Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma
Abstract
1. Introduction
2. Epidemiology
3. Clinical Presentation and Diagnosis
4. Genomics of AITL
5. Prognosis
| Model | Factors | Score | Impact OS/PFS % |
|---|---|---|---|
| International Prognostic Index (IPI) [12] | -Age ≥ 60 years -Stages III to IV disease -Lactic dehydrogenase (LDH) > normal -Extranodal sites (ENSs) > one -Performance status (PS) ≥ 2 | 0–1 2 3 4–5 | @5 years 56/34 @5 years 38/21 @5 years 20/12 @5 years 25/16 |
| Prognostic Index for Peripheral T cell Lymphoma [12] | -Age ≥ 60 years -PS ≥ 2 -LDH > normal -Bone marrow involvement | 0–1 2 3–4 | @5 years 46/22 @5 years 19/12 @5 years 30/22 |
| Prognostic Index for AITL (PIAI) [12] | -Age > 60 years, -PS ≥ 2, -ENSs > one, -B symptoms, and -Platelet count < 150 × 109/L | Low-risk group (0–1 factors) High-risk group (2–5 factors) | @5 years 44/28 @5 years 24/15 |
| Eladl et al., 2020 [35] | -EBER negative status, -Thrombocytopenia -Elevated serum IgA level | Low risk (0–1 factor) High risk (2–3 factors) | @3 years 91/49 @3 years 18/0 |
| AITL score [32] | -Age -PS -C-reactive protein -β2 microglobulin | Low risk Intermediate High | @5 years 63/- @5 years 54/- @5 years 21/- |
| Iqbal et al. (Gene expression model) [38] | -B cell (GCB cell signature) -Monocytic/dendritic signature -TP53-induced gene signature | Good prognosis Poor prognosis Poor prognosis | @5 years 56–64 @5 years 13–14 @5 years 13–14 |
6. Treatment
6.1. Frontline Therapy
| Author | Agent | No. of patients PTCL/AITL | ORR/CR % | PFS % | OS % |
|---|---|---|---|---|---|
| Kim SJ et al. [58] | Bortezomib | 46/8 | 76/65 | 47@3years | 35@3years |
| Ellin et al. [43] Schmitz et al. [45] d’Amore et al. [66] | Etoposide | 107/18 320/28 160/30 | 81/NA NA 82/52.6 | 40@5 years. 60.7@ 3 years AITL 49@5 years | 47@5 years 67.5@ 3 years AITL 52@5 years |
| Horwitz et al. [50] | Brentuximab vedotin | 226/30 | 83/68 | 57.1@ 3 years | 76.8@3years |
| Altmann et al. [56] Gallamini et al. [52] Kim et al. [51] Binder et al. [54] Buckstein et al. [57] Wulf et al. [55] Kluin-Nelemans et al. [53] | Alemtuzumab | 127/42 24/NA 20/NA 29/NA 20/7 58/24 20/6 | NA/56 75/71 80/65 60.4/58.5 68/37 72/60 90/65 | 33@3years 48@2 years 43.3@1year 42.4@3 years 47.5@2 years 28@3 years 27@2 years | 46@3years 53@2years 44.3@1year 75.1@3 years 78.9@2 years 37@3 years 55@ 2 years |
| Delfau-Larue et al. [36] | Rituximab | NA/25 | 80/44 | 42@ 2 years | 62@ 2 years |
| Ganjoo et al. [62] | Bevacizumab | 39/17 | 90/49 reaching 53 % in AITL | 44 @ 1 year 57 in AITL | 88%@ 1 year in AITL |
| Bachy et al. [63] | Romidespin | 421/NA | 63/41 | Median 12 months | Median 51.8 months |
| Zhang et al. [64] | Chidamide | 113/41 | PTCL 60.2/40.6 AITL 65.9/41.5 | PTCL 52.4@ 3years AITL 30.3 months | PTCL 32.8@ 3years AITL 9.6 months |
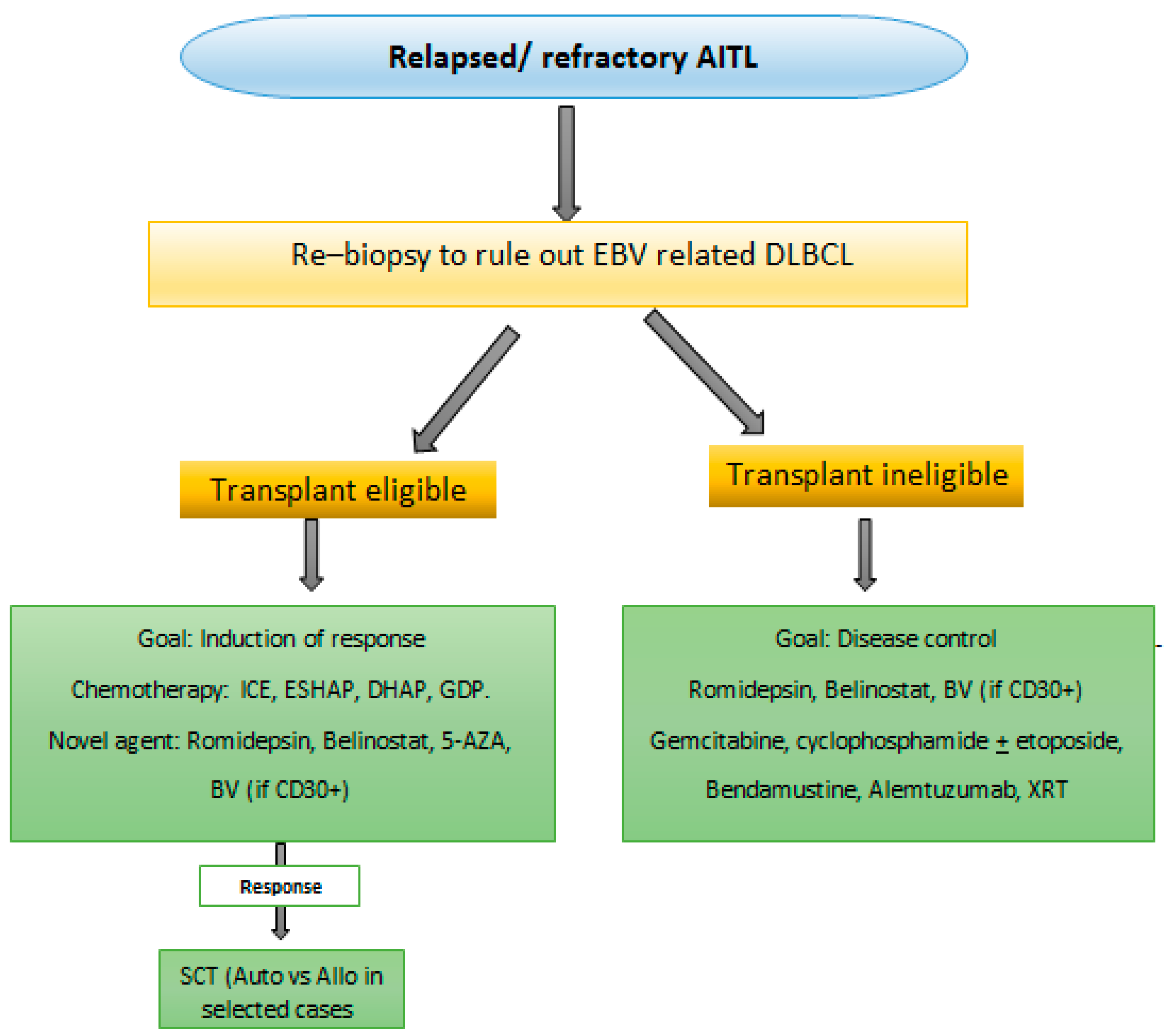
6.2. Relapse or Refractory AITL Therapy
7. Future Directions and Ongoing Trials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sallah, S.; Gagnon, G.A. Angioimmunoblastic lymphadenopathy with dysproteinemia: Emphasis on pathogenesis and treatment. Acta Haematol. 1998, 99, 57–64. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Facchinelli, D.; Polino, A.; Dima, F.; Parisi, A.; Ambrosetti, A.; Veneri, D. Two cases of angioimmunoblastic T-cell lymphoma with concomitant positive serology for acute Epstein-Barr virus infection. Hematol. Rep. 2017, 9, 7088. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Attygalle, A.D.; Chuang, S.S.; Diss, T.; Ye, H.; Liu, H.; Hamoudi, R.A.; Munson, P.; Bacon, C.M.; Dogan, A.; et al. Angioimmunoblastic T-cell lymphoma: Histological progression associates with EBV and HHV6B viral load. Br. J. Haematol. 2007, 138, 44–53. [Google Scholar] [CrossRef]
- Kim, H.J.; Ko, Y.H.; Kim, J.E.; Lee, S.S.; Lee, H.; Park, G.; Paik, J.H.; Cha, H.J.; Choi, Y.D.; Han, J.H.; et al. Epstein-Barr Virus-Associated Lymphoproliferative Disorders: Review and Update on 2016 WHO Classification. J. Pathol. Transl. Med. 2017, 51, 352–358. [Google Scholar] [CrossRef]
- Şimşek, C.; Bostankolu, B.; Özoğul, E.; Sağlam Ayhan, A.; Üner, A.; Büyükaşık, Y. EBV-Related Diffuse Large B-Cell Lymphoma in a Patient with Angioimmunoblastic T-Cell Lymphoma. Turk. J. Hematol. 2019, 36, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Moon, I.J.; Lee, W.J.; Won, C.H.; Chang, S.E.; Choi, J.H.; & Lee, M.W. A Case of Cutaneous Epstein-Barr Virus-Associated Diffuse Large B-Cell Lymphoma in an Angioimmunoblastic T-Cell Lymphoma. Ann. Dermatol. 2016, 28, 789–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiba, S.; Sakata-Yanagimoto, M.J.L. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia 2020, 34, 2592–2606. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.; Chuang, S.S.; Ashton-Key, M.; Ochoa, E.; Bolli, N.; Vassiliou, G.; Gao, Z.; Du, M.Q. Angioimmunoblastic T cell lymphoma: Novel molecular insights by mutation profiling. Oncotarget 2017, 8, 17763–17770. [Google Scholar] [CrossRef]
