Abstract
Purpose: To review the successes and challenges of integrating systematic reviews (SRs) into the Rethinking Clinical Trials (REaCT) Program. Methods: All REaCT program SRs were evaluated and descriptive summaries presented. Results: Twenty-two SRs have been performed evaluating standard of care interventions for the management of: breast cancer (n = 15), all tumour sites (n = 4), breast and prostate cancers (n = 2), and prostate cancer (n = 1). The majority of SRs were related to supportive care (n = 14) and survivorship (n = 5) interventions and most (19/22, 86%) confirmed the existence of uncertainty relating to the clinical question addressed in the SR. Most SRs (15/22, 68%) provided specific recommendations for future studies and results were incorporated into peer-reviewed grant applications (n = 6) and clinical trial design (n = 12). In 12/22 of the SRs, the first author was a trainee. All SRs followed PRISMA guidelines. Conclusion: SRs are important for identifying and confirming clinical equipoise and designing trials. SRs provide an excellent opportunity for trainees to participate in research.
1. Introduction
The Rethinking Clinical Trials (REaCT) program is a novel Canadian-led clinical trials platform that focuses on comparing existing standard of care interventions for cancer management [1]. The program was established in 2014 to overcome many of the traditional challenges in clinical trial performance [2,3,4]. The REaCT process begins with end-user surveys to identify areas of clinical equipoise (i.e., topics involving varied clinical perspectives and a lack of consensus among the clinical community)and the identification research topics that patients, their families, and health care providers feel to be important [2,5,6,7].
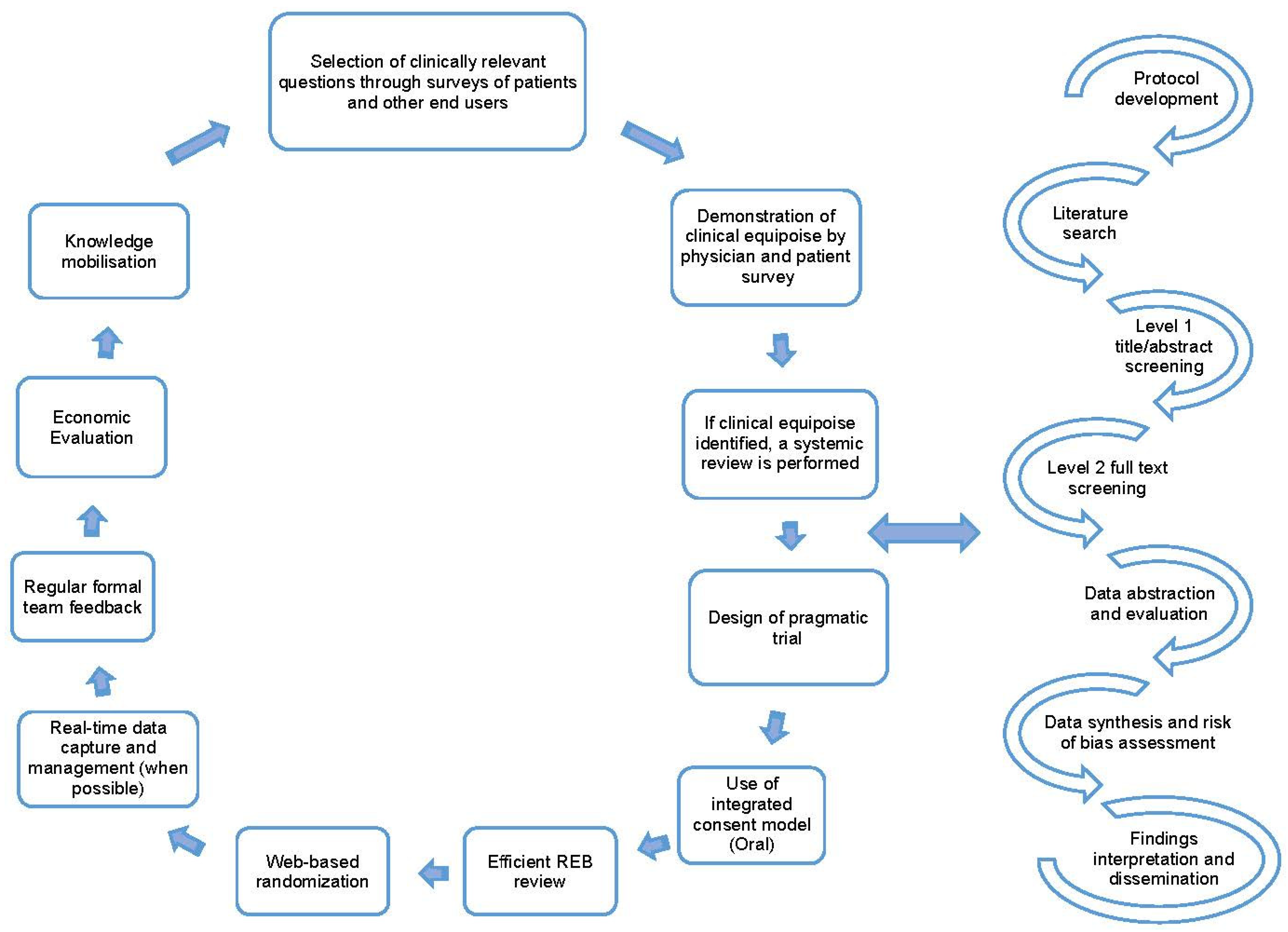
Once clinical equipoise is identified, and the topic is deemed important to patient care, a literature search is performed to identify if an up-to-date systematic review on the topic is available. With these findings we then perform either an updated systematic review (when an older review is available) or a de novo review (when no prior reviews are identified) [1,8]. A systematic review is a comprehensive approach to reviewing the available evidence [9] and follow a well-established process that includes protocol development, search design and execution, level 1 and 2 screening of citations/full-text articles, data abstraction of study characteristics and outcomes, summary and synthesis of results, interpretation of findings, and dissemination of findings [10]. The key steps in the REaCT program process and the processes followed when performing systematic reviews are detailed in Figure 1.
