Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria of NET-QUBIC Study
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gupta, B.; Johnson, N.W.; Kumar, N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Alho, O.-P.; Teppo, H.; Mäntyselkä, P.; Kantola, S. Head and neck cancer in primary care: Presenting symptoms and the effect of delayed diagnosis of cancer cases. CMAJ 2006, 174, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Ford, P.; Farah, C. “I have quality of life… but…”: Exploring support needs important to quality of life in head and neck cancer. Eur. J. Oncol. Nurs. 2014, 18, 192–200. [Google Scholar] [CrossRef]
- Macfarlane, T.V.; Wirth, T.; Ranasinghe, S.; Ah-See, K.W.; Renny, N.; Hurman, D. Head and neck cancer pain: Systematic review of prevalence and associated factors. J. Oral Maxillofac. Res. 2012, 3, e1. [Google Scholar] [CrossRef]
- Santoso, A.M.; Jansen, F.; Lissenberg-Witte, B.I.; de Jong, R.J.B.; Langendijk, J.A.; Leemans, C.R.; Smit, J.H.; Takes, R.P.; Terhaard, C.H.; van Straten, A. Poor sleep quality among newly diagnosed head and neck cancer patients: Prevalence and associated factors. Support. Care Cancer 2021, 29, 1035–1045. [Google Scholar] [CrossRef]
- Haisfield-Wolfe, M.E.; McGuire, D.B.; Soeken, K.; Geiger-Brown, J.; De Forge, B.R. Prevalence and correlates of depression among patients with head and neck cancer: A systematic review of implications for research. Oncol. Nurs. Forum 2009, 36, E107–E125. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, V.; Lal, P.; Verma, M.; Yadav, R.; Singh, N.; Kumar, S. Evaluation of fatigue in head and neck cancer patients undergoing (intensity modulated radiation therapy) radiotherapy: A prospective study. Asian J. Oncol. 2015, 1, 44–48. [Google Scholar] [CrossRef]
- So, W.K.; Law, B.M.; Ng, M.S.; He, X.; Chan, D.N.; Chan, C.W.; McCarthy, A.L. Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Med. 2021, 10, 2531–2565. [Google Scholar] [CrossRef]
- Tometich, D.B.; Small, B.J.; Carroll, J.E.; Zhai, W.; Luta, G.; Zhou, X.; Kobayashi, L.C.; Ahles, T.; Saykin, A.J.; Clapp, J.D. Pretreatment psychoneurological symptoms and their association with longitudinal cognitive function and quality of life in older breast cancer survivors. J. Pain Symptom Manag. 2019, 57, 596–606. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355. [Google Scholar] [CrossRef]