- Vose, J.; Armitage, J.; Weisenburger, D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar]
- Xu, B.; Liu, P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: A population-based study of 1207 cases. PLoS ONE 2014, 9, e92585. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Rudiger, T.; Bellei, M.; Nathwani, B.N.; Luminari, S.; Coiffier, B.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Savage, K.J.; et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: Analysis of the international peripheral T-cell lymphoma project. J. Clin. Oncol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Park, B.B.; Ryoo, B.Y.; Lee, J.H.; Kwon, H.C.; Yang, S.H.; Kang, H.J.; Kim, H.J.; Oh, S.Y.; Ko, Y.H.; Huh, J.R.; et al. Clinical features and treatment outcomes of angioimmunoblastic T-cell lymphoma. Leuk. Lymphoma 2007, 48, 716–722. [Google Scholar] [CrossRef]
- Xie, Y.; Jaffe, E.S. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am. J. Clin. Pathol. 2021, 156, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.D.; Kyriakou, C.; Dupuis, J.; Grogg, K.L.; Diss, T.C.; Wotherspoon, A.C.; Chuang, S.S.; Cabeçadas, J.; Isaacson, P.G.; Du, M.Q.; et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: Clinical correlation and insights into natural history and disease progression. Am. J. Surg. Pathol. 2007, 31, 1077–1088. [Google Scholar] [CrossRef]
- Attygalle, A.; Al-Jehani, R.; Diss, T.C.; Munson, P.; Liu, H.; Du, M.Q.; Isaacson, P.G.; Dogan, A. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood 2002, 99, 627–633. [Google Scholar] [CrossRef]
- Dupuis, J.; Boye, K.; Martin, N.; Copie-Bergman, C.; Plonquet, A.; Fabiani, B.; Baglin, A.C.; Haioun, C.; Delfau-Larue, M.H.; Gaulard, P. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): A new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am. J. Surg. Pathol. 2006, 30, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.M.; Brown, J.A.; Shahsafaei, A.; Freeman, G.J. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2006, 30, 802–810. [Google Scholar] [CrossRef]
- Leung, C.Y.; Ho, F.C.; Srivastava, G.; Loke, S.L.; Liu, Y.T.; Chan, A.C. Usefulness of follicular dendritic cell pattern in classification of peripheral T-cell lymphomas. Histopathology 1993, 23, 433–437. [Google Scholar] [CrossRef]
- Onaindia, A.; Martínez, N.; Montes-Moreno, S.; Almaraz, C.; Rodríguez-Pinilla, S.M.; Cereceda, L.; Revert, J.B.; Ortega, C.; Tardio, A.; González, L.; et al. CD30 Expression by B and T Cells: A Frequent Finding in Angioimmunoblastic T-Cell Lymphoma and Peripheral T-Cell Lymphoma-Not Otherwise Specified. Am. J. Surg. Pathol. 2016, 40, 378–385. [Google Scholar] [CrossRef]
- Nelson, M.; Horsman, D.E.; Weisenburger, D.D.; Gascoyne, R.D.; Dave, B.J.; Loberiza, F.R.; Ludkovski, O.; Savage, K.J.; Armitage, J.O.; Sanger, W.G. Cytogenetic abnormalities and clinical correlations in peripheral T-cell lymphoma. Br. J. Haematol. 2008, 141, 461–469. [Google Scholar] [CrossRef]
- Heavican, T.B.; Bouska, A.; Yu, J.; Lone, W.; Amador, C.; Gong, Q.; Zhang, W.; Li, Y.; Dave, B.J.; Nairismägi, M.L.; et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood 2019, 133, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Nguyen, T.B.; Chiba, S.; Sakata-Yanagimoto, M. Review of the biologic and clinical significance of genetic mutations in angioimmunoblastic T-cell lymphoma. Cancer Sci. 2018, 109, 490–496. [Google Scholar] [CrossRef]
- Odejide, O.; Weigert, O.; Lane, A.A.; Toscano, D.; Lunning, M.A.; Kopp, N.; Kim, S.; van Bodegom, D.; Bolla, S.; Schatz, J.H.; et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 2014, 123, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, M.; Abdul Hamid, M.; Winkens, B.; zur Hausen, A. Mutational heterogeneity of angioimmunoblastic T-cell lymphoma indicates distinct lymphomagenic pathways. Blood Cancer J. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakata-Yanagimoto, M. Multistep tumorigenesis in peripheral T cell lymphoma. Int. J. Hematol. 2015, 102, 523–527. [Google Scholar] [CrossRef]
- Lewis, N.E.; Petrova-Drus, K.; Huet, S.; Epstein-Peterson, Z.D.; Gao, Q.; Sigler, A.E.; Baik, J.; Ozkaya, N.; Moskowitz, A.J.; Kumar, A.; et al. Clonal hematopoiesis in angioimmunoblastic T-cell lymphoma with divergent evolution to myeloid neoplasms. Blood Adv. 2020, 4, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Vallois, D.; Dobay, M.P.; Morin, R.D.; Lemonnier, F.; Missiaglia, E.; Juilland, M.; Iwaszkiewicz, J.; Fataccioli, V.; Bisig, B.; Roberti, A.; et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell–derived lymphomas. Blood 2016, 128, 1490–1502. [Google Scholar] [CrossRef]
- Zhu, W.; He, Q.Y.; Lu, C.; Fu, C.Y.; Zhou, J.H.; Liu, S.; Tao, Y.G.; Xiao, D.S. Detection of immunoglobulin and T-cell receptor gene rearrangements in angioimmunoblastic T-cell lymphoma. Int. J. Clin. Exp. Pathol. 2018, 11, 2642–2653. [Google Scholar]
