J.; Siebert, R.; et al. Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYC low BCL2 low subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol. 2013, 126, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Ha, S.Y.; Yoo, H.Y.; Oh, D.; Kim, S.J.; Kim, W.S.; Ko, Y.H. Prognostic impact of MYC protein expression in central nervous system diffuse large B-cell lymphoma: Comparison with MYC rearrangement and MYC mRNA expression. Mod. Pathol. 2016, 30, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.L.; Goodlad, J.R.; Calaminici, M.; Dotlic, S.; Montes-Moreno, S.; Oschlies, I.; Ponzoni, M.; Traverse-Glehen, A.; Ott, G.; Ferry, J.A. Lymphomas arising in immune-privileged sites: Insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020, 476, 647–665. [Google Scholar] [CrossRef] [PubMed]


| Features | Total (n = 87) | Recurrence (+) (n = 28) | Recurrence (−) (n = 59) | p Value |
|---|---|---|---|---|
| Age | 0.068 | |||
| <60 years | 49 (56.3%) | 10 (20.4%) | 39 (79.6%) | |
| ≥60 years | 38 (43.7%) | 18 (47.7%) | 20 (52.6%) | |
| Gender | 0.923 | |||
| Male | 45 (51.7%) | 14 (31.1%) | 31 (68.9%) | |
| Female | 42 (48.3%) | 14 (33.3%) | 28 (66.7%) | |
| Ocular involvement | 0.917 | |||
| Yes | 6 (6.9%) | 2 (33.3%) | 4 (66.7%) | |
| No | 81 (93.1%) | 26 (32.1%) | 55 (67.9%) | |
| Elevated serum LDH | 0.132 | |||
| Yes | 32 (36.8%) | 13 (40.6%) | 19 (59.4%) | |
| Mo | 55 (63.2%) | 15 (27.3%) | 40 (72.7%) | |
| Elevated protein in CSF | 0.426 | |||
| Yes | 54 (62.1%) | 19 (35.2%) | 35 (64.8%) | |
| No | 33 (37.9%) | 9 (27.3%) | 24 (72.7%) | |
| ECOG performance score | 0.037 | |||
| 0–1 | 62 (71.3%) | 15 (24.2%) | 47 (75.8%) | |
| 2–3 | 25 (28.7%) | 13 (52.0%) | 12 (48.0%) | |
| Risk of IELSG | 0.048 | |||
| Low (0–1) | 28 (32.2%) | 2 (7.1%) | 26 (92.9%) | |
| Intermediate (2–3) | 34 (39.1%) | 13 (38.2%) | 21 (61.8%) | |