- Buckley, T.M.; Schatzberg, A.F. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 2005, 90, 3106–3114. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Jiang, Y.; Rodriguez-Stanley, J.; Almeida, D.M.; Engeland, C.G.; Zilioli, S. Perceived stress is linked to heightened biomarkers of inflammation via diurnal cortisol in a national sample of adults. Brain Behav. Immun. 2021, 93, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.; Voiculescu, V.M.; Caruntu, C.; Lupu, M.; Popa, A.; Ilie, M.A.; Albulescu, R.; Caruntu, A.; Tanase, C.; Constantin, C. Neuroendocrine factors and head and neck squamous cell carcinoma: An affair to remember. Dis. Markers 2018, 2018, 9787831. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-P.; Lin, C.-C. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Eur. J. Oncol. Nurs. 2017, 29, 79–84. [Google Scholar] [CrossRef]
- Clevenger, L.; Schrepf, A.; Christensen, D.; DeGeest, K.; Bender, D.; Ahmed, A.; Goodheart, M.J.; Penedo, F.; Lubaroff, D.M.; Sood, A.K. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav. Immun. 2012, 26, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Ose, J.; Lin, T.; Warby, C.A.; Gigic, B.; Steindorf, K.; Schrotz-King, P.; Abbenhardt-Martin, C.; Zielske, L.; Boehm, J. Inflammation-and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur. J. Cancer Care 2019, 28, e13055. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.O.; Anatriello, E.; Azevedo, L.R.; Cordeiro, J.F.; Peria, F.M.; Flória-Santos, M.; Pereira-da-Silva, G. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine 2018, 104, 72–77. [Google Scholar] [CrossRef]
- Xiao, C.; Beitler, J.J.; Higgins, K.A.; Conneely, K.; Dwivedi, B.; Felger, J.; Wommack, E.C.; Shin, D.M.; Saba, N.F.; Ong, L.Y. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav. Immun. 2016, 52, 145–152. [Google Scholar] [CrossRef]
- Oliveira, K.; von Zeidler, S.; Lamas, A.; de Podestá, J.; Sena, A.; Souza, E.; Lenzi, J.; Lemos, E.; Gouvea, S.; Bissoli, N. Relationship of inflammatory markers and pain in patients with head and neck cancer prior to anticancer therapy. Braz. J. Med. Biol. Res. 2014, 47, 600–604. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Immune-enhancing effects of glucocorticoids in response to day–night cycles and stress. Int. Immunol. 2020, 32, 703–708. [Google Scholar] [CrossRef]
- Mac Giollabhui, N. Inflammation and depression: Research designs to better understand the mechanistic relationships between depression, inflammation, cognitive dysfunction, and their shared risk factors. Brain Behav. Immun.-Health 2021, 15, 100278. [Google Scholar] [CrossRef]
- Contreras, A.; Nieto, I.; Valiente, C.; Espinosa, R.; Vazquez, C. The study of psychopathology from the network analysis perspective: A systematic review. Psychother. Psychosom. 2019, 88, 71–83. [Google Scholar] [CrossRef]
