The Impact of Computed Tomography Measurements of Sarcopenia on Postoperative and Oncologic Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
Abstract
:1. Introduction
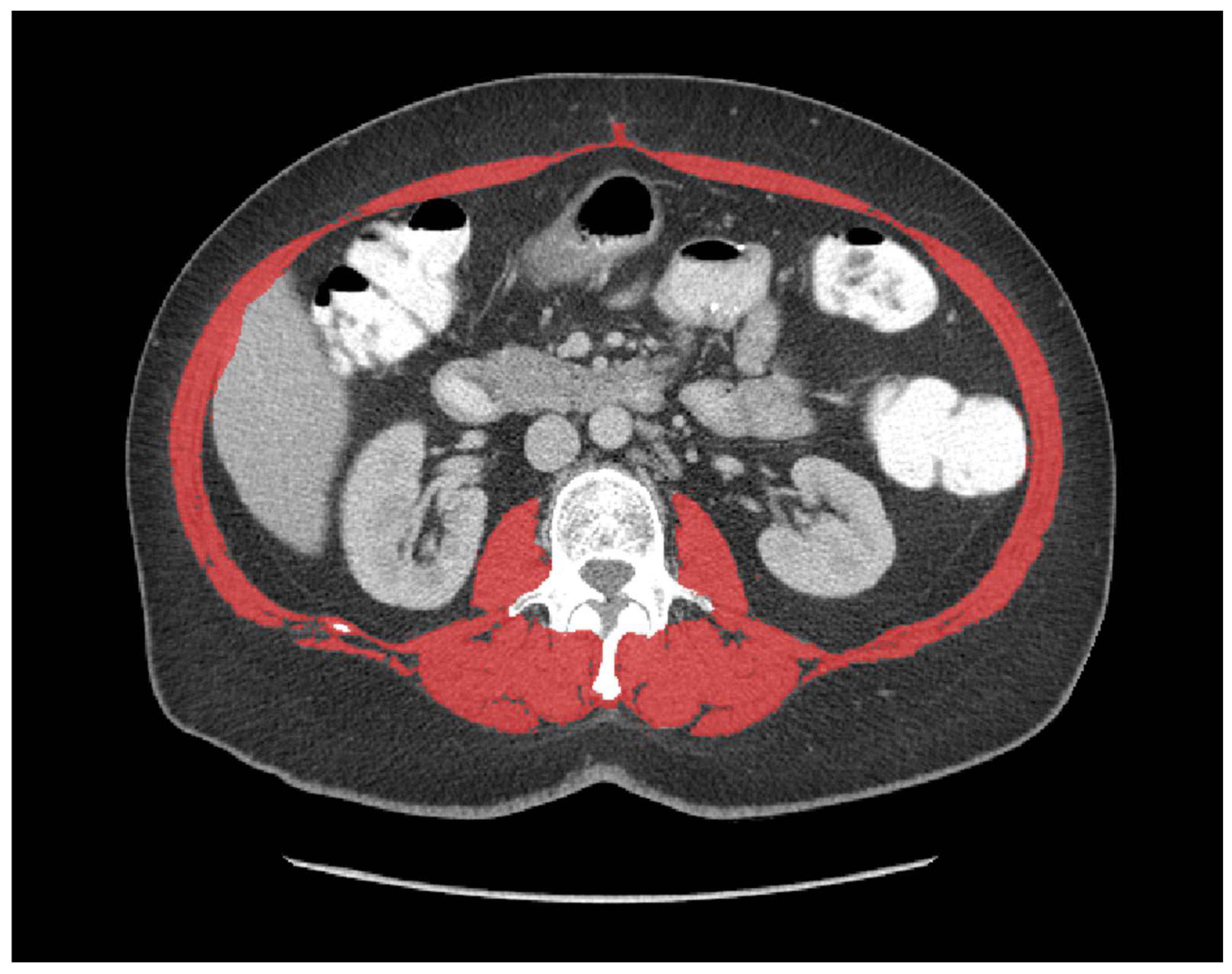
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Postoperative Outcomes
3.3. Multivariate Analysis and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, J.; Lussiez, A.; Sullivan, J.; Wang, S.; Englesbe, M. Implications of Sarcopenia in Major Surgery. Nutr. Clin. Pract. 2015, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, Dynapenia, and the Impact of Advancing Age on Human Skeletal Muscle Size and Strength; a Quantitative Review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voron, T.; Tselikas, L.; Pietrasz, D.; Pigneur, F.; Laurent, A.; Compagnon, P.; Salloum, C.; Luciani, A.; Azoulay, D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2015, 261, 1173–1183. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.D.; van Vledder, M.G.; Tsai, S.; de Jong, M.C.; Makary, M.; Ng, J.; Edil, B.H.; Wolfgang, C.L.; Schulick, R.D.; Choti, M.A.; et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. Hpb 2011, 13, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of Sarcopenia on Outcomes Following Resection of Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Jones, K.; Gordon-Weeks, A.; Coleman, C.; Silva, M. Radiologically Determined Sarcopenia Predicts Morbidity and Mortality Following Abdominal Surgery: A Systematic Review and Meta-Analysis. World J. Surg. 2017, 41, 2266–2279. [Google Scholar] [CrossRef] [Green Version]
- Bushati, M.; Rovers, K.P.; Sommariva, A.; Sugarbaker, P.H.; Morris, D.L.; Yonemura, Y.; Quadros, C.A.; Somashekhar, S.P.; Ceelen, W.; Dubé, P.; et al. The Current Practice of Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 2018, 44, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Bonomi, S.; Grassi, A.; Virzì, S.; Leo, E.; Deraco, M. Postoperative Complications After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Affect Long-term Outcome of Patients With Peritoneal Metastases From Colorectal Cancer. Dis. Colon Rectum 2014, 57, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Votanopoulos, K.I.; Newman, N.A.; Russell, G.; Ihemelandu, C.; Shen, P.; Stewart, J.H.; Levine, E.A. Outcomes of Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients Older Than 70 Years; Survival Benefit at Considerable Morbidity and Mortality. Ann. Surg. Oncol. 2013, 20, 3497–3503. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the Treatment of Peritoneal Carcinomatosis by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Still be Regarded as a Highly Morbid Procedure?: A Systematic Review of Morbidity and Mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef]
