Barriers to Oncofertility Care among Female Adolescent Cancer Patients in Canada
Abstract
:1. Introduction
2. Background
2.1. Cancer Treatment and Infertility Risk
2.2. Fertility Preservation Procedures
3. Psychosocial Impact of Cancer-Related Infertility
4. Unmet Needs
5. Barriers
5.1. Parent Barriers
5.2. Patient Barriers
5.3. Health System Barriers
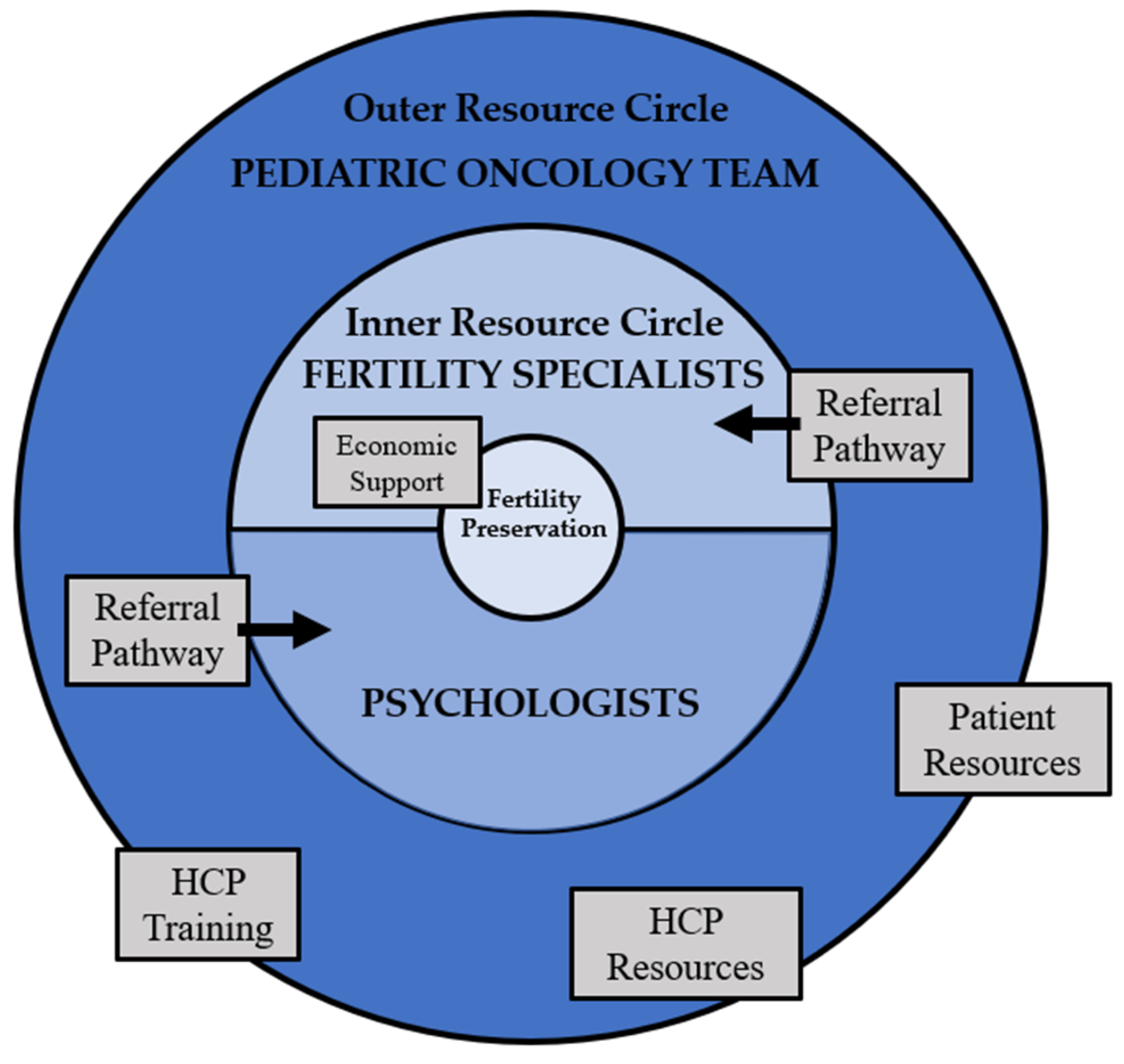
6. Recommendations for Clinical Practice
6.1. Decision Aids
6.2. Health Care Provider Training
6.3. Interdisciplinary Collaboration
6.4. Clinical Models of Care
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Canadian Partnership Against Cancer. Adolescents & Young Adults with Cancer April 2017. 2017. Available online: https://www.partnershipagainstcancer.ca/topics/adolescents-young-adults-with-cancer/ (accessed on 1 February 2022).
