Thin Cartilage Cap May Be Related to the Spontaneous Regression in Pediatric Patients with Osteochondroma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assessment of Osteochondroma
2.2. Statistical Analysis
3. Results
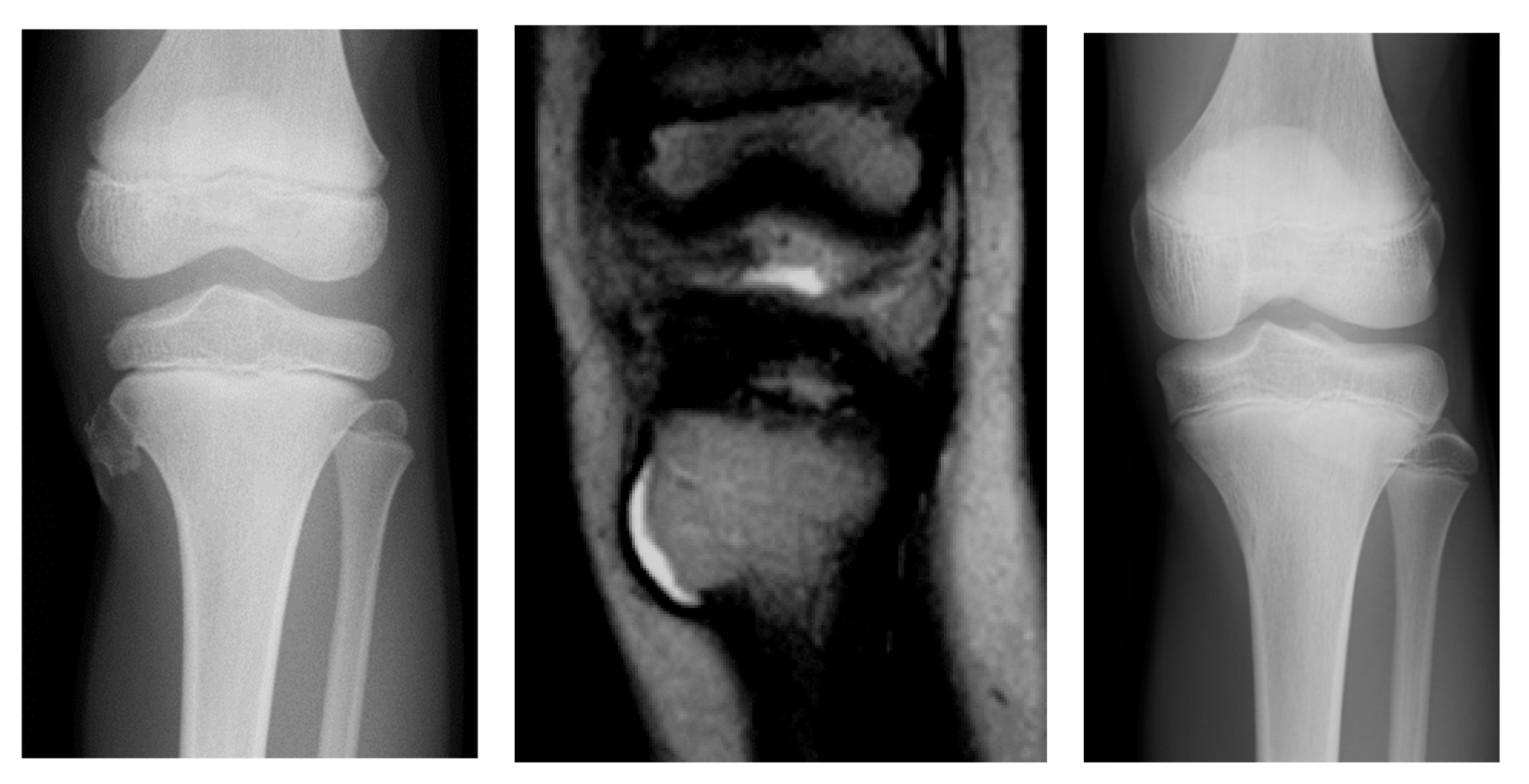
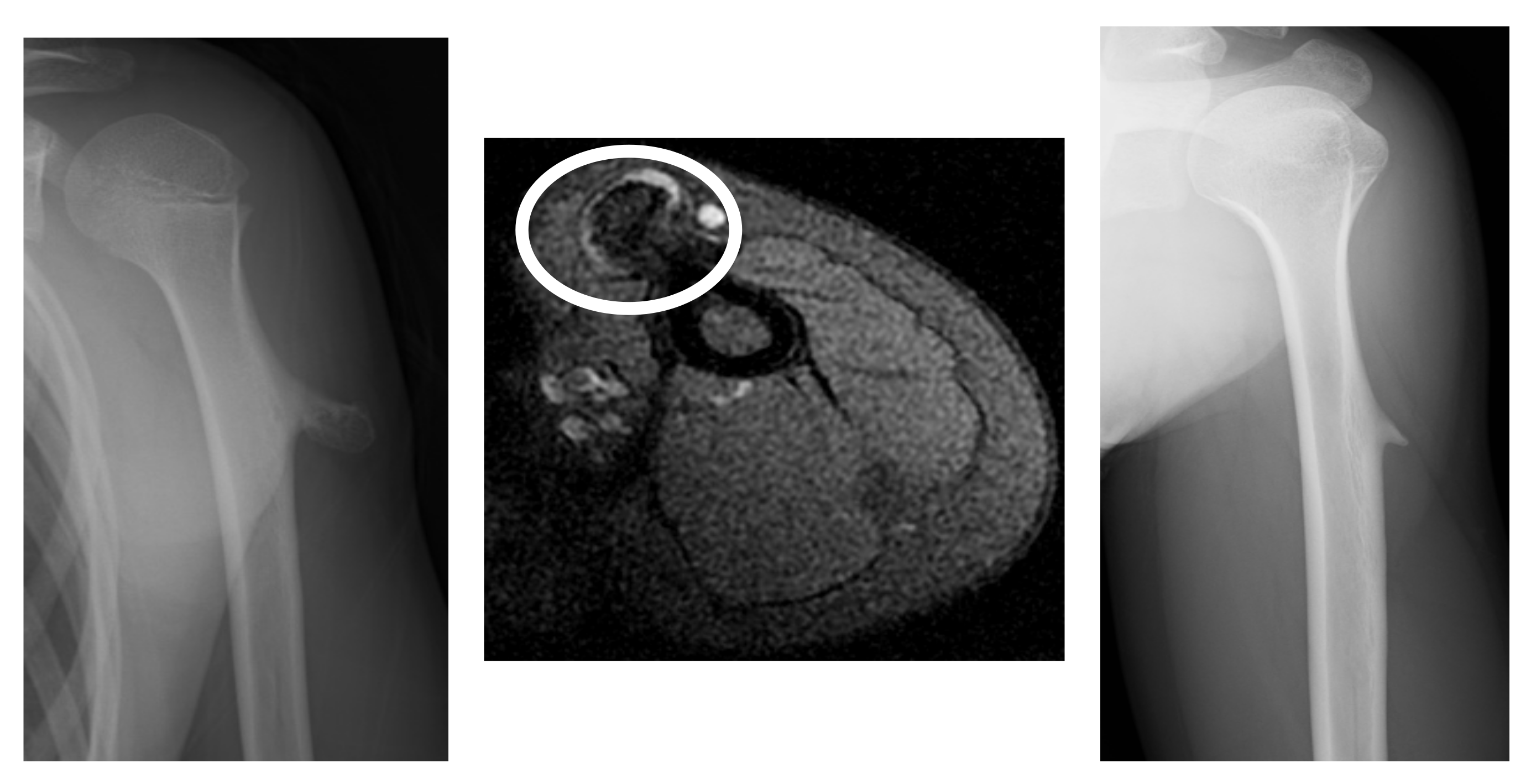
3.1. Case Presentations
3.1.1. Case 1
3.1.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailescu, I.; Popescu, M.; Sarafoleanu, L.R.; Bondari, S.; Sabetay, C.; Mitroi, M.R.; Tuculina, M.J.; Albulescu, D.M. Diagnosis and evolution of the benign tumor osteochondroma. Exp. Ther. Med. 2022, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Kitsoulis, P.; Galani, V.; Stefanaki, K.; Paraskevas, G.; Karatzias, G.; Agnantis, N.J.; Bai, M. Osteochondromas: Review of the clinical, radiological and pathological features. In Vivo 2008, 22, 633–646. [Google Scholar] [PubMed]
- Khurana, J.; Abdul-Kari, F.; Bovey, J.V.M.G. Osteochondroma. In World Health Organization Classification of Tumors: Pathology and Genetics; Flecher, C.D.M., Unni, K.K., Mertens, F., Eds.; Tumors of Soft Tissue and Bone; International Agency for Research on Cancer: Lyon, France, 2002; pp. 234–236. [Google Scholar]
- Wootton-Gorges, S.L. MR imaging of primary bone tumors and tumor-like conditions in children. Magn. Reson. Imaging Clin. N. Am. 2009, 17, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Bovee, J.V. Multiple osteochondromas. Orphanet. J. Rare Dis. 2008, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostas, T.; Georgios, P.; Aikaterini, K.; Theodoros, T.; Alexandra, B.; Konstantinos, V.; Panagiotis, K.; Panagiotis, K. Osteochondromas: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features and Treatment Options. In Vivo 2021, 35, 681–691. [Google Scholar] [CrossRef]