- Galan, A.; Rousset, P.; Mercier, F.; Képénékian, V.; Valette, P.-J.; Glehen, O.; Passot, G. Overall survival of pseudomyxoma peritonei and peritoneal mesothelioma patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy can be predicted by computed tomography quantified sarcopenia. Eur. J. Surg. Oncol. 2018, 44, 1818–1823. [Google Scholar] [CrossRef]
- Agalar, C.; Sokmen, S.; Arslan, C.; Altay, C.; Basara, I.; Canda, A.E.; Obuz, F. The impact of sarcopenia on morbidity and long-term survival among patients with peritoneal metastases of colorectal origin treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A 10-year longitudinal analysis of a single-center experience. Tech. Coloproctol. 2020, 24, 301–308. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.A.; Braam, H.J.; van Oudheusden, T.R.; Vestering, A.; Bollen, T.L.; Wiezer, M.J.; de Hingh, I.H.J.T.; van Ramshorst, B.; Boerma, D. Skeletal Muscle Depletion is Associated with Severe Postoperative Complications in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 3625–3631. [Google Scholar] [CrossRef]
- Chemama, S.; Bayar, M.A.; Lanoy, E.; Ammari, S.; Stoclin, A.; Goéré, D.; Elias, D.; Raynard, B.; Antoun, S. Sarcopenia is Associated with Chemotherapy Toxicity in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Banaste, N.; Rousset, P.; Mercier, F.; Rieussec, C.; Valette, P.-J.; Glehen, O.; Passot, G. Preoperative nutritional risk assessment in patients undergoing cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for colorectal carcinomatosis. Int. J. Hyperther. 2017, 34, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portilla, A.G.; Shigeki, K.; Dario, B.; Marcello, D. The intraoperative staging systems in the management of peritoneal surface malignancy. J. Surg. Oncol. 2008, 98, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef]
- Strijker, D.; Meijerink, W.J.H.J.; Bremers, A.J.A.; de Reuver, P.; van Laarhoven, C.J.H.M.; van den Heuvel, B. Prehabilitation to improve postoperative outcomes in patients with peritoneal carcinomatosis undergoing hyperthermic intraperitoneal chemotherapy (HIPEC): A scoping review. Eur. J. Surg. Oncol. 2021, 48, 657–665. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; de Carvalho, I.A.; Thiyagarajan, J.A.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Bahat, G.; Tufan, A.; Kilic, C.; Karan, M.A.; Cruz-Jentoft, A.J. Prevalence of sarcopenia and its components in community-dwelling outpatient older adults and their relation with functionality. Aging Male 2018, 23, 424–430. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Zhang, L.; Zhou, D.; Tian, F.; Gao, T.; Tian, H.; Hu, H.; Gong, F.; Guo, D.; et al. Effect of Early vs. Late Supplemental Parenteral Nutrition in Patients Undergoing Abdominal Surgery. JAMA Surg. 2022, 157, 384–393. [Google Scholar] [CrossRef]
