Plasmacytic Pleural Effusion as a Major Presentation of Angioimmunoblastic T-Cell Lymphoma: A Case Report
Abstract
:1. Introduction
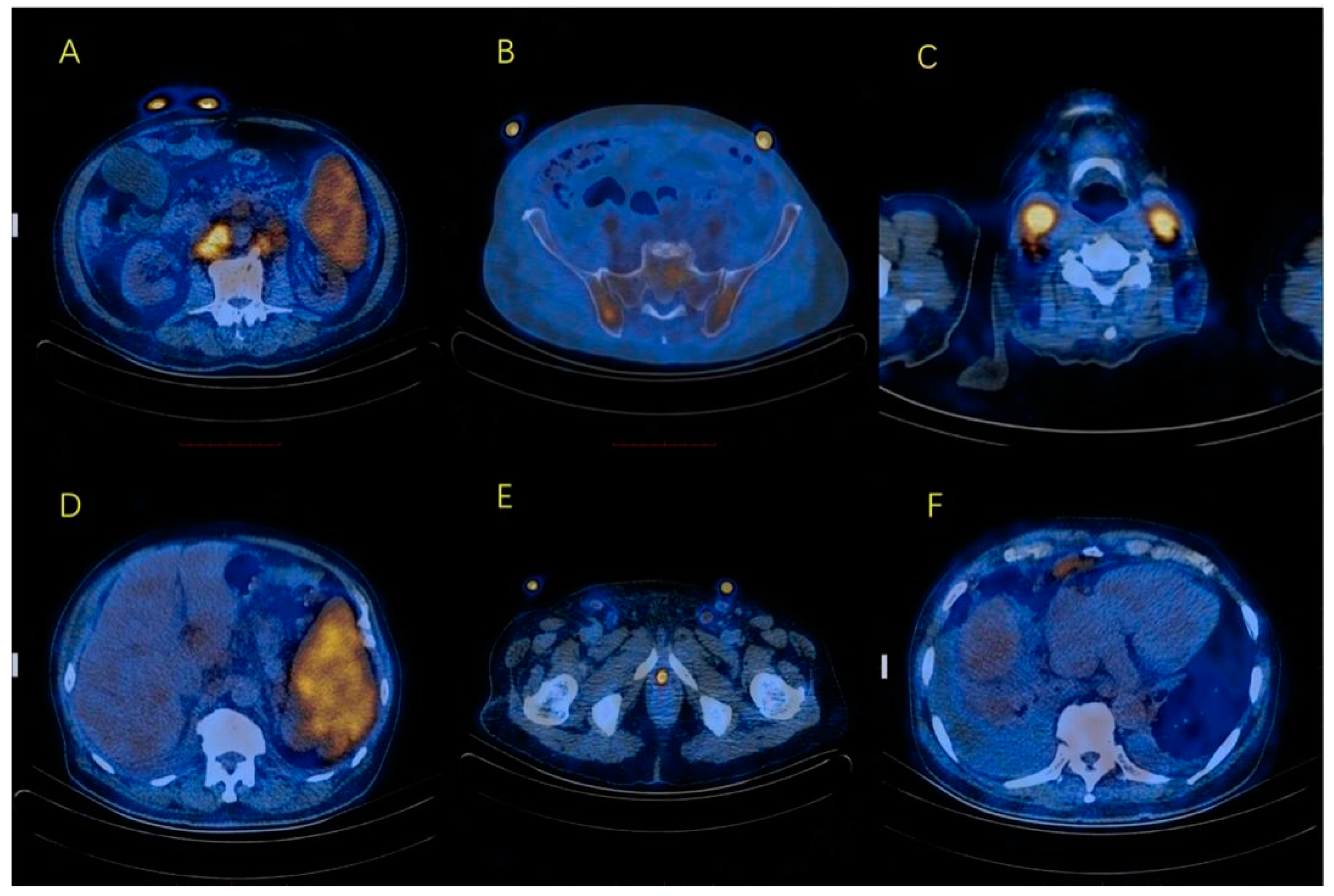
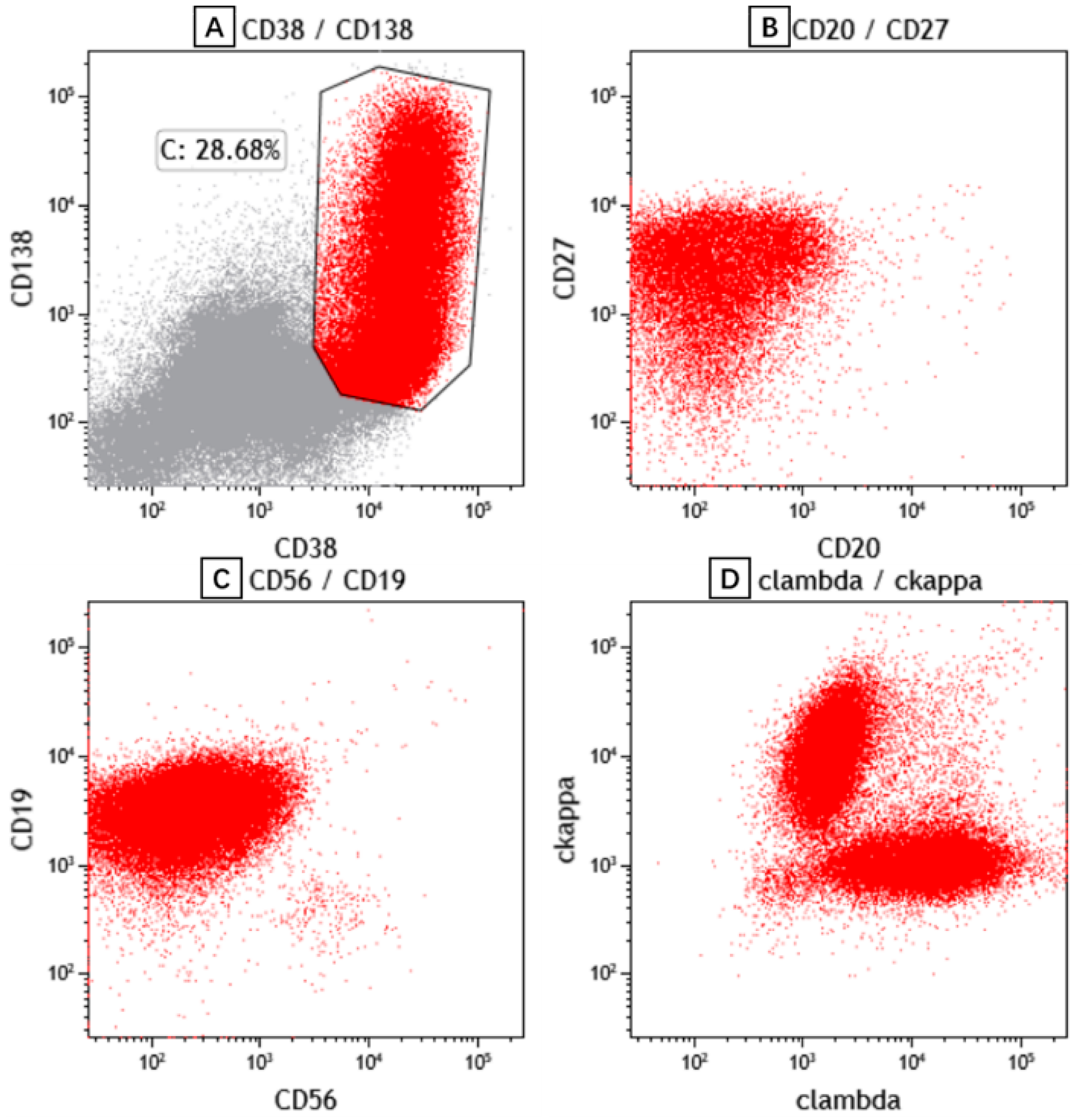
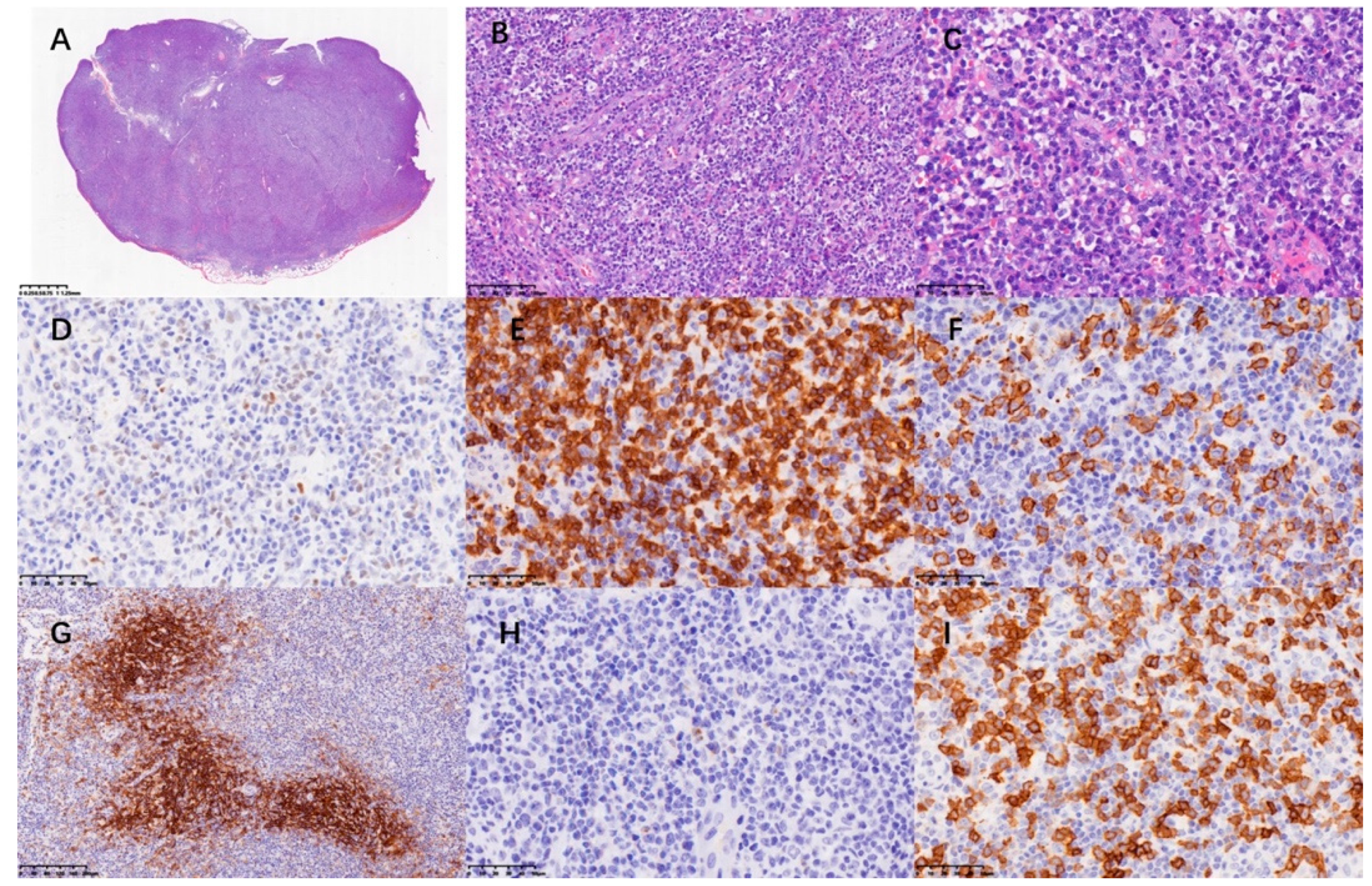
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frizzera, G.; Kaneko, Y.; Sakurai, M. Angioimmunoblastic lymphadenopathy and related disorders: A retrospective look in search of definitions. Leukemia 1989, 3, 1–5. [Google Scholar] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, S.; Sakata-Yanagimoto, M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia 2020, 34, 2592–2606. [Google Scholar] [CrossRef]
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Jaffe, E.S. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am. J. Clin. Pathol. 2021, 156, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lunning, M.A.; Vose, J.M. Angioimmunoblastic T-cell lymphoma: The many-faced lymphoma. Blood 2017, 129, 1095–1102. [Google Scholar] [CrossRef]
- Ahsanuddin, A.N.; Brynes, R.K.; Li, S. Peripheral blood polyclonal plasmacytosis mimicking plasma cell leukemia in patients with angioimmunoblastic T-cell lymphoma: Report of 3 cases and review of the literature. Int. J. Clin. Exp. Pathol. 2011, 4, 416–420. [Google Scholar] [PubMed]
- Yamane, A.; Awaya, N.; Shimizu, T.; Ikeda, Y.; Okamoto, S. Angioimmunoblastic T-cell lymphoma with polyclonal proliferation of plasma cells in peripheral blood and marrow. Acta Haematol. 2007, 117, 74–77. [Google Scholar] [CrossRef]
- Kojima, M.; Kaneko, Y.; Masawa, N.; Sugihara, S. Tuberculous pleural effusion containing numerous reactive plasma cells and their precursors: A case report. Diagn. Cytopathol. 2012, 40, 941–942. [Google Scholar] [CrossRef]