| High (4–5) | 25 (28.7%) | 13 (52.0%) | 12 (48.0%) | |
| Adjuvant cytarabine treatment | 0.602 | |||
| Yes | 19 (21.8%) | 7 (36.8%) | 12 (63.2%) | |
| No | 68 (78.2%) | 21 (30.8%) | 47 (69.2%) |
| Features | Total (n = 87) | Recurrence (+) (n = 28) | Recurrence (−) (n = 59) | p Value |
|---|---|---|---|---|
| Patterns | 0.674 | |||
| Unifocal | 57 (65.5%) | 17 (29.8%) | 40 (70.2%) | |
| Multifocal | 30 (34.5%) | 11 (36.7%) | 19 (63.3%) | |
| Deep brain involvement | 0.392 | |||
| Yes | 38 (43.7%) | 14 (36.8%) | 24 (63.2%) | |
| No | 49 (56.3%) | 14 (28.6%) | 35 (71.4%) | |
| CSF seeding | 0.882 | |||
| Yes | 5 (5.7%) | 2 (40.0%) | 3 (60.0%) | |
| No | 82 (94.3%) | 26 (31.7%) | 56 (68.3%) | |
| Maximal size of tumor | 0.071 | |||
| ≥3 cm | 40 (46.0%) | 18 (45.0%) | 22 (55.5%) | |
| <3 cm | 47 (54.0%) | 10 (21.3%) | 37 (78.7%) | |
| Peritumoral edema | 0.338 | |||
| ≥2 cm | 33 (37.9%) | 13 (39.4%) | 20 (60.6%) | |
| <2 cm | 54 (62.1%) | 15 (27.8%) | 39 (72.2%) | |
| Enhancement patterns | 0.042 | |||
| Homogeneous | 19 (21.8%) | 3 (15.8%) | 16 (84.2%) | |
| Heterogeneous | 68 (78.2%) | 25 (36.8%) | 43 (63.2%) | |
| Surgical extent | 0.036 | |||
| Gross total resection | 31 (35.6%) | 6 (19.4%) | 25 (80.6%) | |
| Biopsy and partial resection | 56 (64.4%) | 22 (39.3%) | 34 (60.7%) |
| Features | Total (n = 87) | Recurrence (+) (n = 28) | Recurrence (−) (n = 59) | p Value |
|---|---|---|---|---|
| Pathological classification | 0.821 | |||
| Diffuse large B-cell | 74 (85.1%) | 24 (32.4%) | 50 (67.6%) | |
| Others * | 13 (14.9%) | 4 (30.8%) | 9 (69.2%) | |
| Molecular subgroup | 0.672 | |||
| GCB | 32 (36.8%) | 12 (37.5%) | 20 (62.5%) | |
| ABC | 55 (63.2%) | 16 (29.1%) | 39 (70.9%) | |
| Bcl-2 | 0.188 | |||
| Positive | 66 (75.9%) | 23 (34.8%) | 43 (65.2%) | |
| Negative | 21 (24.1%) | 5 (23.8%) | 16 (76.2%) | |
| Bcl-6 | 0.078 | |||
| Positive | 69 (79.3%) | 25 (36.2%) | 41 (59.4%) | |