- Smeenk, R.M.; Verwaal, V.J.; Zoetmulder, F.A.N. Learning curve of combined modality treatment in peritoneal surface disease. Br. J. Surg. 2007, 94, 1408–1414. [Google Scholar] [CrossRef]


| Variable | All (n = 312) | Sarcopenia (n = 88) | No Sarcopenia (n = 224) | p Value |
|---|---|---|---|---|
| Mean age at time of surgery, years (SD) | 57.6 (10.3) | 58.1 (10.7) | 57.5 (10.2) | 0.63 |
| (min–max) | (21.7–79.3) | (21.7–73.7) | (24.7–79.3) | |
| Male sex, n (%) | 107 (34.3) | 41 (46.6) | 66 (29.5) | 0.0053 |
| Mean delay from scan to surgery, days (SD) | 44.2 (37.0) | 42.1 (28.6) | 45.0 (39.9) | 0.53 |
| (min, max) | (1–245) | (1–122) | (1–245) | |
| Mean BMI, kg/m2 (SD) | 26.6 (5.5) | 23.6 (3.9) | 27.7 (5.7) | <0.0001 |
| (min–max) | (15.5–46.9) | (20.7–25.5) | (17.5–46.9) | |
| Mean SMI, cm2/m2 (SD) | 48.7 (10.3) | 41.4 (7.3) | 51.2 (9.8) | <0.0001 |
| (min–max) | (30.0–81.3) | (30.0–75.1) | (38.5–81.3) | |
| Origin of PC, n (%) | 0.43 | |||
| Colorectal | 126 (40.4) | 37 (42.0) | 89 (39.7) | |
| Appendix | 88 (28.2) | 27 (30.7) | 61 (27.2) | |
| Ovarian | 66 (21.2) | 15 (17.0) | 51 (22.8) | |
| Peritoneal | 24 (7.7) | 5 (5.7) | 19 (8.5) | |
| Other * | 8 (2.6) | 4 (4.5) | 4 (1.8) | |
| Timing of PC, n (%) | 0.45 | |||
| Synchronous | 168 (53.9) | 52 (59.0) | 116 (51.8) | |
| Metachronous | 120 (38.5) | 31 (35.2) | 89 (39.7) | |
| Primary | 24 (7.7) | 5 (5.7) | 19 (8.5) |
| Variable | All (n = 312) | Sarcopenia (n = 88) | No Sarcopenia (n = 224) | p Value |
|---|---|---|---|---|
| Mean operative time, min (SD) | 423.8 (118.8) | 422.1 (128.8) | 424.5 (114.6) | 0.87 |
| (min–max) | (100–870) | (180–840) | (100–870) | |
| Mean estimated blood loss, ml (SD) | 877.1 (739.2) | 929.3 (909.9) | 856.6 (661.5) | 0.44 |
| (min–max) | (50–4800) | (50–4800) | (50–4000) | |
| CC score | 0.059 | |||
| 0 | 273 (87.5%) | 82 (93.2%) | 191 (85.3%) | |
| 1–2 | 39 (12.5%) | 6 (6.8%) | 33 (14.7%) | |
| Mean intraoperative PCI (SD) | 12.0 (8.9) | 11.2 (9.0) | 12.3 (8.8) | 0.34 |
| (min–max) | (0–39) | (0–39) | (0–39) | |
| Clavien-Dindo ≥ III, n (%) | 66 (21.2%) | 14 (15.9%) | 52 (23.2%) | 0.17 |
| Median CCI score [IQR] | 20.9 [30.8] | 20.9 [29.6] | 20.9 [32.0] | 0.47 |
| (min–max) | (0–100) | (0–100) | (0–100) | |
| HIPEC-related toxicity, n (%) | 37 (11.9%) | 9 (10.2%) | 28 (12.5%) | 0.70 |
| Mean length of stay, days (SD) | 17.4 (10.9) | 17.9 (12.3) | 17.2 (10.3) | 0.60 |
| (min–max) | (3–80) | (3–80) | (5–65) | |