- Douis, H.; Saifuddin, A. The imaging of cartilaginous bone tumours. I. Benign lesions. Skelet. Radiol. 2012, 41, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Liua, H.; Wang, X.; Zhong, Z.; Cao, S.; Zhong, C.; Yang, Y.; Wang, G. Osteochondroma: Review of 431 patients from one medical institution in South China. J. Bone Oncol. 2017, 8, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Florez, B.; Mönckeberg, J.; Castillo, G.; Beguiristain, J. Solitary osteochondroma long-term follow-up. J. Pediatr. Orthop. B 2008, 17, 91–94. [Google Scholar] [CrossRef]
- Paling, M.R. The “disappearing” osteochondroma. Skelet. Radiol. 1983, 10, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Durán-Serrano, M.; Gómez-Palacio, V.E.; Parada-Avendaño, I.; Gil-Albarova, J. Spontaneous regression of solitary osteochondroma in children: An option to consider in clinical practice. Jt. Dis. Relat. Surg. 2021, 31, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Yamada, S.; Yamamoto, N.; Hayashi, K.; Miwa, S.; Tsuchiya, H.; Otsuka, T. Spontaneous shrinkage of solitary osteochondromas. Skelet. Radiol. 2018, 47, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Woertler, K.; Lindner, N.; Gosheger, G.; Brinkschmidt, C.; Heindel, W. Osteochondroma: MR imaging of tumor-related complications. Eur. Radiol. 2000, 10, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Stacy, G.S.; Heck, R.K.; Peabody, T.D.; Dixon, L.B. Neoplastic and tumorlike lesions detected on MR imaging of the knee in patients with suspected internal derangement: Part I, intraosseous entities. AJR Am. J. Roentgenol. 2002, 178, 589–594. [Google Scholar] [CrossRef]
- Motamedi, K.; Seeger, L.L. Benign bone tumors. Radiol. Clin. N. Am. 2011, 49, 1115–1134. [Google Scholar] [CrossRef]