- Wang, C.; McKeithan, T.W.; Gong, Q.; Zhang, W.; Bouska, A.; Rosenwald, A.; Gascoyne, R.D.; Wu, X.; Wang, J.; Muhammad, Z.; et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood 2015, 126, 1741–1752. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sakata-Yanagimoto, M.; Asabe, Y.; Matsubara, D.; Kano, J.; Yoshida, K.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Miyano, S.; et al. Identification of cell-type-specific mutations in nodal T-cell lymphomas. Blood Cancer J. 2017, 7, e516. [Google Scholar] [CrossRef]
- Advani, R.H.; Skrypets, T.; Civallero, M.; Spinner, M.A.; Manni, M.; Kim, W.S.; Shustov, A.R.; Horwitz, S.M.; Hitz, F.; Cabrera, M.E.; et al. Outcomes and prognostic factors in angioimmunoblastic T-cell lymphoma: Final report from the international T-cell Project. Blood 2021, 138, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Iida, H.; Yoshida, I.; Komeno, T.; Sawamura, M.; Matsumoto, M.; Sekiguchi, N.; Hishita, T.; Sunami, K.; Shimomura, T.; et al. Comparison of prognostic scores in transplant-ineligible patients with peripheral T-cell lymphoma not otherwise specified and angioimmunoblastic T-cell lymphoma: A retrospective study from the national hospital organization in Japan. Leuk. Lymphoma 2021, 62, 819–827. [Google Scholar] [CrossRef]
- Tokunaga, T.; Shimada, K.; Yamamoto, K.; Chihara, D.; Ichihashi, T.; Oshima, R.; Tanimoto, M.; Iwasaki, T.; Isoda, A.; Sakai, A.; et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: A multicenter cooperative study in Japan. Blood 2012, 119, 2837–2843. [Google Scholar] [CrossRef]
- Eladl, A.E.; Shimada, K.; Suzuki, Y.; Takahara, T.; Kato, S.; Kohno, K.; Elsayed, A.A.; Wu, C.C.; Tokunaga, T.; Kinoshita, T.; et al. EBV status has prognostic implication among young patients with angioimmunoblastic T-cell lymphoma. Cancer Med. 2020, 9, 678–688. [Google Scholar] [CrossRef]
- Delfau-Larue, M.H.; de Leval, L.; Joly, B.; Plonquet, A.; Challine, D.; Parrens, M.; Delmer, A.; Salles, G.; Morschhauser, F.; Delarue, R.; et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica 2012, 97, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Chiba, S.; Sakata-Yanagimoto, M. Recent Progress in the Understanding of Angioimmunoblastic T-cell Lymphoma. J. Clin. Exp. Hematop. 2017, 57, 109–119. [Google Scholar] [CrossRef]
- Iqbal, J.; Wright, G.; Wang, C.; Rosenwald, A.; Gascoyne, R.D.; Weisenburger, D.D.; Greiner, T.C.; Smith, L.; Guo, S.; Wilcox, R.A.; et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014, 123, 2915–2923. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Wu, T.; Xue, Y.; Zhang, D.; Xu, H. The Incremental Prognostic Value of Baseline 18F-FDG PET/CT Imaging in Angioimmunoblastic T-Cell Lymphoma. BioMed Res. Int. 2020, 2020, 4502489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Zhao, X.; Hu, Y.; Tan Su Yin, E.; Chen, D.; Wang, H.; Zhao, K. The role of pre-treatment and mid-treatment 18F-FDG PET/CT imaging in evaluating prognosis of peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS). BMC Med. Imaging 2021, 21, 145. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, A.Y.; Kim, W.S.; Kim, S.J.; Cho, Y.S.; Choe, Y.S.; Kim, B.T.; Lee, K.H. Value of interim FDG PET/CT for predicting outcome of patients with angioimmunoblastic T-cell lymphoma. Leuk. Lymphoma 2017, 58, 1341–1348. [Google Scholar] [CrossRef]
- Deng, S.; Lin, S.; Shen, J.; Zeng, Y. Comparison of CHOP vs. CHOPE for treatment of peripheral T-cell lymphoma: A meta-analysis. OncoTargets Ther. 2019, 12, 2335–2342. [Google Scholar] [CrossRef]
- Ellin, F.; Landström, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014, 124, 1570–1577. [Google Scholar] [CrossRef]
- Savage, K.J.; Chhanabhai, M.; Gascoyne, R.D.; Connors, J.M. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann. Oncol. 2004, 15, 1467–1475. [Google Scholar] [CrossRef]
- Schmitz, N.; Trümper, L.; Ziepert, M.; Nickelsen, M.; Ho, A.D.; Metzner, B.; Peter, N.; Loeffler, M.; Rosenwald, A.; Pfreundschuh, M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010, 116, 3418–3425. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Byun, J.M.; Park, K.; Bae, G.H.; Lee, D.; Kim, D.S.; Yoon, S.S.; Koh, Y. Redefining the role of etoposide in first-line treatment of peripheral T-cell lymphoma. Blood Adv. 2017, 1, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Nickelsen, M.; Ziepert, M.; Zeynalova, S.; Glass, B.; Metzner, B.; Leithaeuser, M.; Mueller-Hermelink, H.K.; Pfreundschuh, M.; Schmitz, N. High-dose CHOP plus etoposide (MegaCHOEP) in T-cell lymphoma: A comparative analysis of patients treated within trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Ann. Oncol. 2009, 20, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Mhaidly, R.; Krug, A.; Gaulard, P.; Lemonnier, F.; Ricci, J.-E.; Verhoeyen, E.J.O. New preclinical models for angioimmunoblastic T-cell lymphoma: Filling the GAP. Oncogenesis 2020, 9, 73. [Google Scholar] [CrossRef]