- Cancer in Children and Adolescents—National Cancer Institute. 10 November 2021. Available online: https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet (accessed on 1 February 2022).
- Letourneau, J.; Chan, S.W.; Rosen, M.P. Accelerating Ovarian Age: Cancer Treatment in the Premenopausal Woman. Semin. Reprod. Med. 2013, 31, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oktay, K.; Harvey, B.; Partridge, A.H.; Quinn, G.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Korkidakis, A.; Lajkosz, K.; Green, M.; Strobino, D.; Velez, M.P. Patterns of Referral for Fertility Preservation Among Female Adolescents and Young Adults with Breast Cancer: A Population-Based Study. J. Adolesc. Young-Adult Oncol. 2019, 8, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.E.; Pudwell, J.; McClintock, C.; Korkidakis, A.; Green, M.; Velez, M.P. Modest Increase in Fertility Consultations in Female Adolescents and Young Adults with Lymphoma: A Population-Based Study. J. Adolesc. Young-Adult Oncol. 2021, 10, 342–345. [Google Scholar] [CrossRef]
- Warner, E.; Yee, S.; Kennedy, E.; Glass, K.; Foong, S.; Seminsky, M.; Quan, M.L. Oncofertility Knowledge, Attitudes, and Practices of Canadian Breast Surgeons. Ann. Surg. Oncol. 2016, 23, 3850–3859. [Google Scholar] [CrossRef]
- Velez, M.P.; Richardson, H.; Baxter, N.N.; McClintock, C.; Greenblatt, E.; Barr, R.; Green, M. Risk of infertility in female adolescents and young adults with cancer: A population-based cohort study. Hum. Reprod. 2021, 36, 1981–1988. [Google Scholar] [CrossRef]
- Rappaport, R.; Brauner, R.; Czernichow, P.; Thibaud, E.; Renier, D.; Zucker, J.M.; Lemerle, J. Effect of Hypothalamic and Pituitary Irradiation on Pubertal Development in Children with Cranial Tumors. J. Clin. Endocrinol. Metab. 1982, 54, 1164–1168. [Google Scholar] [CrossRef]
- Constine, L.S.; Woolf, P.D.; Cann, D.; Mick, G.; McCormick, K.; Raubertas, R.F.; Rubin, P. Hypothalamic-Pituitary Dysfunction after Radiation for Brain Tumors. N. Engl. J. Med. 1993, 328, 87–94. [Google Scholar] [CrossRef]
- Tsai-Turton, M.; Luong, B.T.; Tan, Y.; Luderer, U. Cyclophosphamide-Induced Apoptosis in COV434 Human Granulosa Cells Involves Oxidative Stress and Glutathione Depletion. Toxicol. Sci. 2007, 98, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Keating, A.F. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol. Appl. Pharmacol. 2016, 292, 65–74. [Google Scholar] [CrossRef]
- Petrillo, S.K.; Desmeules, P.; Truong, T.-Q.; Devine, P.J. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol. Appl. Pharmacol. 2011, 253, 94–102. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Thomson, A.B.; Kelsey, T. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Ma, Y.; Jin, J.; Ren, P.; Zhou, H.; Xu, S.; Zhang, Y.; Hu, Z.; Rong, Y.; Dai, Y.; et al. Cyclophosphamide Exposure Causes Long-Term Detrimental Effect of Oocytes Developmental Competence Through Affecting the Epigenetic Modification and Maternal Factors’ Transcription During Oocyte Growth. Front. Cell Dev. Biol. 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Myers, M.; Anderson, R. The dynamics of the primordial follicle reserve. Reproduction 2013, 146, R205–R215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Anastácio, A.; Liu, K.; Rodriguez-Wallberg, K.A. Ovarian Follicle Depletion Induced by Chemotherapy and the Investigational Stages of Potential Fertility-Protective Treatments—A Review. Int. J. Mol. Sci. 2019, 20, 4720. [Google Scholar] [CrossRef] [Green Version]