- Hoshi, M.; Takami, M.; Hashimoto, R.; Okamoto, T.; Yanagida, I.; Matsumura, A.; Noguchi, K. Spontaneous regression of osteochondromas. Skelet. Radiol. 2007, 36, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, M.; Shih, F.; Boarini, M.; Pedrini, E.; Gnoli, M.; Antonioli, D.; Tremosini, M.; Sangiorgi, L. The natural history of multiple osteochondromas in a large Italian cohort of pediatric patients. Bone 2020, 139, 115499. [Google Scholar] [CrossRef]
- Komura, S.; Matsumoto, K.; Hirakawa, A.; Akiyama, H. Natural history and characteristics of hand exostoses in multiple hereditary exostoses. J. Hand Surg. Am. 2021, 46, 815.e1–815.e15. [Google Scholar] [CrossRef] [PubMed]

| Tumor Regression | |
|---|---|
| Vanished | >70% decrease from baseline |
| Regressed | A 30–70% decrease from baseline |
| Assessment Method for Mode of Tumor Regression | |
| Incorporation | The height of the tumor |
| Absorption | The stalk of the tumor |
| Fracture | History of fracture |
| Age (Years) | Sex | Thickness of Cartilage Cap (mm) | Type | Site | Age at Shrinkage (Years) |
|---|---|---|---|---|---|
| 2 | Female | 3.84 | Sessile | Tibia | 8 |
| 7 | Male | 2.83 | Pedunculated | Tibia | 11 |
| 9 | Male | 4.57 | Sessile | Humerus | 13 |
| 9 | Female | 1 | Sessile | Femur | 15 |
| 8 | Male | 1 | Sessile | Tibia | 9 |
| 3 | Male | 1.61 | Sessile | Femur | 12 |
| 10 | Female | 2.25 | Pedunculated | Humerus | 14 |
| 10 | Male | 1.91 | Pedunculated | Femur | 11 |
| 11 | Female | 1.37 | Sessile | Humerus | 12 |
| 7 | Female | 1 | Sessile | phalanx | 9 |
| Variables | Category | Regression | Stable or Progression | p-Value |
|---|---|---|---|---|
| Sex | Male | 5 | 10 | 1.0 |
| Female | 5 | 8 | ||