- Moskowitz, A.J. Practical Treatment Approach for Angioimmunoblastic T-Cell Lymphoma. J. Oncol. Pract. 2019, 15, 137–143. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Kim, J.G.; Sohn, S.K.; Chae, Y.S.; Cho, Y.Y.; Yang, D.H.; Lee, J.J.; Kim, H.J.; Shin, H.J.; Chung, J.S.; Cho, G.J.; et al. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: A phase II study. Cancer Chemother. Pharmacol. 2007, 60, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Zaja, F.; Patti, C.; Billio, A.; Specchia, M.R.; Tucci, A.; Levis, A.; Manna, A.; Secondo, V.; Rigacci, L.; et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007, 110, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Kluin-Nelemans, H.C.; van Marwijk Kooy, M.; Lugtenburg, P.J.; van Putten, W.; Luten, M.; Oudejans, J.; van Imhoff, G.W. Intensified alemtuzumab–CHOP therapy for peripheral T-cell lymphoma. Ann. Oncol. 2011, 22, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Ziepert, M.; Pfreundschuh, M.; Dührsen, U.; Eimermacher, H.; Aldaoud, A.; Rosenwald, A.; Loeffler, M.; Schmitz, N.; Truemper, L.; et al. CHO(E)P-14 followed by alemtuzumab consolidation in untreated peripheral T cell lymphomas: Final analysis of a prospective phase II trial. Ann. Hematol. 2013, 92, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Wulf, G.G.; Altmann, B.; Ziepert, M.; D’Amore, F.; Held, G.; Greil, R.; Tournilhac, O.; Relander, T.; Viardot, A.; Wilhelm, M.; et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: The DSHNHL2006-1B/ACT-2 trial. Leukemia 2020, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Wulf, G.; Truemper, L.; d’Amore, F.; Relander, T.; Toldbod, H.; Delabie, J.M.A.; Rosenwald, A.; Ziepert, M.; Loeffler, M. Alemtuzumab Added to CHOP for Treatment of Peripheral T-Cell Lymphoma (PTCL) in Previously Untreated Young and Elderly Patients: Pooled Analysis of the International ACT-1/2 Phase III Trials. Blood 2018, 132 (Suppl. 1), 1622. [Google Scholar] [CrossRef]
- Buckstein, R.; Fraser, G.; Cheung, M.; Kukreti, V.; Kuruvilla, J.; Imrie, K.; Piliotis, E.; Pond, G.; Windsor, J.; Ghorab, Z.; et al. Alemtuzumab and CHOP Chemotherapy for the Treatment of Aggressive Histology Peripheral T Cell Lymphomas: A Multi-Center Phase I Study. Clin. Lymphoma Myeloma Leuk. 2016, 16, 18–28.e4. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, D.H.; Kang, H.J.; Kim, J.S.; Park, S.K.; Kim, H.J.; Lee, J.; Ryoo, B.Y.; Ko, Y.H.; Huh, J.; et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur. J. Cancer 2012, 48, 3223–3231. [Google Scholar] [CrossRef]
- Lemonnier, F.; Safar, V.; de Leval, L.; Cottereau, A.S.; Pelletier, L.; Robe, C.; Bachy, E.; Cartron, G.; Moles, M.-P.; Letourneau, A.; et al. Lenalidomide in Combination with CHOP in Patients with Angioimmunoblastic T-Cell Lymphoma (AITL): Final Analysis of Clinical and Molecular Data of a Phase 2 Lysa Study. Blood 2018, 132 (Suppl. 1), 999. [Google Scholar] [CrossRef]
- Hu, P.; Ben, Y.; Liu, J.; Zheng, W.; Yan, X.; Zhang, Y.; Shi, W. Promising Response to Lenalidomide-Combination Therapy in a Discordant Lymphoma Consisting of EBV-Positive Diffuse Large B-Cell Lymphoma and Angioimmunoblastic T-Cell Lymphoma: A Case Report. OncoTargets Ther. 2021, 14, 2489–2495. [Google Scholar] [CrossRef]
- Siegert, W.; Nerl, C.; Meuthen, I.; Zahn, T.; Brack, N.; Lennert, K.; Huhn, D. Recombinant human interferon-alpha in the treatment of angioimmunoblastic lymphadenopathy: Results in 12 patients. Leukemia 1991, 5, 892–895. [Google Scholar]
- Ganjoo, K.; Hong, F.; Horning, S.J.; Gascoyne, R.D.; Natkunam, Y.; Swinnen, L.J.; Habermann, T.M.; Kahl, B.S.; Advani, R.H. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: An Eastern Cooperative Oncology Group study (E2404). Leuk. Lymphoma 2014, 55, 768–772. [Google Scholar] [CrossRef]
- Bachy, E.; Camus, V.; Thieblemont, C.; Casasnovas, R.O.; Ysebaert, L.; Damaj, G.L.; Guidez, S.; Pica, G.M.; Kim, W.S.; Lim, S.T.; et al. Final Analysis of the Ro-CHOP Phase III Study (Conducted by LYSA): Romidepsin Plus CHOP in Patients with Peripheral T-Cell Lymphoma. Blood 2020, 136 (Suppl. 1), 32–33. [Google Scholar] [CrossRef]
- Zhang, W.; Su, L.; Liu, L.; Gao, Y.; Wang, Q.; Su, H.; Song, Y.; Zhang, H.; Shen, J.; Jing, H.; et al. The combination of chidamide with the CHOEP regimen in previously untreated patients with peripheral T-cell lymphoma: A prospective, multicenter, single arm, phase 1b/2 study. Cancer Biol. Med. 2021, 18, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Li, X.; Zhang, L.; Wang, X.; Fu, X.; Sun, Z.; Zhang, X.; Li, Z.; Wu, J.; et al. Outcomes of GDPT (gemcitabine, cisplatin, prednisone, thalidomide) versus CHOP in newly diagnosed peripheral T-cell lymphoma patients. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923829. [Google Scholar] [CrossRef]