- Kamble, R.; Wilson, C.S.; Fassas, A.; Desikan, R.; Siegel, D.S.; Tricot, G.; Anderson, P.; Sawyer, J.; Anaissie, E.; Barlogie, B. Malignant pleural effusion of multiple myeloma: Prognostic factors and outcome. Leuk. Lymphoma 2005, 46, 1137–1142. [Google Scholar] [CrossRef]
- Nagoshi, H.; Kuroda, J.; Kobayashi, T.; Maegawa, S.; Chinen, Y.; Kiyota, M.; Nakayama, R.; Mizutani, S.; Shimura, Y.; Yamamoto-Sugitani, M.; et al. Clinical manifestation of angioimmunoblastic T-cell lymphoma with exuberant plasmacytosis. Int. J. Hematol. 2013, 98, 366–374. [Google Scholar] [CrossRef]
- Desborough, M.J.; Grech, H. Epstein-Barr virus-driven bone marrow aplasia and plasmacytosis mimicking a plasma cell neoplasm. Br. J. Haematol. 2014, 165, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Maeba, H.; Ikawa, Y.; Hashida, Y.; Okumura, A.; Shibata, F.; Tone, Y.; Inoue, M.; Koizumi, S.; Takatori, H.; et al. Reactive peripheral blood plasmacytosis in a patient with acute hepatitis A. Int. J. Hematol. 2007, 85, 191–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koduri, P.R.; Naides, S.J. Transient blood plasmacytosis in parvovirus B19 infection: A report of two cases. Ann. Hematol. 1996, 72, 49–51. [Google Scholar] [CrossRef]
- Moon, Y.; Jang, W.R.; Yi, H.G.; Park, I.S.; Nahm, C.H.; Choi, J.W.; Kim, J.J.; Han, S.B. Klebsiella pneumoniae associated extreme plasmacytosis. Infect. Chemother. 2013, 45, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Shtalrid, M.; Shvidel, L.; Vorst, E. Polyclonal reactive peripheral blood plasmacytosis mimicking plasma cell leukemia in a patient with Staphylococcal sepsis. Leuk. Lymphoma 2003, 44, 379–380. [Google Scholar] [CrossRef]
- Okabe, M.; Hirono, K.; Tamura, K.; Ichida, F.; Kanegane, H. Reactive peripheral blood plasmacytosis in Kawasaki disease. Pediatr. Int. 2018, 60, 884–885. [Google Scholar] [CrossRef]