- Verdonck-de Leeuw, I.; Jansen, F.; Brakenhoff, R.; Langendijk, J.; Takes, R.; Terhaard, C.; de Jong, R.B.; Smit, J.; Leemans, C. Advancing interdisciplinary research in head and neck cancer through a multicenter longitudinal prospective cohort study: The NETherlands QUality of life and BIomedical Cohort (NET-QUBIC) data warehouse and biobank. BMC Cancer 2019, 19, 765. [Google Scholar]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Kalaoja, M.; Corbin, L.J.; Tan, V.Y.; Ahola-Olli, A.V.; Havulinna, A.S.; Santalahti, K.; Pitkänen, N.; Lehtimäki, T.; Lyytikäinen, L.P.; Raitoharju, E. The role of inflammatory cytokines as intermediates in the pathway from increased adiposity to disease. Obesity 2021, 29, 428–437. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Beck, S.L.; Schwartz, A.L.; Towsley, G.; Dudley, W.; Barsevick, A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J. Pain Symptom Manag. 2004, 27, 140–148. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Vodermaier, A.; Linden, W.; Siu, C. Screening for emotional distress in cancer patients: A systematic review of assessment instruments. J. Natl. Cancer Inst. 2009, 101, 1464–1488. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Sullivan, M. EORTC QLQ–C30 Scoring Manual; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 1995. [Google Scholar]
- Smets, E.; Garssen, B.; Bonke, B.d.; De Haes, J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Adam, E.K.; Kumari, M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Peeters, C.F.; Bilgrau, A.E.; van Wieringen, W.N. rags2ridges: A One-Stop-Shop for Graphical Modeling of High-Dimensional Precision Matrices. arXiv 2020, arXiv:2010.05619. [Google Scholar] [CrossRef]
- Van Wieringen, W.N.; Peeters, C.F. Ridge estimation of inverse covariance matrices from high-dimensional data. Comput. Stat. Data Anal. 2016, 103, 284–303. [Google Scholar] [CrossRef]
- Fruchterman, T.M.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef]
- Newman, M.E.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 026113. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Effects of sleep on memory for conditioned fear and fear extinction. Psychol. Bull. 2015, 141, 835. [Google Scholar] [CrossRef]
- McQuestion, M.; Fitch, M. Patients’ experience of receiving radiation treatment for head and neck cancer: Before, during and after treatment. Can. Oncol. Nurs. J. 2016, 26, 325. [Google Scholar] [CrossRef]
- Recine, T.S. Contributions of Fatigue, Pain, and Functional Limitation to Future Depression in Cancer Survivors. Ph.D. Thesis, Florida Institute of Technology, Melbourne, FL, USA, 2016. [Google Scholar]