- Anazodo, A.; Ataman-Millhouse, L.; Jayasinghe, Y.; Woodruff, T.K. Oncofertility-An emerging discipline rather than a special consideration. Pediatr. Blood Cancer 2018, 65, e27297. [Google Scholar] [CrossRef]
- Behringer, K.; Breuer, K.; Reineke, T.; May, M.; Nogova, L.; Klimm, B.; Schmitz, T.; Wildt, L.; Diehl, V.; Engert, A. Secondary Amenorrhea After Hodgkin’s Lymphoma Is Influenced by Age at Treatment, Stage of Disease, Chemotherapy Regimen, and the Use of Oral Contraceptives During Therapy: A Report from the German Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2005, 23, 7555–7564. [Google Scholar] [CrossRef]
- McClam, M.; Xiao, S. Preserving Oocytes in Oncofertility. Biol. Reprod. 2022, 106, 328–337. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2016, 23, 139–155. [Google Scholar] [CrossRef]
- Bahroudi, Z.; Zarnaghi, M.R.; Izadpanah, M.; Abedelahi, A.; Niknafs, B.; Nasrabadi, H.T.; Seghinsara, A.M. Review of ovarian tissue cryopreservation techniques for fertility preservation. J. Gynecol. Obstet. Hum. Reprod. 2021, 51, 102290. [Google Scholar] [CrossRef]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the development and future of oocyte IVM in clinical practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Ba, B.E.O.; Goodwin, T.; Kiernan, M.; Hudson, M.M.; Dahl, G.V. Concerns about infertility risks among pediatric oncology patients and their parents. Pediatr. Blood Cancer 2007, 50, 85–89. [Google Scholar] [CrossRef]
- Ellis, S.J.; Wakefield, C.E.; McLoone, J.K.; Robertson, E.G.; Cohn, R.J. Fertility concerns among child and adolescent cancer survivors and their parents: A qualitative analysis. J. Psychosoc. Oncol. 2016, 34, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Knapp, C.; Murphy, D.; Sawczyn, K.; Sender, L. Congruence of Reproductive Concerns Among Adolescents with Cancer and Parents: Pilot Testing an Adapted Instrument. Pediatrics 2012, 129, e930–e936. [Google Scholar] [CrossRef] [Green Version]
- Stinson, J.N.; Jibb, L.; Greenberg, M.; Barrera, M.; Luca, S.; White, M.E.; Gupta, A. A Qualitative Study of the Impact of Cancer on Romantic Relationships, Sexual Relationships, and Fertility: Perspectives of Canadian Adolescents and Parents During and After Treatment. J. Adolesc. Young-Adult Oncol. 2015, 4, 84–90. [Google Scholar] [CrossRef]
- Gorman, J.R.; Su, H.I.; Mph, S.C.R.; Dominick, S.A.; Malcarne, V. Experiencing reproductive concerns as a female cancer survivor is associated with depression. Cancer 2014, 121, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, L.; Dogan-Ates, A.; Habbal, R.; Berkowitz, R.; Goldstein, D.P.; Bernstein, M.; Kluhsman, B.C.; Osann, K.; Newlands, E.; Seckl, M.J.; et al. Defining and measuring reproductive concerns of female cancer survivors. J. Natl. Cancer Inst. Monogr. 2005, 34, 94–98. [Google Scholar] [CrossRef]
- Crawshaw, M.; Sloper, P. ‘Swimming against the tide’—The influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur. J. Cancer Care 2010, 19, 610–620. [Google Scholar] [CrossRef]
- Petropanagos, A.; Campo-Engelstein, L. Tough Talk: Discussing Fertility Preservation with Adolescents and Young Adults with Cancer. J. Adolesc. Young-Adult Oncol. 2015, 4, 96–99. [Google Scholar] [CrossRef]
- Zebrack, B.J.; Casillas, J.; Nohr, L.; Adams, H.; Zeltzer, L.K. Fertility issues for young adult survivors of childhood cancer. Psycho-Oncology 2004, 13, 689–699. [Google Scholar] [CrossRef]