| Mean duration of parenteral nutrition, days (SD) | 13.5 (11.7) | 11.3 (7.1) | 11.1 (7.6) | 0.83 |
| (min–max) | (2–60) | (2–51) | (3–60) | |
| Death (Clavien-Dindo V), n (%) | 3 (1.0%) | 1 (1.1%) | 2 (0.9%) | 0.84 |
| Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Sex (female) | 1.10 | 0.59–2.06 | 0.77 |
| Age (years) | 1.02 | 0.99–1.05 | 0.29 |
| BMI (kg/m2) | 1.02 | 0.97–1.08 | 0.42 |
| Intraoperative PCI | 1.05 | 1.01–1.08 | 0.007 |
| Sarcopenia | 0.70 | 0.33–1.41 | 0.33 |
| Blood loss | 1.00 | 0.99–1.00 | 0.59 |
| Variable | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| Sex (female) | 0.78 | 0.46–1.34 | 0.37 |
| Age (years) | 1.00 | 0.98–1.03 | 0.77 |
| BMI (kg/m2) | 0.99 | 0.95–1.04 | 0.82 |
| Intraoperative PCI | 1.08 | 1.05–1.12 | <0.0001 |
| Sarcopenia | 1.17 | 0.68–2.0 | 0.57 |
| Origin of PC | |||
| Colorectal vs. appendix | 0.19 | 0.09–0.37 | <0.0001 |
| Colorectal vs. ovarian | 0.37 | 0.18–0.77 | <0.0001 |
| Colorectal vs. peritoneal | 0.12 | 0.04–0.32 | 0.0090 |
| Colorectal vs. other * | 1.93 | 0.64–4.75 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khaldi, M.; Fellouah, M.; Drolet, P.; Côté, J.; Trilling, B.; Brind’Amour, A.; Dugas, A.; Tremblay, J.-F.; Fortin, S.; De Guerké, L.; et al. The Impact of Computed Tomography Measurements of Sarcopenia on Postoperative and Oncologic Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Curr. Oncol. 2022, 29, 9314-9324. https://doi.org/10.3390/curroncol29120730
Al Khaldi M, Fellouah M, Drolet P, Côté J, Trilling B, Brind’Amour A, Dugas A, Tremblay J-F, Fortin S, De Guerké L, et al. The Impact of Computed Tomography Measurements of Sarcopenia on Postoperative and Oncologic Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Current Oncology. 2022; 29(12):9314-9324. https://doi.org/10.3390/curroncol29120730
Chicago/Turabian StyleAl Khaldi, Maher, Massine Fellouah, Pierre Drolet, Julien Côté, Bertrand Trilling, Alexandre Brind’Amour, Alexandre Dugas, Jean-François Tremblay, Suzanne Fortin, Lara De Guerké, and et al. 2022. "The Impact of Computed Tomography Measurements of Sarcopenia on Postoperative and Oncologic Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy" Current Oncology 29, no. 12: 9314-9324. https://doi.org/10.3390/curroncol29120730
APA StyleAl Khaldi, M., Fellouah, M., Drolet, P., Côté, J., Trilling, B., Brind’Amour, A., Dugas, A., Tremblay, J.-F., Fortin, S., De Guerké, L., Auclair, M.-H., Dubé, P., Soucisse, M., & Sideris, L. (2022). The Impact of Computed Tomography Measurements of Sarcopenia on Postoperative and Oncologic Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Current Oncology, 29(12), 9314-9324. https://doi.org/10.3390/curroncol29120730