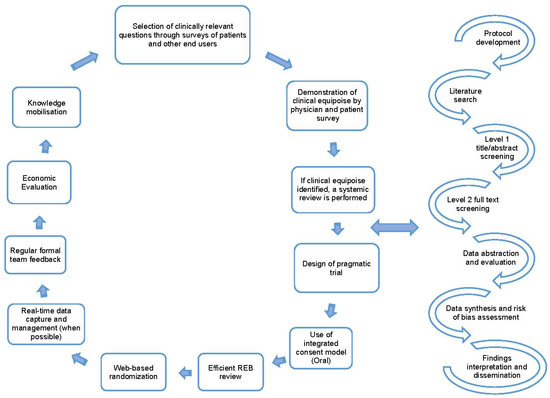
Figure 1.
Key tenants for the REaCT Program; adapted with permission from [1,8] and the road map for systematic reviews.
Systematic reviews can lead to meta-analyses of outcome data to produce a single quantitative estimate from a synthesis of two or more studies [10]. The approach used to pool results from different studies depends on the similarity of the different study populations, the study methods and the clinical outcomes reported between the studies [11,12,13,14]. If, however, there are no overlapping clinical outcomes, or the presence of siginificant clinical heterogeneity in study populations evaluated in the different trials, descriptive methods for synthesis can also used [15]. Through these processes systematic reviews can provide a valuable summary of the available evidence for a research question of interest to patients, researchers, clinicians, and other stakeholders and can also be valuable in the process of trial design.
The current study evaluated the successes and challenges of integrating systematic reviews into the REaCT program. We also hope to demonstrate how systematic reviews should be a crucial step in the REaCT process, allowing us to confirm whether areas of patient and health care provider perceived clinical equipoise exist. In addition, we will show how performing a systematic review can assist in the design of potential clinical trials through the identification of previously unanswered questions and the choice of endpoints for such trials.
2. Materials and Methods
2.1. Search Strategy and Data Extraction
We reviewed all of the systematic reviews that have performed through the REaCT program from instigation until December 2021. As several co-authors (MC, BH, LV) have been involved in all the systematic reviews conducted by the REaCT program no formal database search to identify these reviews was required. The data was extracted by two authors (BA and MC) and reviewed by other team members (BH, ML, LV). The data extracted from each systematic review included: the primary research question, cancer care setting (e.g., adjuvant, metastatic, survivorship, palliative care), reporting strategy, approach to synthesis, and summary of findings. In addition, data was collected evaluating: the number of abstracts screened (phase I), articles (phase II) and the included studies, number of authors (staff and trainees), and training level of the first author (resident, fellow, staff) for each of the systematic reviews.
2.2. Review Outcomes
The authors were interested in whether or not the individual systematic reviews identified, confirmed or answered the clinical questions identified by stakeholders (patients, their families and healthcare provider surveys). Furthermore, the outcome of each systematic review was also sought, including recommendations to apply for peer-reviewed grant funding to answer the particular clinical question as well as if systematic review affected future and clinical trial design, and/or the decision to try and perform a definitive trial. Descriptive summaries were used to present the study findings.