- Janson, C.; Leisenring, W.; Cox, C.; Termuhlen, A.M.; Mertens, A.C.; Whitton, J.A.; Goodman, P.; Zeltzer, L.; Robison, L.L.; Krull, K.R.; et al. Predictors of Marriage and Divorce in Adult Survivors of Childhood Cancers: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2626–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawshaw, M.; Glaser, A.; Hale, J.; Sloper, P. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. Eur. J. Cancer Care 2009, 18, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.A.; Braun, I.M.; Meyer, F.L. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: A systematic review. Cancer 2015, 121, 3938–3947. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.; Abrol, K.; McDonald, M.; Tonelli, M.; Liu, K.E. Addressing Oncofertility Needs: Views of Female Cancer Patients in Fertility Preservation. J. Psychosoc. Oncol. 2012, 30, 331–346. [Google Scholar] [CrossRef] [PubMed]
- McKillop, S.; Henning, J.; Prud’homme, N.; Schulte, F.; Labelle, L.; Turner, J. Adolescent and Young Adult (AYA) Cancer: Clinical Practice Guideline SUPP-020; Alberta Health Services: Edmonton, AB, Canada, 2020. [Google Scholar]
- Roberts, J.; Ronn, R.; Tallon, N.; Holzer, H.E.G. Fertility Preservation in Reproductive-Age Women Facing Gonadotoxic Treatments. Curr. Oncol. 2015, 22, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Pediatric Oncology Group of Ontario. Guideline for Fertility Preservation for Patients with Cancer. 2019. Available online: https://www.pogo.ca/healthcare/practiceguidelines/fertility-preservation (accessed on 1 February 2022).
- Logan, S.; Perz, J.; Ussher, J.; Peate, M.; Anazodo, A. A systematic review of patient oncofertility support needs in reproductive cancer patients aged 14 to 45 years of age. Psycho-Oncology 2017, 27, 401–409. [Google Scholar] [CrossRef]
- Rae, C.; Pole, J.; Gupta, S.; Digout, C.; Szwajcer, D.; Flanders, A.; Srikanthan, A.; Hammond, C.; Schacter, B.; Barr, R.D.; et al. Development of System Performance Indicators for Adolescent and Young Adult Cancer Care and Control in Canada. Value Health 2019, 23, 74–88. [Google Scholar] [CrossRef]
- Wright, C.; Coad, J.; Morgan, S.; Stark, D.; Cable, M. ‘Just in case’: The fertility information needs of teenagers and young adults with cancer. Eur. J. Cancer Care 2013, 23, 189–198. [Google Scholar] [CrossRef]
- Zebrack, B. Information and service needs for young adult cancer survivors. Support. Care Cancer 2008, 17, 349–357. [Google Scholar] [CrossRef]
- Anderson, R.A.; Weddell, A.; Spoudeas, H.A.; Douglas, C.; Shalet, S.M.; Levitt, G.; Wallace, W.H.B. Do doctors discuss fertility issues before they treat young patients with cancer? Hum. Reprod. 2008, 23, 2246–2251. [Google Scholar] [CrossRef] [Green Version]
- Peddie, V.; Porter, M.; Barbour, R.; Culligan, D.; Macdonald, G.; King, D.; Horn, J.; Bhattacharya, S. Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaczkowski, G.; White, V.; Thompson, K.; Bibby, H.; Coory, M.; Orme, L.M.; Conyers, R.; Phillips, M.B.; Osborn, M.; Harrup, R.; et al. Factors influencing the provision of fertility counseling and impact on quality of life in adolescents and young adults with cancer. J. Psychosoc. Oncol. 2018, 36, 1–19. [Google Scholar] [CrossRef]
- Canadian Partnership Against Cancer. Canadian Framework for the Care and Support of Adolescents and Young Adults with Cancer 2019. 2019. Available online: https://www.partnershipagainstcancer.ca/topics/framework-adolescents-young-adults/ (accessed on 1 February 2022).