- D’Amore, F.; Relander, T.; Lauritzsen, G.F.; Jantunen, E.; Hagberg, H.; Anderson, H.; Holte, H.; Österborg, A.; Merup, M.; Brown, P.; et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J. Clin. Oncol. 2012, 30, 3093–3099. [Google Scholar] [CrossRef]
- Park, S.I.; Horwitz, S.M.; Foss, F.M.; Pinter-Brown, L.C.; Carson, K.R.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019, 125, 1507–1517. [Google Scholar] [CrossRef]
- Mamez, A.C.; Dupont, A.; Blaise, D.; Chevallier, P.; Forcade, E.; Ceballos, P.; Mohty, M.; Suarez, F.; Beguin, Y.; Peffault De Latour, R.; et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: A retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J. Hematol. Oncol. 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Bolwell, B.J.; Rybicki, L.A.; Brown, S.; Dean, R.; Kalaycio, M.; Sobecks, R.; Andresen, S.; Hsi, E.D.; Pohlman, B.; et al. Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant. 2007, 40, 239–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horwitz, S.M.; Moskowitz, C.H.; Kewalramani, T.; Hamlin, P.A.; Straus, D.J.; O’Connor, O.A.; Noy, A.; Portlock, C.S.; Nimer, S.D.; Palomba, M.L.; et al. Second-Line Therapy with ICE Followed by High Dose Therapy and Autologous Stem Cell Transplantation for Relapsed/Refractory Peripheral T-Cell Lymphomas: Minimal Benefit When Analyzed by Intent to Treat. Blood 2005, 106, 2679. [Google Scholar] [CrossRef]
- Norasetthada, L.; Tantiworawit, A.; Rattanathammethee, T.; Chai-Adisaksopha, C.; Chaipoh, T.; Rattarittamrong, E. Efficacy of ESHAP Regimen in Transplant Ineligible Patients with Relapsed/Refractory T-Cell Lymphoma. J. Hematol. 2018, 7, 131–139. [Google Scholar] [CrossRef]
- Smith, S.M.; Burns, L.J.; van Besien, K.; Lerademacher, J.; He, W.; Fenske, T.S.; Suzuki, R.; Hsu, J.W.; Schouten, H.C.; Hale, G.A.; et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 3100–3109. [Google Scholar] [CrossRef]
- Le Gouill, S.; Milpied, N.; Buzyn, A.; De Latour, R.P.; Vernant, J.P.; Mohty, M.; Moles, M.P.; Bouabdallah, K.; Bulabois, C.E.; Dupuis, J.; et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: A study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J. Clin. Oncol. 2008, 26, 2264–2271. [Google Scholar] [CrossRef]
- Jacobsen, E.D.; Kim, H.T.; Ho, V.T.; Cutler, C.S.; Koreth, J.; Fisher, D.C.; Armand, P.; Alyea, E.P.; Freedman, A.S.; Soiffer, R.J.; et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann. Oncol. 2011, 22, 1608–1613. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Chou, J.; Maloy, M.; Zhang, Z.; Moskowitz, A.J.; Moskowitz, C.H.; Barker, J.N.; Giralt, S.; Perales, M.A.; Horwitz, S.M.; et al. Successful Treatment of Peripheral T-Cell Lymphoma with Allogeneic Stem Cell Transplantation: A Large Single-Center Experience. Blood 2015, 126, 4392. [Google Scholar] [CrossRef]
- Dodero, A.; Spina, F.; Narni, F.; Patriarca, F.; Cavattoni, I.; Benedetti, F.; Ciceri, F.; Baronciani, D.; Scimè, R.; Pogliani, E.; et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: Long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia 2012, 26, 520–526. [Google Scholar] [CrossRef]
- Kyriakou, C.; Canals, C.; Finke, J.; Kobbe, G.; Harousseau, J.L.; Kolb, H.J.; Novitzky, N.; Goldstone, A.H.; Sureda, A.; Schmitz, N. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: A retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J. Clin. Oncol. 2009, 27, 3951–3958. [Google Scholar] [CrossRef] [PubMed]
- Corradini, P.; Dodero, A.; Zallio, F.; Caracciolo, D.; Casini, M.; Bregni, M.; Narni, F.; Patriarca, F.; Boccadoro, M.; Benedetti, F.; et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin’s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J. Clin. Oncol. 2004, 22, 2172–2176. [Google Scholar] [CrossRef]
- Epperla, N.; Ahn, K.W.; Litovich, C.; Ahmed, S.; Battiwalla, M.; Cohen, J.B.; Dahi, P.; Farhadfar, N.; Farooq, U.; Freytes, C.O.; et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: A CIBMTR analysis. J. Hematol. Oncol. 2019, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Pro, B.; Pinter-Brown, L.; Bartlett, N.; Popplewell, L.; Coiffier, B.; Lechowicz, M.J.; Savage, K.J.; Shustov, A.R.; Gisselbrecht, C.; et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J. Clin. Oncol. 2011, 29, 1182–1189. [Google Scholar] [CrossRef]
- Pro, B.; Horwitz, S.M.; Prince, H.M.; Foss, F.M.; Sokol, L.; Greenwood, M.; Caballero, D.; Morschhauser, F.; Wilhelm, M.; Iyer, S.P.; et al. Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Hematol. Oncol. 2017, 35, 914–917. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V.; et al. Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 2018, 132, 2305–2309. [Google Scholar] [CrossRef]