3. Results
3.1. Reviews Characteristics
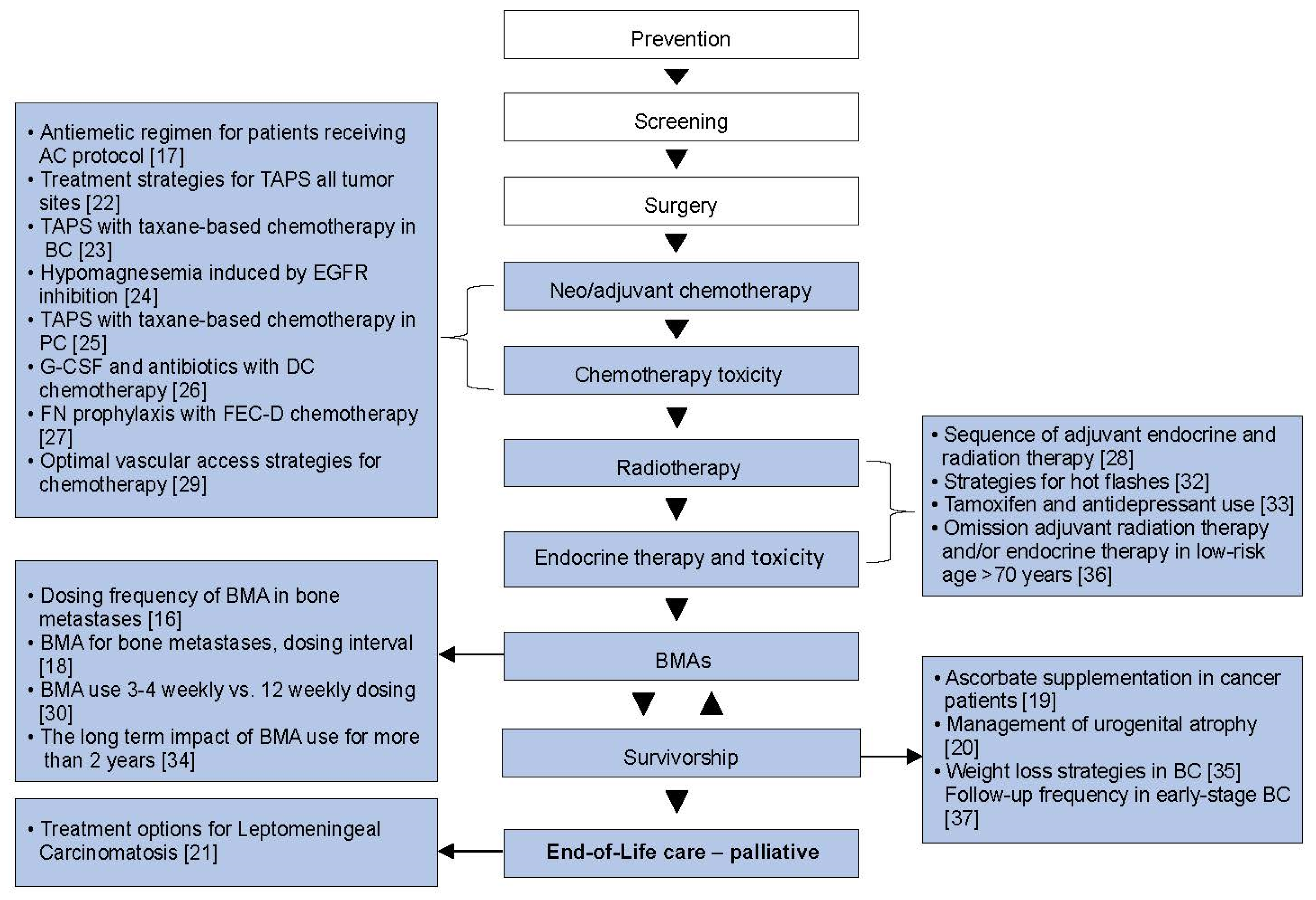
Out of 28 areas of clinical equipoise identified from surveys of patients and healthcare providers [7], 22 systemic reviews have been performed and published [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Of these, 18 were new reviews, while 4 were updated reviews of previously published reviews. The reviews were related to areas of clinical equipoise in: breast cancer (n = 15), all tumour sites (n = 4), breast and prostate cancers (n = 2), and prostate cancer (n = 1) management. These reviews covered topics regarding; adjuvant therapy [28,36], adjuvant supportive care [17,21,26,27,29], metastatic [21], palliative supportive care [16,18,25,30,34], both adjuvant and palliative supportive care [19,22,23,24,31], and survivorship [20,32,33,35,37]. The details of each review are shown in Table 1, and where each question lay in the cancer journey is shown in Figure 2.

Table 1.
Summary of the systematic review (n = 22).
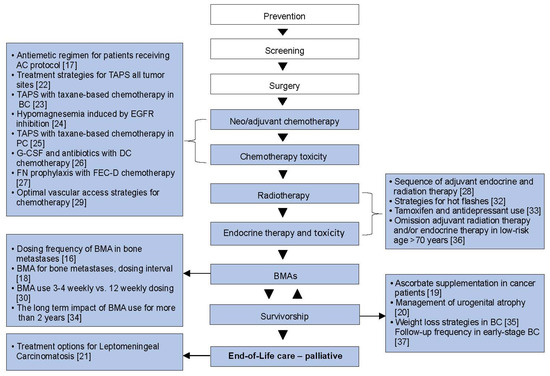
Figure 2.