- Stepanski, E.J.; Walker, M.S.; Schwartzberg, L.S.; Blakely, L.J.; Ong, J.C.; Houts, A.C. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J. Clin. Sleep Med. 2009, 5, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.M.; France, T.J.; Teknos, T.N.; Kumar, P. Interleukin-6 role in head and neck squamous cell carcinoma progression. World J. Otorhinolaryngol.-Head Neck Surg. 2016, 2, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 2006, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its behavioral and metabolic correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Xiao, C.; Eldridge, R.C.; Beitler, J.J.; Higgins, K.A.; Chico, C.E.; Felger, J.C.; Wommack, E.C.; Knobf, T.; Saba, N.F.; Shin, D.M. Association Among Glucocorticoid Receptor Sensitivity, Fatigue, and Inflammation in Patients with Head and Neck Cancer. Psychosom. Med. 2020, 82, 508–516. [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C. Measuring salivary markers of inflammation in health research: A review of methodological considerations and best practices. Psychoneuroendocrinology 2020, 124, 105069. [Google Scholar] [CrossRef]

| Characteristics | Patients with Complete Data | Patients with Incomplete Data | Adjusted p-Value a |
|---|---|---|---|
| (n = 264) | (n = 475) | ||
| Age (mean, SD) | 65 (8.2) | 62 (10.4) | 0.007 |
| Men, No. (%) | 209 (79.2%) | 340 (71.6%) | 0.112 |
| Education level, No. (%) b | |||
| Low | 107 (40.5%) | 172 (44.8%) | 0.560 |
| Middle | 77 (29.2%) | 94 (24.5%) | |
| High | 80 (30.3%) | 118 (30.7%) | |
| Living together, No. (%) b | 215 (81.4%) | 270 (70.1%) | 0.007 |
| HNC location, No. (%) | |||
| Oral cavity | 64 (24.2%) | 135 (28.4%) | 0.427 |
| Oropharynx | 98 (37.1%) | 164 (34.5%) | |
| Hypopharynx | 17 (6.4%) | 35 (7.4%) | |
| Larynx | 81 (30.7%) | 124 (26.1%) | |
| Unknown primary | 4 (1.5%) | 17 (3.6%) | |
| HNC stage, No. (%) | |||
| I | 67 (25.4%) | 96 (20.2%) | 0.559 |
| II | 48 (18.2%) | 84 (17.7%) | |
| III | 40 (15.2%) | 87 (18.3%) | |
| IV | 109 (41.3%) | 208 (43.8%) | |
| ECOG performance status, No. (%) | |||
| 0 | 191 (72.3%) | 316 (66.5%) | 0.238 |
| 1 or more | 73 (27.7%) | 159 (33.5%) | |
| Comorbidity, No. (%) b | |||
| None | 86 (33.7%) | 118 (26.6%) | 0.427 |
| Mild | 93 (36.5%) | 171 (38.5%) | |
| Moderate | 51 (20%) | 104 (23.4%) | |
| Severe | 25 (9.8%) | 51 (11.5%) | |
| Excessive alcohol consumption, No. (%) b | 58 (22.0%) | 71 (22.8%) | 0.805 |
| Smoking daily, No. (%) b | 57 (21.7%) | 70 (22.7%) | 0.805 |
| BMI (kg/m2) b, mean (SD) | 26.1 (4.5) | 25.3 (4.6) | 0.112 |
| Sleep (PSQI score) b, median (IQR) | 5.0 (3.0–7.0) | 5.0 (3.0–9.0) | 0.600 |
| Depression (HADS-D score) b, median (IQR) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 0.791 |