- Gregory, G.P.; Dickinson, M.; Yannakou, C.K.; Wong, J.; Blombery, P.; Corboy, G.; Kats, L.; Crozier, T.; Kumar, B.; Prince, H.M.; et al. Rapid and Durable Complete Remission of Refractory AITL with Azacitidine Treatment in Absence of TET2 Mutation or Concurrent MDS. Hemasphere 2019, 3, e187. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- Sawhney, R.; Volkmer, R.D., II; Cooper, B. Relapsed angioimmunoblastic T-cell lymphoma with large pericardial effusion. Bayl. Univ. Med. Cent. Proc. 2020, 33, 62–64. [Google Scholar] [CrossRef]
- Beckers, M.M.; Huls, G. Therapy refractory angioimmunoblastic T-cell lymphoma in complete remission with lenalidomide. Eur. J. Haematol. 2013, 90, 162–163. [Google Scholar] [CrossRef]
- Ohmoto, A.; Fuji, S. Cyclosporine for angioimmunoblastic T-cell lymphoma: A literature review. Expert Rev. Hematol. 2019, 12, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Reboursiere, E.; Damaj, G. Bendamustine in peripheral T-cell lymphoma. Ann. Lymphoma 2018, 2. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sakata-Yanagimoto, M.; Fujisawa, M.; Nuhat, S.T.; Miyoshi, H.; Nannya, Y.; Hashimoto, K.; Fukumoto, K.; Bernard, O.A.; Kiyoki, Y.; et al. Dasatinib Is an Effective Treatment for Angioimmunoblastic T-cell Lymphoma. Cancer Res. 2020, 80, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.D.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Hancock, H.; Davey, T.; Obadi, O.; et al. Final Results of a Phase II Biomarker-Driven Study of Ruxolitinib in Relapsed and Refractory T-Cell Lymphoma. Blood 2019, 134 (Suppl. 1), 4019. [Google Scholar] [CrossRef]
- Krishnan, C.; Warnke, R.A.; Arber, D.A.; Natkunam, Y. PD-1 expression in T-cell lymphomas and reactive lymphoid entities: Potential overlap in staining patterns between lymphoma and viral lymphadenitis. Am. J. Surg. Pathol. 2010, 34, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Bennani, N.N.; Pederson, L.D.; Atherton, P.; Micallef, I.; Colgan, J.P.; Thanarajasingam, G.; Nowakowski, G.; Witzig, T.E.; Feldman, A.L.; Ansell, S.M. A Phase II Study of Nivolumab in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma. Blood 2019, 134 (Suppl. 1), 467. [Google Scholar] [CrossRef]
- Barta, S.K.; Zain, J.; MacFarlane, A.W., 4th; Smith, S.M.; Ruan, J.; Fung, H.C.; Tan, C.R.; Yang, Y.; Alpaugh, R.K.; Dulaimi, E.; et al. Phase II Study of the PD-1 Inhibitor Pembrolizumab for the Treatment of Relapsed or Refractory Mature T-cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 356–364.e3. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, J.; Wang, Z.; Zhang, L.; Wang, Z.; Zhang, M.; Cen, H.; Peng, Z.; Li, Y.; Fan, L.; et al. Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J. Hematol. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef]
- Neuwelt, A.; Al-Juhaishi, T.; Davila, E.; Haverkos, B. Enhancing antitumor immunity through checkpoint blockade as a therapeutic strategy in T-cell lymphomas. Blood Adv. 2020, 4, 4256–4266. [Google Scholar] [CrossRef]
- Iyer, S.P.; Neelapu, S.S.; Burns, E.; Nair, R.; Hosing, C.; Nieto, Y.; Westin, J.R.; Parmar, S.; Fowler, N.H.; Nastoupil, L.J.; et al. A Phase I/II Study to Examine the Safety and Efficacy of Pembrolizumab 200 mg Fixed Dose Administered Every 3 Weeks (Q3W) in Combination with Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma (PTCL). Blood 2019, 134 (Suppl. 1), 1546. [Google Scholar] [CrossRef]
- Berg, H.; Otteson, G.E.; Corley, H.; Shi, M.; Horna, P.; Jevremovic, D.; Olteanu, H. Flow cytometric evaluation of TRBC1 expression in tissue specimens and body fluids is a novel and specific method for assessment of T-cell clonality and diagnosis of T-cell neoplasms. Cytom. Part B Clin. Cytom. 2021, 100, 361–369. [Google Scholar] [CrossRef]

| Author | No. of Patients PTCL/AITL | OS % | PFS % |
|---|---|---|---|
| Smith et al. [72] | 126/12 | 83@3 years | 67@3years |
| Le Gouill et al. [73] | 77/11 | 57(80)@5years ** | 53(80)@5years ** |
| Mehta-shah et al. [75] | 65/11 | 59@2years | 48@2years |
| Jacobsen et al. [74] | 52/5 | 41@3years | 30@3years |
| Mamez et al. [68] * | 285/83 | 67@2years | 64@2years |
| Dodero et al. [76] | 52/9 | 66@5years | 44@5years |
| Kyriakou et al. [77] | 0/45 | 64@3years | 54@3years |
| Corradini et al. [78] | 17/4 | 81@3years | 64@3years |
| Epperla et al. [79] | NA/249 | 56@4years | 49@4years |
| ClinicalTrials.gov Identifier | Title | Disease Status | Intervention | Status |
|---|---|---|---|---|
| NCT03853044 | A Phase 2, open-label study to evaluate the safety and efficacy of chidamide combined with CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisone) in untreated subjects with AITL | First line | Chidamide + CHOP; single arm | Recruiting |
| NCT02879526 | A phase 2, chidamide combined with cyclophosphamide, prednisone, thalidomide in treatment of fragile patients with R/R PTCL | R/R | Chidamide + CPT; single arm | Recruiting |
| NCT03617432 | A phase 2, chidamide combined with CHOPE regimen for PTCL Patients | R/R | Chi + CHOPE; single arm | Recruiting |