The cancer journey: where the systemic reviews fit. BC, breast cancer; BMAs, bone-modifying agents; DC, docetaxel–cyclophosphamide; EGFR, epidermal growth factor receptor; FEC-D, 5-fluorouracil, epirubicin; PC, prostate cancer; TAPS = taxane-associated pain syndrome [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,32,33,34,35,36,37].
The median number of abstracts assessed for phase I screening was 1447 (range, 113–3860), while a median of 65 (range, 7–640) full-text articles were reviewed for phase II screening. The median number of included articles was 11 (range, 4–173). All reviews followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. However, one review did not specify the reporting guidelines, but the methodology was consistent with PRISMA guidelines [24]. The median number of authors in each publication was 12 (range, 5–18), and of these, 5 (range, 1–9) were trainees (i.e., a resident or fellow). The first author was a trainee in 54% (12/22) of reviews.
3.2. Impact of Systemic Reviews
Most of the systematic reviews (19/22, 86%) confirmed the presence of clinical equipoise, and only three provided strong recommendations [25,27,30]. LeVasseur et al. conducted a systematic review with network meta-analyses that included 98 randomised controlled trials (RCTs) and concluded that dietary and combination interventions of diet and exercise significantly improved anthropometric measures compared to standard care [35]. Surujballi et al. performed a systematic review that included six RCTs and one prospective cohort study, and concluded that reduced frequency of follow-up of early stage breast cancer has no adverse effects on breast cancer-related outcomes [37]. Bradbury et al. conducted a systematic review that included 15 studies (2 RCTs, 10 retrospective cohorts, and 3 case–control) that concluded that concurrent use of tamoxifen and antidepressants is most likely safe for breast cancer-related outcomes [33].
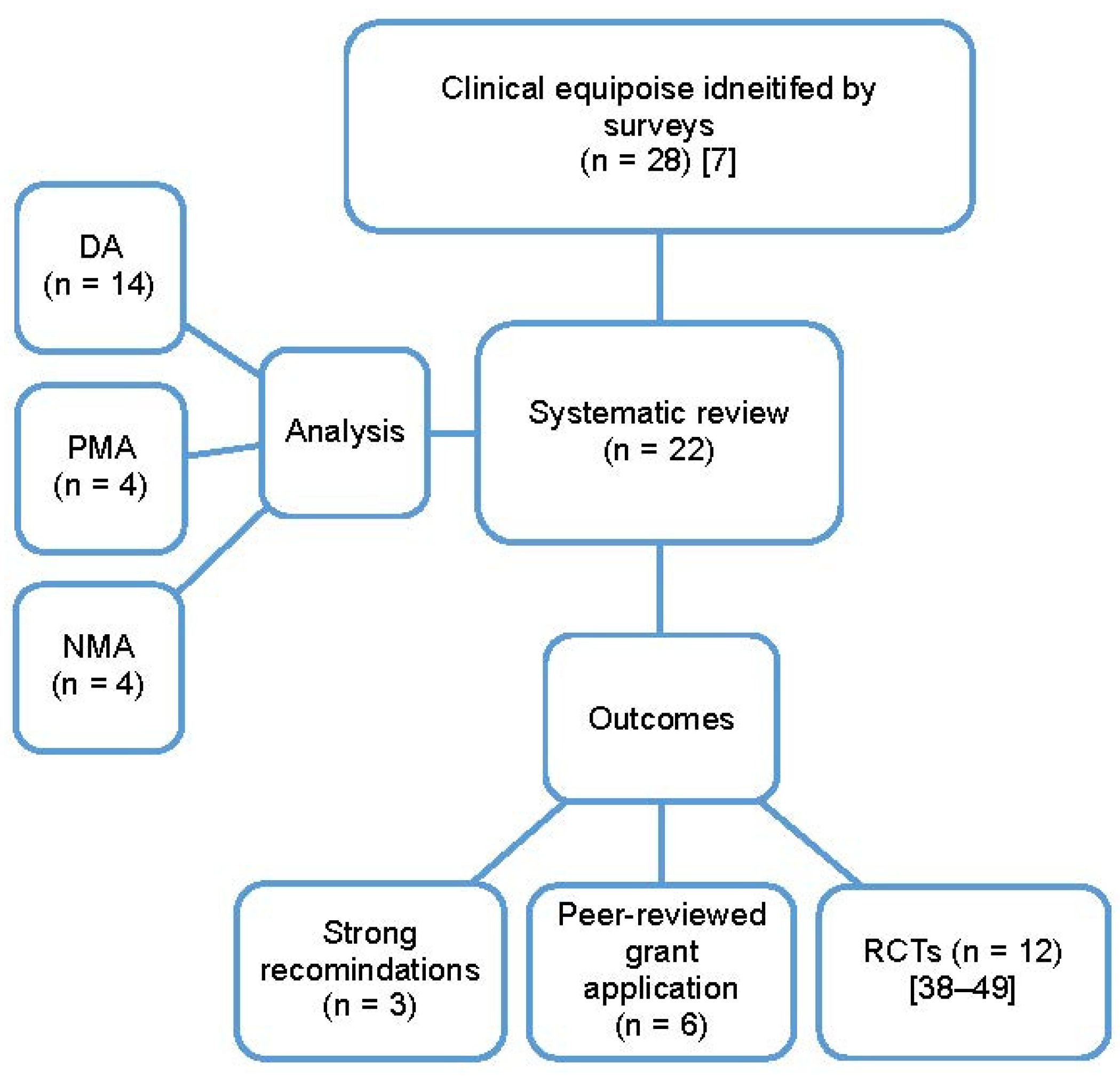
The cited factors that led to the inability to provide strong recommendations from systematic reviews included: heterogeneity in the reporting of outcomes between the included studies (16/22, 72%), differences in study types and populations (14/22, 63%), as well as the lack of reporting or variation in the reporting of variables potentially associated with different outcomes (16/22, 72%). Other limitations included the paucity of relevant published studies (10/22, 45%), and the lack of RCT data (7/22, 31%). Most of the systematic reviews performed cited that multiple limiting factors to the interpretation of data were present. Most studies (n = 14) used a descriptive approach to synthesis due to clinical, methodologic and/or statistical heterogeneity amongst the included studies. There was sufficient data for 4 reviews with pairwise meta-analysis [17,20,32,35] and 4 for network meta-analyses [18,30,33,36]. For all the systematic reviews performed, the authors recommended that further studies were needed, even for those