- Quinn, G.P.; Vadaparampil, S.T. Fertility Preservation and Adolescent/Young Adult Cancer Patients: Physician Communication Challenges. J. Adolesc. Health 2009, 44, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Vadaparampil, S.; Quinn, G.; King, L.; Wilson, C.; Nieder, M. Barriers to fertility preservation among pediatric oncologists. Patient Educ. Couns. 2008, 72, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jayasinghe, Y.; Kemertzis, M.A.; Moore, P.; Peate, M. Fertility Preservation in Pediatric and Adolescent Oncology Patients: The Decision-Making Process of Parents. J. Adolesc. Young-Adult Oncol. 2017, 6, 213–222. [Google Scholar] [CrossRef]
- Ussher, J.M.; Cummings, J.R.; Dryden, A.; Perz, J. Talking about fertility in the context of cancer: Health care professional perspectives. Eur. J. Cancer Care 2015, 25, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Kashal, P.; Quinn, G.; Sawczyn, K.; Termuhlen, A. Development of a Spanish Language Fertility Educational Brochure for Pediatric Oncology Families. J. Pediatr. Adolesc. Gynecol. 2013, 27, 202–209. [Google Scholar] [CrossRef]
- Quinn, G.P.; Murphy, D.; Knapp, C.; Stearsman, D.K.; Bradley-Klug, K.L.; Sawczyn, K.; Clayman, M.L. Who Decides? Decision Making and Fertility Preservation in Teens with Cancer: A Review of the Literature. J. Adolesc. Health 2011, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.; Davies, S.; Wright, D.; Chapman, C.; Mbe, M.W. The experiences of teenagers and young adults with cancer—Results of 2004 conference survey. Eur. J. Oncol. Nurs. 2007, 11, 362–368. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T.; Gwede, C.K.; Miree, C.; King, L.M.; Clayton, H.B.; Wilson, C.; Munster, P. Discussion of fertility preservation with newly diagnosed patients: Oncologists’ views. J. Cancer Surviv. 2007, 1, 146–155. [Google Scholar] [CrossRef]
- Tam, M.W. Queering reproductive access: Reproductive justice in assisted reproductive technologies. Reprod. Health 2021, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vadaparampil, S.T.; Quinn, G.P.; Clayton, H.B.; King, L.M.; Miree, C.A. Institutional Availability of Fertility Preservation. Clin. Pediatr. 2008, 47, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Kroon, L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J. Natl. Compr. Cancer Netw. 2013, 11, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, A.; Laws, P.; Logan, S.; Saunders, C.; Travaglia, J.; Gerstl, B.; Bradford, N.; Cohn, R.; Birdsall, M.; Barr, R.; et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum. Reprod. Update 2018, 25, 159–179. [Google Scholar] [CrossRef] [Green Version]
- Clayman, M.L.; Harper, M.M.; Quinn, G.P.; Reinecke, J.; Shah, S. Oncofertility resources at NCI-designated comprehensive cancer centers. J. Natl. Compr. Cancer Netw. 2013, 11, 1504–1509. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, N.; Ghuman, N.; Sandher, R.; Herbert, M.; Stewart, J. Barriers and facilitators towards fertility preservation care for cancer patients: A meta-synthesis. Eur. J. Cancer Care 2015, 27, e12428. [Google Scholar] [CrossRef] [Green Version]
- Funding by Province. Fertility Matters Canada (FMC). Available online: https://fertilitymatters.ca/funding-by-province (accessed on 1 February 2022).