| NCT03593018 | Randomized phase 3 study evaluating the efficacy and the safety of oral azacitidine (CC-486) Compared to investigator’s choice therapy in patient with relapsed or refractory AITL | R/R | Oral Azacitidine Vs Romedpsin or Bendamustine or Gemcitabine | Recruiting |
| NCT01998035 | A phase 1/2; romidepsin plus oral 5-Azacitidine in relapsed/refractory lymphoid malignancies | R/R | Oral azacitadine + romidepsin | Terminated (PI left institution) |
| NCT04480125 | A phase 2, azacitidine iv combined with chidamide in the treatment of newly diagnosed PTCL unfit for conventional chemotherapy | First line | Azacitadine + Chidamide; single arm | Recruiting |
| NCT04251065 | A phase 2, open label, multicenter trial of Daratumumab in combination with gemcitabine, dexamethasone and cisplatin (D-GDP) in patients with relapsed/refractory CD38 positive PTCL-NOS, AITL and other nodal lymphomas of TFH cell origin | R/R | D-GDP; single arm | Not yet recruiting |
| NCT02520791 | A phase I trial of MEDI-570 in patients with relapsed/refractory PTCL follicular variant and AITL | R/R | MEDI-570 (ICOS monoclonal antibody); single arm | Recruiting |
| NCT04319601 | A single-arm, multiple centers, phase II study evaluating Rituximab in combination with chidamide and lenalidomide for relapsed or refractory AITL | R/R | Rituximab + Chidamide + Lenalidomide; single arm | Recruiting |
| NCT03703375 | Randomized phase 3 study evaluation the efficacy and safety of oral azacitidine(CC-486) compared to investigator’s choice therapy in patients with relapsed or refractory AITL | R/R | Oral Azacitadine vs Romedepsin or Gemcitabine | Recruiting |
| NCT03552692 | Use of venetoclax as single agent in patients with relapsed/refractory BCL-2 Positive peripheral T cell lymphoma. | R/R | Venetoclax; single arm | Terminated |
| NCT03590574 | A single arm, open label, multi-center, phase I/II study evaluating the safety and clinical activity of AUTO4, a CAR T cell treatment targeting TRBC1, in patients with relapsed or refractory TRBC1 positive selected T cell non-Hodgkin lymphoma | R/R | AUTO4 (CAR T cell against TRBC1); single arm | Recruiting |
| NCT01719835 | CHEMO-T: Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone (CHOP) versus Gemcitabine, Cisplatin and Methyl Prednisolone (GEM-P) in the first line treatment of T cell lymphoma, a multicenter randomized phase II study | First line | CHOP vs GEM-P | Active not Recruiting |
| NCT02223208 | Romidepsin in combination with CHOEP as first line treatment before Hematopoietic Stem Cell Transplantation in young patients with nodal peripheral T cell lymphomas: a phase I-II study | First line | Romidepsin + CHOEP; single arm | Recruiting |
| NCT03598998 | A phase 1/2 study of Pembrolizumab plus Pralatrexate for treatment of relapsed or refractory PTCL | R/R | Pemrbroliumab + Pralatrexate; single arm | Recruiting |
| NCT02588651 | A phase II study of single agent Brentuximab Vedotin in relapsed/refractory CD30 Low (<10%) mature T cell lymphoma (TCL) | R/R | Brentuximb Vedotin; single arm | Recruiting |
| NCT04447027 | A phase 1 study of Romidepsin, CC-486 (5-azacitidine), Dexamethasone, and Lenalidomide (RAdR) for relapsed/refractory T cell malignancies | R/R | RAdR; single arm | Not yet recruiting |
| NCT01755975 | A phase1/2; Romidepsin in combination with Lenalidomide in adults with relapsed or refractory lymphomas and myeloma | R/R | Romidepsin + Lenalidomide | Active, not recruiting |
| NCT02783625 | A phase 1; trial of Duvelisib in combination with either Romidepsin or Bortezomib in relapsed/refractory T cell lymphomas | R/R | Duvelisib + romidepsin or Bortezomib | Recruiting |
| NCT03372057 | A multi-Center, Phase 2, open-label, parallel Cohort study of efficacy and safety of Duvelisib in Patients with relapsed or refractory PTCL | R/R | Duvelisib; single arm | Active, not recruiting |
| NCT04639843 | A phase 1 study of Doxorubicin, CC-486 (5-azacitidine), Romidepsin, and Duvelisib (hARD) for T cell lymphoma | First line and R/R | hARD; single arm | Not yet recruiting |
| NCT04803201 | A randomized phase II study of CHO(E)P vs CC-486-CHO(E)P vs Duvelisib-CHO(E)P in previously untreated CD30 negative peripheral T cell lymphomas | First line | CHOEP vs Duvelisib + CHOEP | Recruiting |
| NCT05010005 | Phase I multicenter study of Ruxolitinib and Duvelisib in relapsed or refractory T- or NK-cell lymphomas | R/R | Ruxolitinib + Duvelisib | Recruiting |
| NCT02974647 | A phase II multicenter study of Ruxolitinib in patients with T or NK cell lymphoma that has either come back or not responded to treatment | R/R | Ruxolitinib; single arm | Recruiting |
| NCT03017820 | Phase I trial of systemic administration of Vesicular Stomatitis Virus Genetically Engineered to Express NIS and human Interferon, in Patients With relapsed or refractory multiple myeloma, acute myeloid leukemia, and T cell neoplasms | R/R | VSV-hIFNbeta-NIS; single arm | Recruiting |