| Age | Mean (range) | 9 (2–12) | 10 (2–16) | 0.346 |
| Tumor site | Long bone | 9 | 12 | 0.362 |
| Flat bone | 1 | 6 | ||
| Shape of tumor | Pedunculated | 3 | 4 | 0.674 |
| Sessile | 7 | 14 | ||
| Cartilage cap | Mean (range) | 2.29 mm (1.0–4.57) | 3.65 mm (1.6–7.65) | 0.038 |
| Clinical Variables | Category | Mean Cartilage Cap | p-Value |
|---|---|---|---|
| Sex | Male | 2.86 | 0.351 |
| Female | 2.09 | ||
| Age | 10> | 2.93 | 0.162 |
| 10≤ | 2.01 | ||
| Tumor site | Long bone | 2.7 | 0.362 |
| Flat bone | 1.35 | ||
| Shape of tumor | Pedunculated | 3.24 | 0.674 |
| Sessile | 2.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, R.; Nakamura, T.; Asanuma, K.; Hagi, T.; Uchiyama, T.; Sudo, A. Thin Cartilage Cap May Be Related to the Spontaneous Regression in Pediatric Patients with Osteochondroma. Curr. Oncol. 2022, 29, 9884-9890. https://doi.org/10.3390/curroncol29120777
Adachi R, Nakamura T, Asanuma K, Hagi T, Uchiyama T, Sudo A. Thin Cartilage Cap May Be Related to the Spontaneous Regression in Pediatric Patients with Osteochondroma. Current Oncology. 2022; 29(12):9884-9890. https://doi.org/10.3390/curroncol29120777
Chicago/Turabian StyleAdachi, Ryohei, Tomoki Nakamura, Kunihiro Asanuma, Tomohito Hagi, Teruya Uchiyama, and Akihiro Sudo. 2022. "Thin Cartilage Cap May Be Related to the Spontaneous Regression in Pediatric Patients with Osteochondroma" Current Oncology 29, no. 12: 9884-9890. https://doi.org/10.3390/curroncol29120777
APA StyleAdachi, R., Nakamura, T., Asanuma, K., Hagi, T., Uchiyama, T., & Sudo, A. (2022). Thin Cartilage Cap May Be Related to the Spontaneous Regression in Pediatric Patients with Osteochondroma. Current Oncology, 29(12), 9884-9890. https://doi.org/10.3390/curroncol29120777