| Negative | 18 (20.7%) | 3 (16.7%) | 15 (83.3%) | |
| p53 | 0.040 | |||
| Positive | 38 (43.7%) | 18 (47.4%) | 20 (52.6%) | |
| Negative | 49 (56.3%) | 10 (20.4%) | 39 (79.6%) | |
| MUM1 | 0.009 | |||
| Positive | 50 (57.5%) | 25 (50.0%) | 25 (50.0%) | |
| Negative | 37 (42.5%) | 3 (8.1%) | 34 (91.9%) | |
| c-Myc | 0.027 | |||
| Positive | 41 (47.1%) | 19 (46.3%) | 22 (53.7%) | |
| Negative | 46 (52.9%) | 9 (19.6%) | 37 (80.4%) | |
| EBV in situ hybridization | 0.804 | |||
| Positive | 19 (21.8%) | 6 (31.6%) | 13 (68.4%) | |
| Negative | 68 (78.2%) | 22 (32.4%) | 46 (67.6%) | |
| Ki67 | 0.226 | |||
| ≥50% | 56 (64.4%) | 21 (37.5%) | 35 (62.5%) | |
| <50% | 31 (35.6%) | 7 (22.6%) | 24 (77.4%) |
| Factors | Mean PFS (month) | HR (95% CI) | p Value | Mean OS (month) | HR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Age < 60 years | 122.5 (±7.7) | 2.33 (0.82–3.74) | 0.109 | 127.9 (±7.1) | 2.05 (0.78–3.32) | 0.309 |
| ≥60 years | 66.5 (±6.5) | 104.9 (±4.5) | ||||
| Gender Male | 106.1 (±9.5) | 1.98 (0.28–3.68) | 0.988 | 127.6 (±7.6) | 1.97 (0.75–3.19) | 0.390 |
| Female | 99.4 (±8.7) | 132.2 (±6.1) | ||||
| Ocular involve (+) | 105.3 (±6.8) | 1.87 (0.71–3.03) | 0.172 | 134.4 (±4.9) | 1.55 (0.64–2.46) | 0.146 |
| involve (−) | 42.0 (±8.4) | 41.9 (±8.4) | ||||
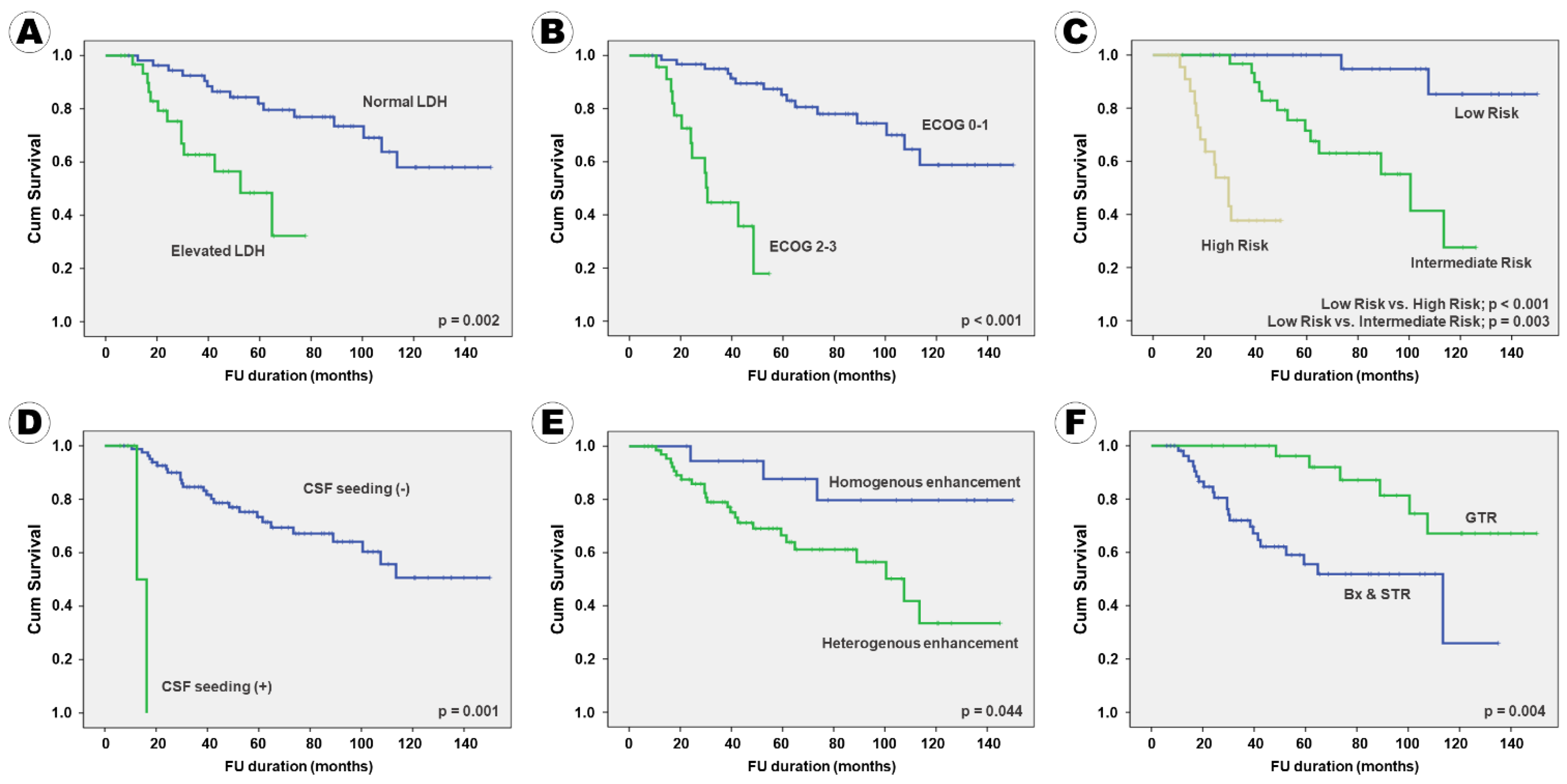
| Serum LDH increase (−) | 116.6 (±7.1) | 6.18 (4.00–8.36) | 0.008 | 142.6 (±4.1) | 7.14 (4.62–9.66) | 0.002 |
| increase (+) | 50.5 (±5.2) | 60.3 (±5.1) | ||||
| CSF protein increase (−) | 112.2 (±10.3) | 1.39 (0.80–1.97) | 0.387 | 137.7 (±6.7) | 1.88 (0.58–3.18) | 0.360 |
| increase (+) | 93.3 (±8.0) | 119.9 (±6.5) | ||||
| ECOG score 0–1 | 118.7 (±6.7) | 8.50 (5.61–11.39) | 0.002 | 145.5 (±3.7) | 9.04 (6.27–11.81) | 0.001 |
| score 2–3 | 34.4 (±3.4) | 40.4 (±3.9) | ||||