that provided a firm conclusion. Reasons for further studies included: specifically designed RCT to measure the magnitude of benefit with different measures [35], to strengthen the current evidence [33], and to assess the impact of health economic aspects [37]. In 15 (68%) reviews, the authors suggested specific recommendations for future clinical trials; the findings of these reviews were incorporated into 6 peer-reviewed grant applications and the design of 12 clinical trials (6 RCTs were published [42,43,44,45,48,50], and 6 are ongoing [38,40,41,46,47,49]), (Figure 3).
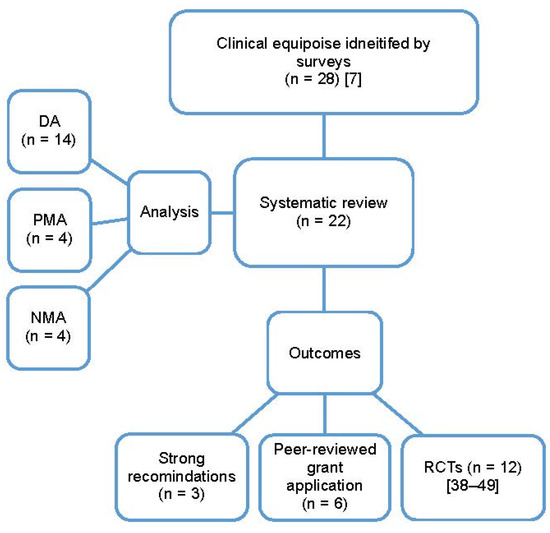
Figure 3.
The type of analysis and outcomes of the systematic reviews performed and published based on clinical equipoise identified by surveys; DA, descriptive analysis; NMA, network meta-analysis; PMA, pairwise meta-analysis; RCTs, randomized clinical trials [7,38,40,41,42,43,44,45,46,47,48,49,50].
4. Discussion
The Rethinking Clinical Trials (REaCT) Program was created to overcome many of the barriers in traditional clinical trial design and performance in oncology. The REaCT program is the largest pragmatic cancer clinical trials program in Canada, with more than 4000 patients participating in clinical trials at 16 Canadian centres [51]. As part of the REaCT process, systematic reviews are a key step to address whether areas of patient- and health care provider-perceived clinical equipoise exist. Systematic reviews can also help in the design of potential future clinical trials through the identification of previously unanswered questions as well as challenges faced in previous studies and the choice of endpoints for such trials. As a result most topics showing clinical equipoise that were identified from surveys of patients and health0care providers [7] resulted in 18 new systematic reviews and 4 updates of previously published systematic reviews. So far, these reviews have led to 6 peer-reviewed grant applications and 12 RCTs [38,40,41,42,43,44,45,46,47,48,49,50]. The involvement of trainees in these reviews was important and in almost half of these reviews, trainees took a senior role in the particular review.
It is important to note that in the majority of systemic reviews, meta-analysis was not feasible, and in most reviews (14), authors used a descriptive approach to synthesize the available data. These numbers reflect the limitations of using the published literature to answer many specific clinical questions. The major limitations of the evidence base identified for synthesis included heterogeneity in; study types, populations and reporting outcomes; as well as the lack of unity in the definitions of study outcomes and the lack of reporting on variables and risk factors that can have important implications for a particular study outcome. Further limitations included the limited number of published studies, and the lack of relevant randomized controlled trials. As a result of all of these variables most reviews are unable to provide firm recommendations.