- Lee, J.; Chang, J.E.; Cho, Y.J.; Han, W.S. A case of reactive plasmacytosis mimicking multiple myeloma in a patient with primary Sjogren’s syndrome. J. Korean Med. Sci. 2005, 20, 506–508. [Google Scholar] [CrossRef] [Green Version]
- Sokol, K.; Kartan, S.; Johnson, W.T.; Alpdogan, O.; Nikbakht, N.; Haverkos, B.M.; Gong, J.; Porcu, P. Extreme Peripheral Blood Plasmacytosis Mimicking Plasma Cell Leukemia as a Presenting Feature of Angioimmunoblastic T-Cell Lymphoma (AITL). Front. Oncol. 2019, 9, 509. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, A.; Miyoshi, H.; Yamauchi, T.; Arakawa, F.; Kawano, R.; Muta, H.; Sugita, Y.; Akashi, K.; Ohshima, K. Composite lymphoma of peripheral T-cell lymphoma and Hodgkin lymphoma, mixed cellularity type; pathological and molecular analysis. Pathol. Int. 2017, 67, 194–201. [Google Scholar] [CrossRef]
- Bashoura, L.; Eapen, G.A.; Faiz, S.A. Pulmonary Manifestations of Lymphoma and Leukemia. Clin. Chest. Med. 2017, 38, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Park, B.B.; Ryoo, B.Y.; Lee, J.H.; Kwon, H.C.; Yang, S.H.; Kang, H.J.; Kim, H.J.; Oh, S.Y.; Ko, Y.H.; Huh, J.R.; et al. Clinical features and treatment outcomes of angioimmunoblastic T-cell lymphoma. Leuk. Lymphoma 2007, 48, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Kim, K.H.; Choi, I.S.; Park, J.H.; Kim, J.S.; Shin, D.Y.; Koh, Y.; Kim, I.; Yoon, S.S.; Lim, H.J. Pleural Effusion in Multiple Myeloma: Characteristics and Practice Patterns. Acta Haematol. 2017, 138, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Nakazato, Y.; Shiino, S.; Kaneko, Y.; Hirabayashi, K.; Masawa, N. Nodal marginal zone B-cell lymphoma showing prominent plasma cell differentiation in the pleural effusion: A case report. Diagn. Cytopathol. 2014, 42, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Funada, H.; Urata, K.; Yosimura, S.; Kotani, Y.; Satouti, M.; Negoro, S.; Takada, Y. A case of malignant lymphoma diagnosed by thoracoscopy with local anesthesia. Nihon Kokyuki Gakkai Zasshi 2005, 43, 622–625. [Google Scholar]