| IELSG risk Low | 141.9 (±5.3) | 13.42 (8.45–18.39) | <0.001 | 145.1 (±7.7) | 9.53 (6.15–12.91) | 0.001 |
| Intermediate | 89.3 (±7.0) | 4.33 (2.64–6.02) | 0.011 | 136.5 (±7.4) | 6.55 (4.09–9.01) | 0.006 |
| High | 31.8 (±3.3) | 14.4 (±1.9) | ||||
| Adjuvant ARA-C Yes | 117.0 (±2.5) | 1.73 (0.75–2.71) | 0.362 | 126.7 (±7.3) | 1.69 (0.61–2.77) | 0.324 |
| No | 101.0 (±7.2) | 133.1 (±6.4) | ||||
| Pattern Unifocal | 113.2 (±7.1) | 2.01 (1.01–3.01) | 0.053 | 145.4 (±3.2) | 4.11 (2.94–5.28) | 0.017 |
| Multifocal | 68.3 (±8.3) | 73.4 (±8.3) | ||||
| Deep location involve (−) | 109.9 (±8.5) | 1.52 (0.69–2.35) | 0.264 | 138.7 (±5.4) | 1.48 (0.51–2.45) | 0.126 |
| (+) | 90.0 (±9.1) | 111.2 (±8.0) | ||||
| CSF seeding (−) | 106.0 (±6.7) | 4.43 (1.58–7.28) | 0.013 | 135.0 (±4.7) | 5.74 (3.61–7.87) | 0.005 |
| (+) | 14.4 (±1.9) | 14.4 (±1.9) | ||||
| Tumor size < 3 cm | 121.1 (±7.8) | 1.92 (0.94–2.89) | 0.089 | 132.2 (±6.7) | 1.08 (0.22–1.94) | 0.980 |
| ≥3 cm | 82.2 (±9.7) | 127.4 (±7.3) | ||||
| Peritumoral edema < 2 cm | 101.6 (±6.9) | 1.30 (0.62–1.97) | 0.401 | 120.9 (±5.4) | 1.52 (0.60–2.44) | 0.594 |
| ≥2 cm | 95.7 (±11.0) | 129.4 (±8.5) | ||||
| Enhance Homogenous | 130.3 (±10.3) | 3.35 (1.27–5.44) | 0.034 | 140.2 (±5.4) | 6.92 (4.05–9.79) | 0.004 |
| Heterogeneous | 91.0 (±7.6) | 14.4 (±1.9) | ||||
| Extent of surgery GTR | 128.6 (±8.2) | 7.12 (4.96–9.28) | 0.004 | 142.3 (±6.1) | 7.13 (4.68–9.58) | 0.003 |
| Bx | 80.5 (±7.4) | 14.4 (±1.9) | ||||
| Pathology DLBCL | 103.9 (±7.3) | 1.10 (0.43–1.77) | 0.919 | 132.8 (±5.3) | 1.21 (0.39–2.03) | 0.720 |
| Others | 92.6 (±11.4) | 103.7 (±11.1) | ||||
| Cell type Non-GCL | 109.9 (±8.2) | 1.57 (0.61–2.53) | 0.304 | 134.8 (±5.8) | 1.48 (0.63–2.33) | 0.407 |
| GCL | 84.8 (±8.7) | 107.7 (±7.5) | ||||
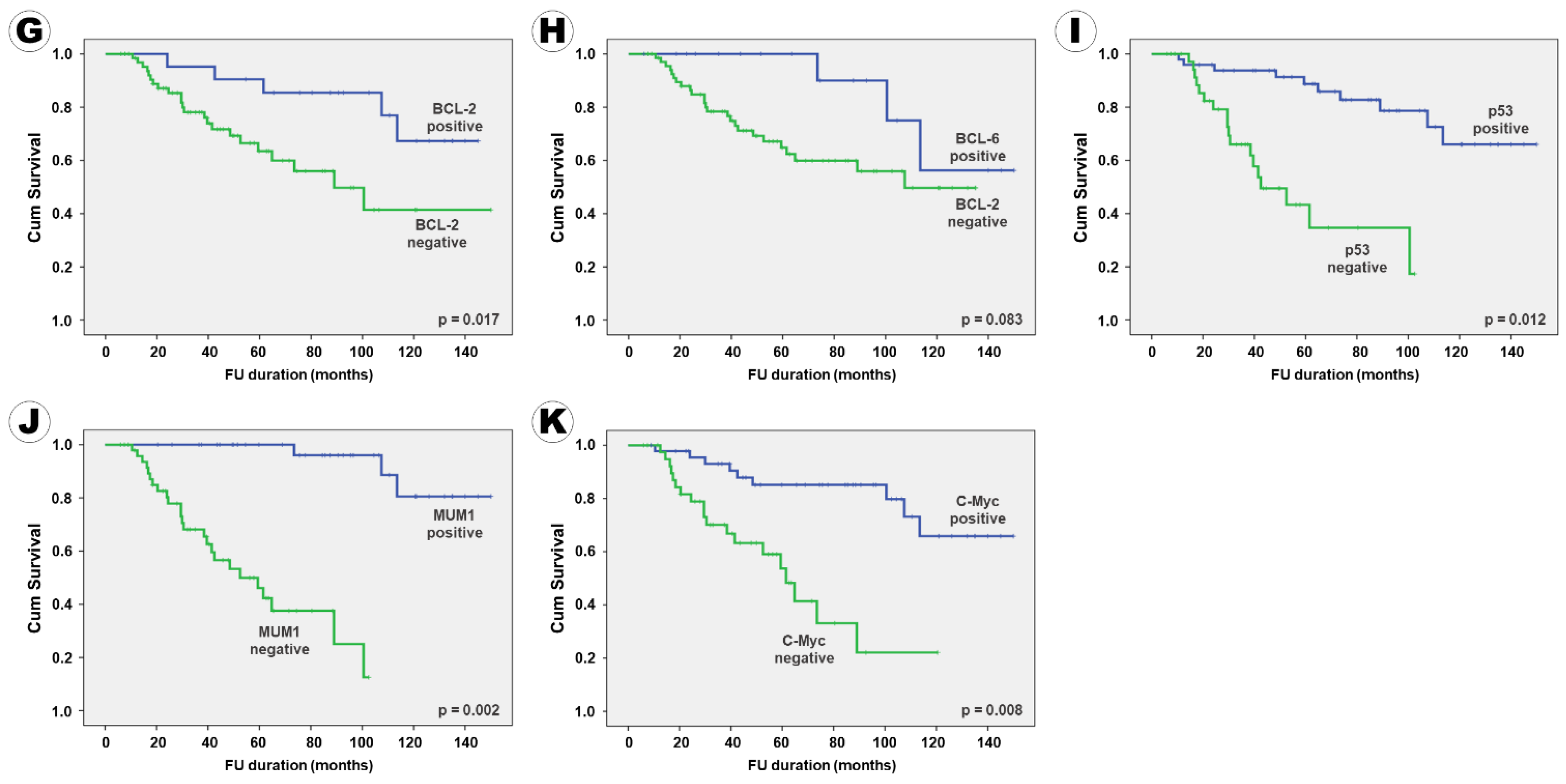
| BCL-2 Negative | 123.9 (±8.4) | 5.65 (3.08–8.21) | 0.011 | 146.4 (±4.8) | 9.74 (6.54–12.94) | <0.001 |