Despite these limitations all the perfomed systematic, reviews were able to highlighted areas of limitations and knowledge gaps in current studies. Not surprisingly the majority of reviews made specific recommendations that future clinical trials if clinical equipoise was to be resolved. For example, Hutton et al. provided a comprehensive review on identifying the optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy. Their systematic review identified 47 different antiemetic regimens and 15 different chemotherapy-induced nausea and vomiting endpoints that were used in these trials. They recommended that future trialists unify emesis outcome definitions and balance patients for risk factors reated to underlying emesis risk [17]. For another example, Fernandes et al. recommended that future studies use validated scores to measure taxane acute pain syndrome to evaluate treatment response [22]. In a review comparing bone modifying agents frequency (q3–4 weeks vs. 12 weeks) in breast cancer patients with bone metastasis, Awan et al. recommended future studies to stratify patients based on bone vs. visceral disease, disease burden, bone metastasis site, and bone turnover markers to allow decreased heterogenicity between trials [30].
5. Conclusions
Systematic reviews provide an important tool in the performance of clinic research. The current manuscript shows how incorporation of systemic review into The REaCT program continues to confirm that in many areas of clinical care identified by patients, their families and healthcare providers that insufficient evidence for optimal practice exist and therefore clinical equipoise is present. Systemic reviews also provide an important tool for designing future potential clinical trials by exploring the challenges and limitations reported in previously reported studies. Systematic review also provide unique opportunities for trainees to be fully incorporated into the research process.
Author Contributions
B.A., B.H., L.V. and M.C. designed the study and prepared the protocol. B.A. and M.L. collected the data. B.A., B.H. and M.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by the Rethinking Clinical Trials (REaCT) Program platform at the Ottawa Hospital which is supported by The Ottawa Hospital Foundation and its generous donors.
Institutional Review Board Statement
Not applicable as all data used in this review was extracted from published manuscripts.
Informed Consent Statement
Not applicable as this was a review of existing published data.
Data Availability Statement
All data was extracted from published manuscripts.
Conflicts of Interest
MC and BH report consulting fees from Cornerstone Research, outside the submitted work. All other authors declare no competing interests.
References
- Hilton, J.; Mazzarello, S.; Fergusson, D.; Joy, A.A.; Robinson, A.; Arnaout, A.; Hutton, B.; Vandermeer, L.; Clemons, M. Novel methodology for comparing standard-of-care interventions in patients with cancer. J. Oncol. Pract. 2016, 12, e1016–e1024. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Whitney, S.N.; Kurzrock, R. Equipoise lost: Ethics, costs, and the regulation of cancer clinical research. J. Clin. Oncol. 2010, 28, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Kuchuk, I.; Bouganim, N.; Pond, G.; Verma, S.; Segal, R.; Dent, S.; Gertler, S.; Song, X.; Kanji, F.; et al. Can the referring surgeon enhance accrual of breast cancer patients to medical and radiation oncology trials? The ENHANCE study. Curr. Oncol. 2016, 23, e276–e279. [Google Scholar] [CrossRef] [PubMed]
- Dilts, D.M.; Cheng, S.K.; Crites, J.S.; Sandler, A.B.; Doroshow, J.H. Phase III clinical trial development: A process of chutes and ladders. Clin. Cancer Res. 2010, 16, 5381–5389. [Google Scholar] [CrossRef]
- Freedman, B. Equipoise and the ethics of clinical research. N. Engl. J. Med. 1987, 317, 141–145. [Google Scholar] [CrossRef]
- Weijer, C.; Shapiro, S.H.; Cranley Glass, K. For and against: Clinical equipoise and not the uncertainty principle is the moral underpinning of the randomised controlled trial. BMJ 2000, 321, 756–758. [Google Scholar] [CrossRef]
- Saunders, D.; Liu, M.; Vandermeer, L.; Alzahrani, M.J.; Hutton, B.; Clemons, M. The rethinking clinical trials (REaCT) program. A Canadian-led pragmatic trials program: Strategies for integrating knowledge users into trial design. Curr. Oncol. 2021, 28, 3959–3977. [Google Scholar] [CrossRef]
- Basulaiman, B.; Awan, A.A.; Fergusson, D.; Vandermeer, L.; Arnaout, A.; Hilton, J.; Hutton, B.; Joy, A.A.; Robinson, A.; Califaretti, N.; et al. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res. Treat. 2019, 177, 93–101. [Google Scholar] [CrossRef]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 14 December 2021).