- OHIP Fertility Coverage—TRIO. TRIO Fertility Treatment Practice. Available online: https://triofertility.com/ohip-fertility-coverage/ (accessed on 1 February 2022).
- Fitch, M.; Longo, C.J. Exploring the impact of out-of-pocket costs on the quality of life of Canadian cancer patients. J. Psychosoc. Oncol. 2018, 36, 582–596. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 2017, CD001431. [Google Scholar] [CrossRef] [Green Version]
- Garvelink, M.M.; Ter Kuile, M.M.; Louwé, L.A.; Hilders, C.G.; Stiggelbout, A. Feasibility and effects of a decision aid about fertility preservation. Hum. Fertil. 2016, 20, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Ehrbar, V.; Germeyer, A.; Nawroth, F.; Dangel, A.; Findeklee, S.; Urech, C.; Rochlitz, C.; Stiller, R.; Tschudin, S. Long-term effectiveness of an online decision aid for female cancer patients regarding fertility preservation: Knowledge, attitude, and decisional regret. Obstet. Gynecol. Scand. 2021, 100, 1132–1139. [Google Scholar] [CrossRef]
- Speller, B.; Metcalfe, K.; Kennedy, E.D.; Facey, M.; Greenblatt, E.; Scheer, A.S.; Warner, E.; Joy, A.A.; Wright, F.C.; Baxter, N.N. The “Begin Exploring Fertility Options, Risks and Expectations” (BEFORE) decision aid: Development and alpha testing of a fertility tool for premenopausal breast cancer patients. BMC Med. Inform. Decis. Mak. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peate, M.; Meiser, B.; Cheah, B.C.; Saunders, C.; Butow, P.; Thewes, B.; Hart, R.; Phillips, K.-A.; Hickey, M.; Friedlander, M. Making hard choices easier: A prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br. J. Cancer 2012, 106, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, V.; Travado, L.; Ferreira, P.; Quinn, G. Protocol for the development and acceptability of a fertility-related decision aid for young women with breast cancer in Portugal. BMJ Open 2019, 9, e030690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Making Your Fertility Preservation Decision. Cancer Fertility and Me. Available online: https://cancerfertilityandme.org.uk/making-my-fertility-preservation-decisionKaGC5 (accessed on 1 February 2022).
