The Feasibility of Immunocryosurgery in the Treatment of Non-Superficial, Facial Basal Cell Carcinoma That Relapsed after Standard Surgical Excision: An Experience Report from Two Centers
Abstract
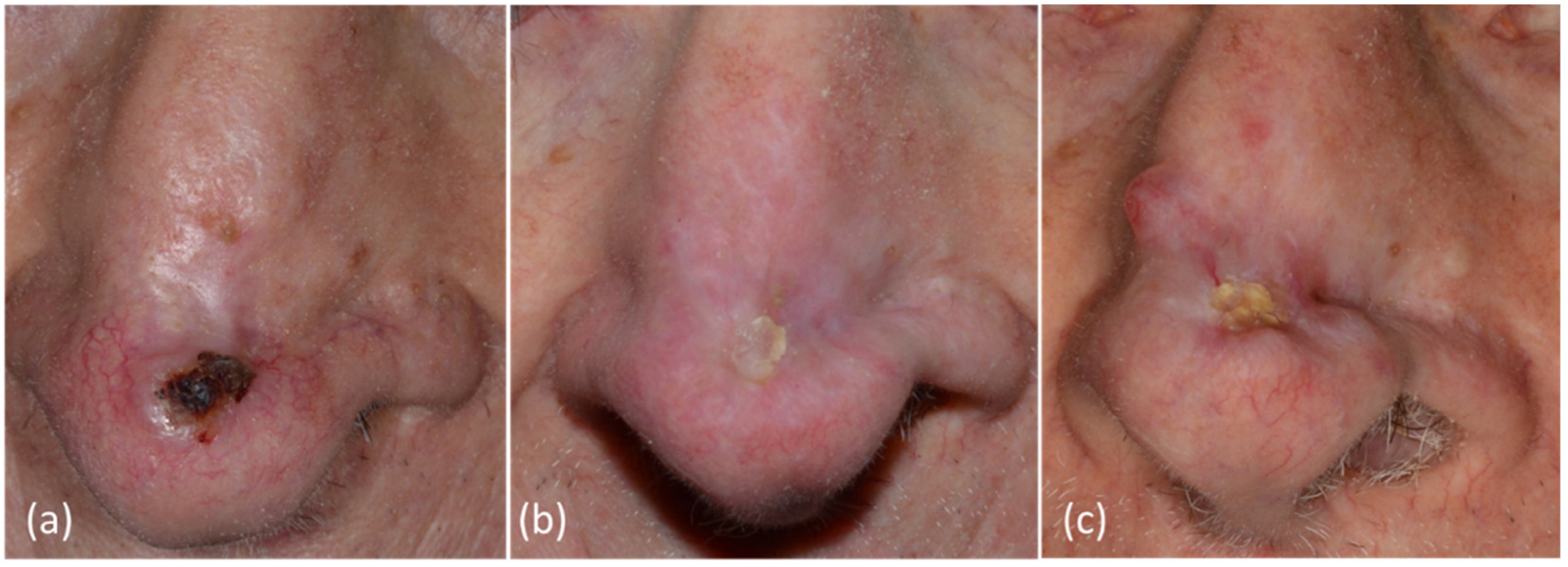
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaitanis, G.; Bassukas, I.D. A Review of Immunocryosurgery and a Practical Guide to Its Applications. Diseases 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Bassukas, I.D. Immunocryosurgery for non-superficial basal cell carcinoma: A prospective, open-label phase III study for tumours ≤2 cm in diameter. Acta Derm.-Venereol. 2014, 94, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Bassukas, I.D. Immunocryosurgery for non-superficial basal cell carcinomas ≤20 mm in maximal diameter: Five-year follow-up. J. Geriatr. Oncol. 2019, 10, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, K.; Krekels, G.A.; Nieman, F.H.; Ostertag, J.U.; Essers, B.A.B.; Dirksen, C.D.; Steijlen, P.M.; Vermeulen, A.; Neumann, H.; Kelleners-Smeets, N.W.J. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008, 9, 1149–1156. [Google Scholar] [CrossRef]
- Bassukas, I.D.; Gaitanis, G. Combination of cryosurgery and topical imiquimod: Does timing matter for successful immunocryosurgery? Cryobiology 2009, 59, 116–117. [Google Scholar] [CrossRef]
- Gaitanis, G.; Alexopoulos, E.C.; Bassukas, I.D. Cryosurgery is more effective in the treatment of primary, non-superficial basal cell carcinomas when applied during and not prior to a five week imiquimod course: A randomized, prospective, open-label study. Eur. J. Dermatol. 2011, 21, 952–958. [Google Scholar] [CrossRef]
- Nomikos, K.; Lampri, E.; Spyridonos, P.; Bassukas, I.D. Alterations in the inflammatory cells infiltrating basal cell carcinomas during immunocryosurgery. Arch. Dermatol. Res. 2019, 311, 499–504. [Google Scholar] [CrossRef]
- Gaitanis, G.; Ganiatsa, A.; Vartholomatos, G.; Karamoutsios, A.; Pappas, P.; Bassukas, I.D. Immunocryosurgery for basal cell carcinoma: Impact on circulating CD4 + CD25 high T regulatory cells and serum cytokines. Eur. J. Dermatol. 2020, 30, 605–606. [Google Scholar] [CrossRef]
- Nakuçi, M.; Bassukas, I.D. Office-based treatment of basal cell carcinoma with immunocryosurgery: Feasibility and efficacy. Acta Dermatovenerol. Alp. Panon. Adriat. 2013, 22, 35–38. [Google Scholar]
- Gaitanis, G.; Bassukas, I.D. Immunocryosurgery-An effective combinational modality for Bowen’s disease. Dermatol. Ther. 2016, 29, 334–337. [Google Scholar] [CrossRef]
- Tsironi, T.; Gaitanis, G.; Pappas, C.; Koutlas, V.; Dounousi, E.; Bassukas, I.D. Immunocryosurgery is a safe and feasible treatment for basal cell carcinoma and Bowen disease in renal transplant recipients. Dermatol. Ther. 2022, 35, e15405. [Google Scholar] [CrossRef] [PubMed]