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Caldwell, D.M.; Ades, A.E.; Higgins, J.P. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 2005, 331, 897–900. [Google Scholar] [CrossRef]
- Li, T.; Puhan, M.A.; Vedula, S.S.; Singh, S.; Dickersin, K.; Ad Hoc Network Meta-analysis Methods Meeting Working Group. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011, 9, 79. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Hutton, B.; Addison, C.L.; Campbell, K.; Fergusson, D.; Mazarello, S.; Clemons, M. A systematic review of dosing frequency with bone-targeted agents for patients with bone metastases from breast cancer. J. Bone Oncol. 2013, 2, 123–131. [Google Scholar] [CrossRef][Green Version]
- Hutton, B.; Clemons, M.; Mazzarello, S.; Kuchuk, I.; Skidmore, B.; Ng, T. Identifying an optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for breast cancer—An inspection of the evidence base informing clinical decision-making. Cancer Treat. Rev. 2015, 41, 951–959. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Mazzarello, S.; Shorr, R.; Vandermeer, L.; Jacobs, C.; Hilton, J.; Hutton, B.; Clemons, M. Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2205–2213. [Google Scholar] [CrossRef]
- Jacobs, C.; Hutton, B.; Ng, T.; Shorr, R.; Clemons, M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 2015, 20, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Mazzarello, S.; Hutton, B.; Ibrahim, M.F.K.; Jacobs, C.; Shorr, R.; Smith, S.; Ng, T.; Clemons, M. Management of urogenital atrophy in breast cancer patients: A systematic review of available evidence from randomized trials. Breast Cancer Res. Treat. 2015, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dudani, S.; Mazzarello, S.; Hilton, J.; Hutton, B.; Vandermeer, L.; Fernandes, R.; Ibrahim, M.F.; Smith, S.; Majeed, H.; Al-Baimani, K.; et al. Optimal management of leptomeningeal carcinomatosis in breast cancer patients—A systematic review. Clin. Breast Cancer 2016, 16, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Majeed, H.; Smith, S.; Shorr, R.; Hutton, B.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Treatment of taxane acute pain syndrome (TAPS) in cancer patients receiving taxane-based chemotherapy—A systematic review. Support. Care Cancer 2016, 24, 1583–1594. [Google Scholar] [CrossRef]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Majeed, H.; Ibrahim, M.F.; Jacobs, C.; Ong, M.; Clemons, M. Taxane acute pain syndrome (TAPS) in patients receiving taxane-based chemotherapy for breast cancer—A systematic review. Support. Care Cancer 2016, 24, 3633–3650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Dennis, K.; Steinmetz, A.; Clemons, M.; Asmis, T.R.; Goodwin, R.A.; Vickers, M.M. Management of epidermal growth factor receptor inhibitor-induced hypomagnesemia: A systematic review. Clin. Colorectal Cancer 2016, 15, e117–e123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Hutton, B.; Shorr, R.; Ibrahim, M.F.K.; Jacobs, C.; Ong, M.; Clemons, M. A systematic review of the incidence and risk factors for taxane acute pain syndrome in patients receiving taxane-based chemotherapy for prostate cancer. Clin. Genitourin. Cancer 2017, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Vandermeer, L.; Dudani, S.; Ibrahim, M.F.; Majeed, H.; Perdrizet, K.; Shorr, R.; Hutton, B.; et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Mazzarello, S.; Stober, C.; Ibrahim, M.F.K.; Dudani, S.; Perdrizet, K.; Majeed, H.; Vandermeer, L.; Shorr, R.; Hutton, B.; et al. Primary febrile neutropenia prophylaxis for patients who receive FEC-D chemotherapy for breast cancer: A systematic review. J. Glob. Oncol. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.F.; Mazzarello, S.; Caudrelier, J.M.; Lima, M.A.G.; Hutton, B.; Sienkiewicz, M.; Stober, C.; Fernandes, R.; Ibrahim, M.F.K.; Vandermeer, L.; et al. Optimal sequence of adjuvant endocrine and radiation therapy in early-stage breast cancer—A systematic review. Cancer Treat. Rev. 2018, 69, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Souied, O.; Bota, A.B.; Levasseur, N.; Stober, C.; Hilton, J.; Kamel, D.; Hutton, B.; Vandermeer, L.; Mazzarello, S.; et al. Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 607–620. [Google Scholar] [CrossRef]
- Awan, A.A.; Hutton, B.; Hilton, J.; Mazzarello, S.; Van Poznak, C.; Vandermeer, L.; Bota, B.; Stober, C.; Sienkiewicz, M.; Fergusson, D.; et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 507–517. [Google Scholar] [CrossRef]
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and harms of medical cannabis: A scoping review of systematic reviews. Syst. Rev. 2019, 8, 320. [Google Scholar] [CrossRef]
- Hutton, B.; Hersi, M.; Cheng, W.; Pratt, M.; Barbeau, P.; Mazzarello, S.; Ahmadzai, N.; Skidmore, B.; Morgan, S.C.; Bordeleau, L.; et al. Comparing interventions for management of hot flashes in patients with breast and prostate cancer: A systematic review with meta-analyses. Oncol. Nurs. Forum 2020, 47, E86–E106. [Google Scholar]