- Aoki, T.; Okita, H.; Kayano, H.; Orikasa, H.; Watanabe, K.; Eyden, B.P.; Yamazaki, K. Anaplastic plasmacytoma with malignant pleural effusion lacking evidence of monoclonal gammopathy. Virchows Arch. 2002, 441, 154–158. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Zhou, X.; Zhang, K.; Yin, P.; Liu, S.; Zou, Y.; Li, Q. Detection of carcinoma in serous effusions: A review. Am. J. Cancer Res. 2021, 11, 43–60. [Google Scholar]
- Savvidou, K.; Dimitrakopoulou, A.; Kafasi, N.; Konstantopoulos, K.; Vassilakopoulos, T.; Angelopoulou, M.; Siakantaris, M.; Korkolopoulou, P.; Kanavaros, P.; Mikou, P. Diagnostic role of cytology in serous effusions of patients with hematologic malignancies. Diagn. Cytopathol. 2019, 47, 404–411. [Google Scholar] [CrossRef]
- Bedier, H.; Lin, J.; Julien, L.A.; Routy, J.P. Concurrent development of HIV-negative Kaposi’s sarcoma and mycosis fungoides in an elderly Inuit from Canada. BMJ Case Rep. 2021, 14, e238644. [Google Scholar] [CrossRef]
- Tajarernmuang, P.; Fiset, P.O.; Routy, J.P.; Beaudoin, S. Intractable pleural effusion in Kaposi sarcoma following antiretroviral therapy in a Caucasian female infected with HIV. BMJ Case Rep. 2020, 13, e233335. [Google Scholar] [CrossRef]
- Wawrzycki, B.; Prystupa, A.; Szumilo, J.; Panasiuk, L.; Krasowska, D. Fever, rash, and eosinophilia—Early signs of angioimmunoblastic T-cell lymphoma. Ann. Agric. Environ. Med. 2021, 28, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Hino, T.; Ohsawa, M.; Ueki, K.; Murao, T.; Li, M.; Cui, Y.; Okigaki, M.; Ito, M.; Ikehara, S. A case of CD10-negative angioimmunoblastic T cell lymphoma with leukemic change and increased plasma cells mimicking plasma cell leukemia: A case report. Oncol. Lett. 2015, 10, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Du, P.Y.; Raso-Barnett, L.; Yao, W.Q.; Chen, Z.; Casa, C.; Ei-Daly, H.; Farkas, L.; Soilleux, E.; Wright, P.; et al. Early detection of T-cell lymphoma with T follicular helper phenotype by RHOA mutation analysis. Haematologica 2022, 107, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.H.; Skrypets, T.; Civallero, M.; Spinner, M.A.; Manni, M.; Kim, W.S.; Shustov, A.R.; Horwitz, S.M.; Hitz, F.; Cabrera, M.E.; et al. Outcomes and prognostic factors in angioimmunoblastic T-cell lymphoma: Final report from the international T-cell Project. Blood 2021, 138, 213–220. [Google Scholar] [CrossRef]
- Delfau-Larue, M.H.; de Leval, L.; Joly, B.; Plonquet, A.; Challine, D.; Parrens, M.; Delmer, A.; Salles, G.; Morschhauser, F.; Delarue, R.; et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica 2012, 97, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Bennani, N.N.; Kim, H.J.; Pederson, L.D.; Atherton, P.J.; Micallef, I.N.; Thanarajasingam, G.; Nowakowski, G.S.; Witzig, T.; Feldman, A.L.; Ansell, S.M. Nivolumab in patients with relapsed or refractory peripheral T-cell lymphoma: Modest activity and cases of hyperprogression. J. Immunother. Cancer 2022, 10, e004984. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Sakata-Yanagimoto, M.; Fujisawa, M.; Nuhat, S.T.; Miyoshi, H.; Nannya, Y.; Hashimoto, K.; Fukumoto, K.; Bernard, O.A.; Kiyoki, Y.; et al. Dasatinib Is an Effective Treatment for Angioimmunoblastic T-cell Lymphoma. Cancer Res. 2020, 80, 1875–1884. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, O.A.; Falchi, L.; Lue, J.K.; Marchi, E.; Kinahan, C.; Sawas, A.; Deng, C.; Montanari, F.; Amengual, J.E.; Kim, H.A.; et al. Oral 5-azacytidine and romidepsin exhibit marked activity in patients with PTCL: A multicenter phase 1 study. Blood 2019, 134, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, K.; Okada, S.; Ichinohasama, R.; Yamaguchi, M.; Uchiyama, B.; Maeyama, T. Angioimmunoblastic