| Positive | 92.9 (±8.8) | 14.4 (±1.9) | ||||
| BCL-6 Negative | 128.1 (±9.9) | 1.83 (0.87–2.79) | 0.102 | 135.0 (±4.7) | 2.33 (0.97–3.69) | 0.084 |
| Positive | 91.4 (±6.7) | 40.4 (±5.6) | ||||
| p53 Negative | 124.5 (±6.9) | 5.23 (2.21–8.24) | 0.014 | 135.0 (±4.7) | 7.50 (3.99–11.01) | 0.003 |
| Positive | 58.2 (±6.8) | 14.4 (±1.9) | ||||
| MUM1 Negative | 140.9 (±4.8) | 8.21 (4.92–11.51) | 0.002 | 139.2 (±5.1) | 10.22 (7.45–12.99) | 0.001 |
| Positive | 59.1 (±5.5) | 20.8 (±5.9) | ||||
| c-Myc Negative | 124.5 (±7.3) | 6.09 (2.38–9.81) | 0.008 | 145.0 (±4.7) | 14.51 (8.77–20.25) | <0.001 |
| Positive | 64.8 (±7.6) | 14.4 (±2.6) | ||||
| EBV Negative | 103.7 (±7.6) | 1.23 (0.29–2.17) | 0.871 | 133.1 (±5.6) | 1.36 (0.55–2.17) | 0.728 |
| Positive | 100.1 (±12.9) | 120.5 (±10.3) | ||||
| Ki67 < 50% | 120.4 (±9.6) | 1.95 (0.96–2.93) | 0.091 | 145.5 (±4.4) | 1.54 (0.86–2.23) | 0.246 |
| ≥50% | 81.6 (±6.3) | 101.0 (±5.7) |
| Factors | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (<60 yrs vs. ≥60 yrs) | 2.23 (0.96–3.49) | 0.098 | 1.89 (0.86–2.92) | 0.209 |
| Ocular involvement (No vs. Yes) | 2.07 (0.92–3.21) | 0.123 | 2.35 (0.95–3.75) | 0.084 |
| Elevation of LDH (No vs. Yes) | 4.32 (1.78–6.86) | 0.017 | 2.76 (1.27–4.25) | 0.043 |
| ECOG score (0–1 vs. 2–3) | 5.18 (2.31–8.04) | 0.008 | 4.26 (2.45–6.07) | 0.011 |
| IELSG risk (Low vs. High) | 7.64 (4.26–11.02) | <0.001 | 8.42 (5.09–11.76) | <0.001 |
| (Intermediate vs. High) | 7.05 (4.19–9.91) | 0.002 | 8.13 (4.28–11.98) | 0.002 |
| Patterns (Unifocal vs. Multifocal) | 1.94 (0.76–3.12) | 0.184 | 2.47 (0.99–3.94) | 0.053 |
| Deep structure involvement (No vs. Yes) | N.A. | 1.92 (0.97–2.87) | 0.126 | |
| CSF seeding | 4.85 (2.54–7.16) | 0.002 | 5.22 (3.14–7.29) | 0.006 |
| Tumor size (<3 cm vs. ≥3 cm) | 2.44 (0.98–3.89) | 0.056 | N.A. | |
| Enhancement (Homogenous vs. Heterogeneous) | 2.73 (1.12–3.87) | 0.047 | 2.23 (0.88–3.48) | 0.091 |