- Bradbury, M.; Hutton, B.; Beltran-Bless, A.A.; Alzahrani, M.; Lariviere, T.; Fernandes, R.; Ibrahim, M.F.; Cole, K.; Hilton, J.; Vandermeer, L.; et al. Time to update evidence-based guideline recommendations about concurrent tamoxifen and antidepressant use? A systematic review. Clin. Breast Cancer 2022, 22, e362–e373. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2021, 29, 925–943. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, N.; Cheng, W.; Mazzarello, S.; Clemons, M.; Vandermeer, L.; Jones, L.; Joy, A.A.; Barbeau, P.; Wolfe, D.; Ahmadzai, N.; et al. Optimising weight-loss interventions in cancer patients—A systematic review and network meta-analysis. PLoS ONE. 2021, 16, e0245794. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.F.; Clemons, M.; Hutton, B.; Jemaan Alzahrani, M.; Caudrelier, J.M.; Vandermeer, L.; Liu, M.; Saunders, D.; Sienkiewicz, M.; Stober, C.; et al. De-escalating adjuvant therapies in older patients with lower risk estrogen receptor-positive breast cancer treated with breast-conserving surgery: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102254. [Google Scholar] [CrossRef] [PubMed]
- Surujballi, J.; Shah, H.; Hutton, B.; Alzahrani, M.; Beltran-Bless, A.A.; Shorr, R.; Larocque, G.; McGee, S.; Cole, K.; Ibrahim, M.F.K.; et al. The COVID-19 pandemic: An opportunity to rethink and harmonise the frequency of follow-up visits for patients with early stage breast cancer. Cancer Treat. Rev. 2021, 97, 102188. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.M.; McGee, S. Evaluating Optimal Timing of Endocrine Therapy and Radiation Therapy in Early-Stage Breast Cancer (REaCT-RETT), NCT03948568, ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03948568 (accessed on 12 November 2021).
- Savard, M.F. Evaluating Harms and Benefits of Endocrine Therapy in Patients ≥70 Years of Age with Lower Risk Breast Cancer, NCT04921137, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04921137 (accessed on 12 November 2021).
- Clemons, M. Individualised Versus Standard Care for Breast Cancer Patients at High-Risk for Chemotherapy-Induced Nausea and Vomiting the ILIAD Study, NCT02861859, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02861859 (accessed on 15 December 2021).
- Clemons, M. Granulocyte-Colony Stimulating Factors or Antibiotics for Primary Prophylaxis for Febrile Neutropenia, NCT02816112, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02816112 (accessed on 12 November 2021).
- Clemons, M.; Fergusson, D.; Simos, D.; Mates, M.; Robinson, A.; Califaretti, N.; Zibdawi, L.; Bahl, M.; Raphael, J.; Ibrahim, M.F.K.; et al. A multicentre, randomised trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy-induced febrile neutropenia in early stage breast cancer. Ann. Oncol. 2020, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Stober, C.; Fergusson, D.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.C.; Saunders, D.; Mazzarello, S.; Vandermeer, L.; et al. A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res. Treat. 2019, 178, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Liu, M.; Stober, C.; Pond, G.; Jemaan Alzahrani, M.; Ong, M.; Ernst, S.; Booth, C.; Mates, M.; Abraham Joy, A.; et al. Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer. J. Bone Oncol. 2021, 30, 100388. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Ong, M.; Stober, C.; Ernst, S.; Booth, C.; Canil, C.; Mates, M.; Robinson, A.; Blanchette, P.; Joy, A.A.; et al. A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur. J. Cancer 2021, 142, 132–140. [Google Scholar] [CrossRef]
- Clemons, M. Comparing a Single-Dose vs. Twice Yearly Zoledronate in Patients with Early Stage Breast Cancer (REaCT-ZOL), NCT03664687, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03664687 (accessed on 12 November 2021).
- Ng, T. Comparing Continuation or De-Escalation of Bone Modifying Agents (BMA) in Patients Treated for Over 2 Years for Bone Metastases from Either Breast or Castration-resistant Prostate Cancer, NCT04549207, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04549207 (accessed on 12 November 2021).
- Clemons, M.; Simos, D.; Sienkiewicz, M.; Ng, T.; Zibdawi, L.; Basulaiman, B.; Awan, A.; Fergusson, D.; Vandermeer, L.; Saunders, D.; et al. A prospective multi-centre, randomized study comparing the addition of tapering dexamethasone to other standard of care therapies for taxane-associated pain syndrome (TAPS) in breast cancer patients. Support. Care Cancer 2021, 29, 5787–5795. [Google Scholar] [CrossRef]
- Vickers, M. Feasibility of Using an Integrated Consent Model to Compare Two Standard of Care Regimens for the Management of Hypomagnesemia from Anti-Cancer Therapies, NCT02690012, ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02690012 (accessed on 12 November 2021).
- Clemons, M.; Dranitsaris, G.; Sienkiewicz, M.; Sehdev, S.; Ng, T.; Robinson, A.; Mates, M.; Hsu, T.; McGee, S.; Freedman, O.; et al. A randomized trial of individualized versus standard of care antiemetic therapy for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. Breast 2020, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- REaCT: REthinking Clinical Trials. Available online: https://react.ohri.ca/ (accessed on 1 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).