T-cell lymphoma developed with lymphocytic pleural effusion. Intern. Med. 2007, 46, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Cheng, J.H.; Lin, M.S.; Dai, Y.C.; Hsiue, T.R. Eosinophilic pleural effusion as the first presentation of angioimmunoblastic T cell lymphoma. J. Formos. Med. Assoc. 2007, 106, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Maeda, K.; Hasunuma, K.; Takahashi, H.; Dambara, T.; Tamayose, K.; Oshimi, K.; Miyamoto, H.; Tominaga, S.; Uekusa, T.; et al. Idiopathic plasmacytic lymphadenopathy with polyclonal hyperimmunoglobulinemia and pleural effusion. Nihon Kokyuki Gakkai Zasshi 2000, 38, 288–292. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Nong, L.; Zhang, J.; Wang, W.; Wang, Q.; Zhang, Y.; Ren, S.; Wang, M. Plasmacytic Pleural Effusion as a Major Presentation of Angioimmunoblastic T-Cell Lymphoma: A Case Report. Curr. Oncol. 2022, 29, 7637-7644. https://doi.org/10.3390/curroncol29100603
Li B, Nong L, Zhang J, Wang W, Wang Q, Zhang Y, Ren S, Wang M. Plasmacytic Pleural Effusion as a Major Presentation of Angioimmunoblastic T-Cell Lymphoma: A Case Report. Current Oncology. 2022; 29(10):7637-7644. https://doi.org/10.3390/curroncol29100603
Chicago/Turabian StyleLi, Borui, Lin Nong, Jianhua Zhang, Wensheng Wang, Qian Wang, Yang Zhang, Shaomin Ren, and Mangju Wang. 2022. "Plasmacytic Pleural Effusion as a Major Presentation of Angioimmunoblastic T-Cell Lymphoma: A Case Report" Current Oncology 29, no. 10: 7637-7644. https://doi.org/10.3390/curroncol29100603
APA StyleLi, B., Nong, L., Zhang, J., Wang, W., Wang, Q., Zhang, Y., Ren, S., & Wang, M. (2022). Plasmacytic Pleural Effusion as a Major Presentation of Angioimmunoblastic T-Cell Lymphoma: A Case Report. Current Oncology, 29(10), 7637-7644. https://doi.org/10.3390/curroncol29100603