| NCT03113500 | A phase 2 study of Brentuximab Vedotin plus Cyclophosphamide, Doxorubicin, Etoposide, and Prednisone (CHEP-BV) followed by BV consolidation in patients With CD30-positive peripheral T cell lymphomas | First line | CHEP-BV followed by BV consolidation; single arm | Recruiting |
| NCT04008394 | Efficacy and safety of anti-CD30 CAR-T therapy in patients with refractory/relapsed lymphocyte malignancies a single-center, open, single-arm clinical study. | R/R | Anti-CD30 CAR- T therapy; single arm | Recruiting |
| NCT02232516 | Phase II study of Romidepsin Plus Lenalidomide for patients with previously untreated PTCL | First line | Romidepsin + Lenalidomide; single arm | Recruiting |
| NCT00416351 | A Phase I/II study of Clofarabine in patients with relapsed T cell and NK-cell lymphomas | R/R | Clofarabine; single arm | Active not recruiting |
| NCT02168140 | Phase I dose-escalation study of CPI-613, in combination with Bendamustine, in patients with relapsed or refractory T cell Non-Hodgkin Lymphoma or classic Hodgkin Lymphoma | R/R | CPI-613 + Bendamustine; single arm | Active not recruiting |
| NCT01261247 | A phase II study of the histone deacetylase (HDAC) inhibitor LBH589 (Panobinostat) in patients with relapsed or refractory non-Hodgkin lymphoma | R/R | Panobinostat; single arm | Active not recruiting |
| NCT01805037 | A phase I-II trial of Brentuximab Vedotin plus Rituximab as frontline therapy for patients with CD30+ and/or EBV+ lymphomas | First line | BV + R; single arm | Active not recruiting |
| NCT01075321 | A phase I/II clinical trial of the mTor Inhibitor RAD001 (Everolimus) in combination with Lenalidomide (Revlimid) for patients with relapsed or refractory lymphoid malignancy | R/R | Everloimus + Lenalidomide; single arm | Active not recruiting |
| NCT01678443 | A phase I study evaluating escalating doses of 90Y-BC8-DOTA (Anti-CD45) antibody followed by autologous Stem Cell Transplantation for relapsed or refractory lymphoid malignancies. | R/R | 90Y-BC8-DOTA (Anti-CD45) then ASCT; single arm | Active not recruiting |
| NCT02561273 | A phase I/II trial of CHOEP Chemotherapy plus Lenalidomide as front line therapy for patients with stage II, III and IV peripheral T cell non-Hodgkin’s lymphoma | First line | CHOEP + Lenalidomide; single arm | Active not recruiting |
| NCT03278782 | A phase I/II study of Pembrolizumab (MK-3475) in combination with Romidepsin in patients with relapsed or refractory PTCL | R/R | Pemborolizumab + Romidepsin; single arm | Active not recruiting |
| NCT03493451 | A Phase 2, open-label study of BGB-A317 in patients with relapsed or refractory mature T- and NK- neoplasms | R/R | BGB-A317; single arm | Active not recruiting |
| NCT02533700 | CEOP/IVE/GDP compared with CEOP as the first-line therapy for newly diagnosed adult patients with PTCL | First line | CEOP/IVE/GDP vs CEOP | Active not recruiting |
| NCT04234048 | A phase 1a/1b trial in relapsed/refractory T cell non-Hodgkin lymphoma to determine the safety profile, pharmacology, and maximum tolerated dose of ST-001, a Fenretinide phospholipid suspension (12.5 mg/mL) for intravenous infusion | R/R | Dose of ST-001, a Fenretinide Phospholipid; single arm, sequential assignment dose escalating | Not yet recruiting |
| NCT04319601 | Rituximab combined With chidamide and Lenalidomide for R/R AITL | R/R | RChR; single arm | Recruiting |
| NCT02341014 | A phase 1/2, combination therapy with Carfilzomib, Romidepsin, Lenalidomide in patients with relapsed or refractory B- and T cell lymphomas | R/R | KRoR; single arm | Active, not recruiting |
| NCT02273739 | A phase 1/2, multicenter, open-label, dose-escalation study of AG-221 in subjects with advanced solid tumors, Including glioma, and with AITL, that harbor an IDH2 mutation | R/R | AG-221(Enasidenib); single arm | completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed Saleh, M.F.; Kotb, A.; Abdallah, G.E.M.; Muhsen, I.N.; El Fakih, R.; Aljurf, M. Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma. Curr. Oncol. 2021, 28, 5480-5498. https://doi.org/10.3390/curroncol28060456
Mohammed Saleh MF, Kotb A, Abdallah GEM, Muhsen IN, El Fakih R, Aljurf M. Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma. Current Oncology. 2021; 28(6):5480-5498. https://doi.org/10.3390/curroncol28060456
Chicago/Turabian StyleMohammed Saleh, Mostafa F., Ahmed Kotb, Ghada E. M. Abdallah, Ibrahim N. Muhsen, Riad El Fakih, and Mahmoud Aljurf. 2021. "Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma" Current Oncology 28, no. 6: 5480-5498. https://doi.org/10.3390/curroncol28060456
APA StyleMohammed Saleh, M. F., Kotb, A., Abdallah, G. E. M., Muhsen, I. N., El Fakih, R., & Aljurf, M. (2021). Recent Advances in Diagnosis and Therapy of Angioimmunoblastic T Cell Lymphoma. Current Oncology, 28(6), 5480-5498. https://doi.org/10.3390/curroncol28060456