| Anxiety (HADS-A score) b, median (IQR) | 5.0 (3.0–7.8) | 5 (3.0–8.0) | 0.600 |
| Oral pain (EORTC-H&N35) b, median (IQR) | 16.7 (8.3–33.3) | 25 (8.3–50.0) | 0.112 |
| Fatigue (MFI general fatigue) b, median (IQR) | 9.0 (5.0–13.0) | 11.5 (6.0–14.0) | 0.007 |
| Cortisol slope (nmol/L/hour) b, median (IQR) | 0.47 (0.25–0.78) | 0.48 (0.28–0.77) | 0.791 |
| CRP (mg/L) b, median (IQR) | 2.8 (2.5–5.5) | 3.1 (2.4–8.0) | 0.238 |
| IL-6 (pg/mL) b, median (IQR) | 1.03 (0.66–1.72) | 1.11 (0.66–1.84) | 0.427 |
| IL-10 (pg/mL) b, median (IQR) | 0.24 (0.18–0.36) | 0.27 (0.19–0.40) | 0.222 |
| TNF-α (pg/mL) b, median (IQR) | 2.72 (2.32–3.37) | 2.79 (2.31–3.44) | 0.600 |
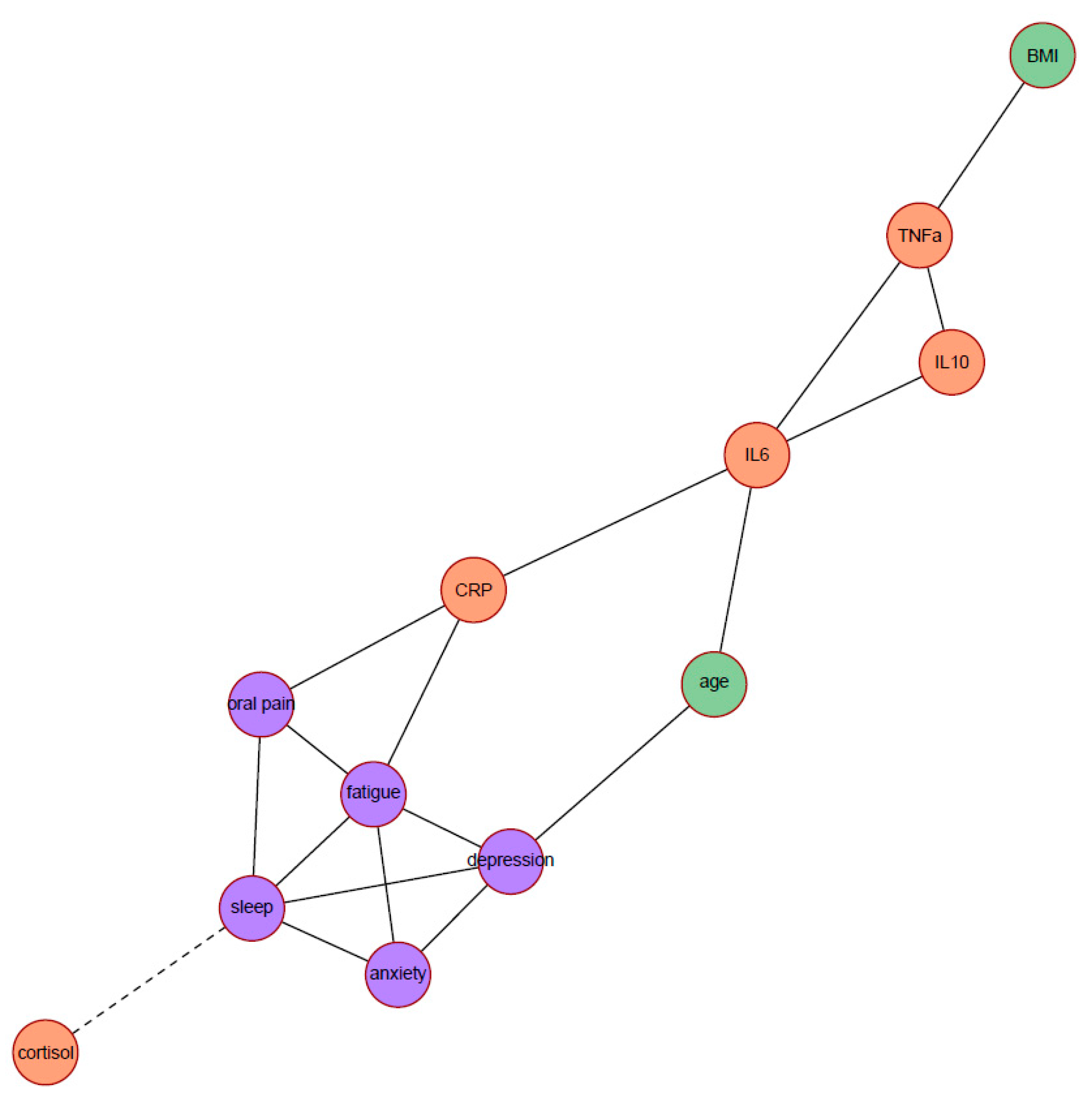
| - | Degree | Betweenness | Closeness |
|---|---|---|---|
| Poor sleep quality | 5 | 11.33 | 0.040 |
| Depression symptoms | 4 | 9.33 | 0.045 |
| Anxiety symptoms | 3 | 0 | 0.037 |
| Oral pain | 3 | 3.67 | 0.042 |
| Fatigue | 5 | 9 | 0.048 |
| Cortisol slope | 1 | 0 | 0.028 |
| CRP | 3 | 15.67 | 0.048 |
| IL-6 | 4 | 25.33 | 0.045 |
| IL-10 | 2 | 0 | 0.033 |
| TNF-α | 3 | 10 | 0.034 |
| Age | 2 | 8.67 | 0.043 |
| BMI | 1 | 0 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoso, A.M.M.; Jansen, F.; Peeters, C.F.W.; Baatenburg de Jong, R.J.; Brakenhoff, R.H.; Langendijk, J.A.; Leemans, C.R.; Takes, R.P.; Terhaard, C.H.J.; van Straten, A.; et al. Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis. Curr. Oncol. 2022, 29, 7109-7121. https://doi.org/10.3390/curroncol29100559
Santoso AMM, Jansen F, Peeters CFW, Baatenburg de Jong RJ, Brakenhoff RH, Langendijk JA, Leemans CR, Takes RP, Terhaard CHJ, van Straten A, et al. Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis. Current Oncology. 2022; 29(10):7109-7121. https://doi.org/10.3390/curroncol29100559
Chicago/Turabian StyleSantoso, Angelina M. M., Femke Jansen, Carel F. W. Peeters, Robert J. Baatenburg de Jong, Ruud H. Brakenhoff, Johannes A. Langendijk, C. René Leemans, Robert P. Takes, Chris H. J. Terhaard, Annemieke van Straten, and et al. 2022. "Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis" Current Oncology 29, no. 10: 7109-7121. https://doi.org/10.3390/curroncol29100559
APA StyleSantoso, A. M. M., Jansen, F., Peeters, C. F. W., Baatenburg de Jong, R. J., Brakenhoff, R. H., Langendijk, J. A., Leemans, C. R., Takes, R. P., Terhaard, C. H. J., van Straten, A., & Verdonck-de Leeuw, I. M. (2022). Psychoneurological Symptoms and Biomarkers of Stress and Inflammation in Newly Diagnosed Head and Neck Cancer Patients: A Network Analysis. Current Oncology, 29(10), 7109-7121. https://doi.org/10.3390/curroncol29100559