| Extent of resection (GTR vs. Bx) | 3.04 (1.28–4.79) | 0.036 | 2.69 (1.14–4.24) | 0.048 |
| BCL-2 (Negative vs. Positive) | 2.95 (1.33–4.57) | 0.042 | 1.74 (0.81–2.65) | 0.164 |
| BCL-6 (Negative vs. Positive) | 2.88 (1.29–4.47) | 0.045 | 1.58 (0.84–2.31) | 0.229 |
| p53 (Negative vs. Positive) | 3.21 (1.18–5.24) | 0.031 | 3.37 (1.66–5.08) | 0.036 |
| MUM-1 (Negative vs. Positive) | 3.94 (2.17–5.71) | 0.028 | 4.62 (2.51–6.73) | 0.017 |
| c-Myc (Negative vs. Positive) | 3.51 (2.33–4.69) | 0.035 | 4.08 (2.16–6.01) | 0.024 |
| Ki67 (<50% vs. ≥50%) | 2.11 (0.88–3.32) | 0.122 | 2.31 (0.85–3.77) | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, T.G.; Lee, H.W.; Kim, S.H.; Park, J.E.; Lee, M.; Kim, Y.Z. Clinical Outcomes of RTOG 9310 Protocol for Primary Central Nervous System Lymphoma: Single-Center Experience with 87 Patients. Curr. Oncol. 2021, 28, 4655-4672. https://doi.org/10.3390/curroncol28060393
Kim J, Kim TG, Lee HW, Kim SH, Park JE, Lee M, Kim YZ. Clinical Outcomes of RTOG 9310 Protocol for Primary Central Nervous System Lymphoma: Single-Center Experience with 87 Patients. Current Oncology. 2021; 28(6):4655-4672. https://doi.org/10.3390/curroncol28060393
Chicago/Turabian StyleKim, Jinuk, Tae Gyu Kim, Hyoun Wook Lee, Seok Hyun Kim, Ji Eun Park, Moonok Lee, and Young Zoon Kim. 2021. "Clinical Outcomes of RTOG 9310 Protocol for Primary Central Nervous System Lymphoma: Single-Center Experience with 87 Patients" Current Oncology 28, no. 6: 4655-4672. https://doi.org/10.3390/curroncol28060393
APA StyleKim, J., Kim, T. G., Lee, H. W., Kim, S. H., Park, J. E., Lee, M., & Kim, Y. Z. (2021). Clinical Outcomes of RTOG 9310 Protocol for Primary Central Nervous System Lymphoma: Single-Center Experience with 87 Patients. Current Oncology, 28(6), 4655-4672. https://doi.org/10.3390/curroncol28060393