- Oro-Ayude, M.; Gonzalez-Sixto, B.; Faraldo-Lorenzo, J.M.; Feal, C.; Flórez, A. Periocular lentigo maligna successfully treated with immunocryosurgery. Dermatol. Ther. 2021, 34, e14561. [Google Scholar] [CrossRef] [PubMed]
- Matas-Nadal, C.; Sòria, X.; García-de-la-Fuente, M.R.; Huerva, V.; Ortega, E.; Vilardell, F.; Gatius, S.; Casanova, J.M.; Martí, R.M. Immunocryosurgery as monotherapy for lentigo maligna or combined with surgical excision for lentigo maligna melanoma. J. Dermatol. 2018, 45, 564–570. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Gaitanis, G.; Markatseli, T.E.; Kempf, W.; Bassukas, I.D. In transit recurrence of merkel cell carcinoma associated with polyarthritis effectively treated with immunocryosurgery. Acta Derm. Venereol. 2014, 94, 739–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitanis, G.; Vlachos, C.; Bassukas, I.D. Individualized, Adjuvant-Intensified Immunocryosurgery for the Treatment of Squamous Cell Carcinoma in the Oldest Old: An Exploratory Case Series. Actas Dermosifiliogr. 2022, 113, 750–753. [Google Scholar] [CrossRef]
- Gaitanis, G.; Kalogeropoulos, C.D.; Bassukas, I.D. Cryosurgery during Imiquimod (Immunocryosurgery) for Periocular Basal Cell Carcinomas: An Efficacious Minimally Invasive Treatment Alternative. Dermatology 2016, 232, 17–21. [Google Scholar] [CrossRef]
- Ceder, H.; Grönberg, M.; Paoli, J. Mohs micrographic surgery for primary versus recurrent or incompletely excised facial high-risk basal cell carcinomas. Acta Derm.-Venereol. 2021, 101, adv00381. [Google Scholar] [CrossRef]
- Trakatelli, M.; Morton, C.; Nagore, E.; Ulrich, C.; Del Marmol, V.; Peris, K.; Basset-Seguin, N. Update of the European guidelines for basal cell carcinoma management. Eur. J. Dermatol. 2014, 24, 312–329. [Google Scholar] [CrossRef]
- Linos, E.; Schroeder, S.A.; Chren, M.M. Potential overdiagnosis of basal cell carcinoma in older patients with limited life expectancy. J. Am. Med. Assoc. 2014, 312, 997–998. [Google Scholar] [CrossRef]
- Gaitanis, G.; Nomikos, K.; Vava, E.; Alexopoulos, E.C.; Bassukas, I.D. Immunocryosurgery for basal cell carcinoma: Results of a pilot, prospective, open-label study of cryosurgery during continued imiquimod application. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1427–1430. [Google Scholar] [CrossRef]
- Gaitanis, G.; Nomikos, K.; Vlachos, C.; Bassukas, I.D. Immunocryosurgery for patients with therapeutically challenging basal cell carcinomas: Report of two representative cases. J. Dermatol. Treat. 2012, 23, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Bath-Hextall, F.; Ozolins, M.; Armstrong, S.J.; Colver, G.B.; Perkins, W.; Miller, P.S.J.; Williams, H.C. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 96–105. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peris, K.; Licitra, L.; Ascierto, P.A.; Corvò, R.; Simonacci, M.; Picciotto, F.; Gualdi, G.; Pellacani, G.; Santoro, A. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: An expert panel consensus. Future Oncol. 2015, 11, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, I.A.; Yoo, S.; Kudchadkar, R.; Guillen, J.; Rogers, G.; Chang, A.L.S.; Guenthner, S.; Raskin, B.; Dawson, K.; Mun, Y.; et al. Real-world assessment and treatment of locally advanced basal cell carcinoma: Findings from the RegiSONIC disease registry. PLoS ONE 2022, 17, e0262151. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 574–597. [Google Scholar] [CrossRef]
- Pellegrini, C.; Maturo, M.; Di Nardo, L.; Ciciarelli, V.; Gutiérrez García-Rodrigo, C.; Fargnoli, M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef] [Green Version]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Schön, M.P.; Schön, M. Imiquimod: Mode of action. Br. J. Dermatol. 2007, 157 (Suppl. S2), 8–13. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef]


| Follow-up [months] | Cases in Follow-up (% of Initial Cases) | BCC Sites not Disease Free (in Interval) | Disease-Free [S.E.] % | |
|---|---|---|---|---|
| All Cases (n = 27) | Cleared BCC (n = 22) # | |||
| 0 * | 22 (81.5) | 5 | 81.5 [7.5] | 100 |
| 12 | 21 (77.8) | 1 &,$ | 77.8 [8.0] | 95.5 [4.4] |
| 18 | 16 (59.3) | 0 | ||
| 24 | 15 (55.6) | 0 | ||
| 36 | 11 (40.7) | 1 &,† | 72.2 [9.2] | |
| 48 | 9 (33.3) | 0 | ||
| 60 | 4 (14.8) | 1 &,$ | 60.2 [13.4] | 81.8 [13.2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitanis, G.; Zampeta, A.; Tsintzou, P.; Fillis, G.; Seretis, K.; Feldmeyer, L.; Bassukas, I. The Feasibility of Immunocryosurgery in the Treatment of Non-Superficial, Facial Basal Cell Carcinoma That Relapsed after Standard Surgical Excision: An Experience Report from Two Centers. Curr. Oncol. 2022, 29, 8475-8482. https://doi.org/10.3390/curroncol29110668
Gaitanis G, Zampeta A, Tsintzou P, Fillis G, Seretis K, Feldmeyer L, Bassukas I. The Feasibility of Immunocryosurgery in the Treatment of Non-Superficial, Facial Basal Cell Carcinoma That Relapsed after Standard Surgical Excision: An Experience Report from Two Centers. Current Oncology. 2022; 29(11):8475-8482. https://doi.org/10.3390/curroncol29110668
Chicago/Turabian StyleGaitanis, Georgios, Athanasia Zampeta, Panagiota Tsintzou, Grigorios Fillis, Konstantinos Seretis, Laurence Feldmeyer, and Ioannis Bassukas. 2022. "The Feasibility of Immunocryosurgery in the Treatment of Non-Superficial, Facial Basal Cell Carcinoma That Relapsed after Standard Surgical Excision: An Experience Report from Two Centers" Current Oncology 29, no. 11: 8475-8482. https://doi.org/10.3390/curroncol29110668