- Jones, G.L.; Hughes, J.; Mahmoodi, N.; Greenfield, D.; Brauten-Smith, G.; Skull, J.; Gath, J.; Yeomanson, D.; Baskind, E.; Snowden, J.A.; et al. Observational study of the development and evaluation of a fertility preservation patient decision aid for teenage and adult women diagnosed with cancer: The Cancer, Fertility and Me research protocol. BMJ Open 2017, 7, e013219. [Google Scholar] [CrossRef]
- Allingham, C.; Gillam, L.; McCarthy, M.; Zacharin, M.; Jayasuriya, S.; Heloury, Y.; Orme, L.; Sullivan, M.; Peate, M.; Jayasinghe, Y. Fertility Preservation in Children and Adolescents with Cancer: Pilot of a Decision Aid for Parents of Children and Adolescents with Cancer. JMIR Pediatr. Parent. 2018, 1, e10463. [Google Scholar] [CrossRef]
- Pecoriello, J.; Klosky, J.L.; Augusto, B.; Santiago-Datil, W.; Sampson, A.; Reich, R.; Vadaparampil, S.; Quinn, G. Evolution and growth of the ECHO (Enriching Communication skills for Health professionals in Oncofertility) program: A 5-year study in the training of oncofertility professionals. J. Cancer Surviv. 2022, 1–7. [Google Scholar] [CrossRef]
- Quinn, G.P.; Curci, M.B.; Reich, R.R.; Gwede, C.K.; Meade, C.D.; Vadaparampil, S.T. The ENRICH/ECHO Working Group; ENRICH/ECHO Working Group Impact of a web-based reproductive health training program: ENRICH (Educating Nurses about Reproductive Issues in Cancer Healthcare). Psycho-Oncology 2019, 28, 1096–1101. [Google Scholar] [CrossRef]
- Quinn, G.P.; Vadaparampil, S.T.; Gwede, C.K.; Reinecke, J.D.; Mason, T.M.; Silva, C. Developing a referral system for fertility preservation among patients with newly diagnosed cancer. J. Natl. Compr. Cancer Netw. 2011, 9, 1219–1225. [Google Scholar] [CrossRef]
- Miller, E.J.N.; Cookingham, L.M.; Woodruff, T.K.; Ryan, G.L.; Summers, K.; Kondapalli, L.A.; Shah, D.K. Fertility preservation training for obstetrics and gynecology fellows: A highly desired but non-standardized experience. Fertil. Res. Pract. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Gardino, S.L.; Jeruss, J.S.; Woodruff, T.K. Using decision trees to enhance interdisciplinary team work: The case of oncofertility. J. Assist. Reprod. Genet. 2010, 27, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Corney, R.H.; Swinglehurst, A.J. Young childless women with breast cancer in the UK: A qualitative study of their fertility-related experiences, options, and the information given by health professionals. Psycho-Oncology 2013, 23, 20–26. [Google Scholar] [CrossRef]
- Bradford, A.; Woodard, T.L. Novel Psychological Intervention for Decision Support in Women Considering Fertility Preservation Before Cancer Treatment. J. Adolesc. Young Adult Oncol. 2017, 6, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Razzano, A.; Revelli, A.; Piane, L.D.; Salvagno, F.; Casano, S.; Randaccio, S.; Benedetto, C. Fertility preservation program before ovarotoxic oncostatic treatments: Role of the psychological support in managing emotional aspects. Gynecol. Endocrinol. 2014, 30, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Perz, J.; Ussher, J.; Peate, M.; Anazodo, A. Clinician provision of oncofertility support in cancer patients of a reproductive age: A systematic review. Psycho-Oncology 2017, 27, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinecke, J.D.; Kelvin, J.F.; Arvey, S.R.; Quinn, G.P.; Levine, J.; Beck, L.N.; Miller, A. Implementing a Systematic Approach to Meeting Patients’ Cancer and Fertility Needs: A Review of the Fertile Hope Centers of Excellence Program. J. Oncol. Pract. 2012, 8, 303–308. [Google Scholar] [CrossRef]
- Dorfman, C.S.; Stalls, J.M.; Mills, C.; Voelkel, S.; Thompson, M.; Acharya, K.S.; Baker, K.C.; Wagner, L.M.; Miller, N.; Boswell, A.; et al. Addressing Barriers to Fertility Preservation for Cancer Patients: The Role of Oncofertility Patient Navigation. J. Oncol. Navig. Surviv. 2021, 12, 332–348. [Google Scholar]
- Cancer Fertility Preservation|PCRM. Available online: https://edmonton.pacificfertility.ca/our-services/fertility-preservation/09ZSx (accessed on 1 February 2022).
- Ronn, R.; Holzer, H.E.G. Oncofertility in Canada: An Overview of Canadian Practice and Suggested Action Plan. Curr. Oncol. 2013, 20, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.M.; Duncan, F.E.; Ataman, L.; Smith, K.; Quinn, G.P.; Chang, R.J.; Finlayson, C.; Orwig, K.; Valli-Pulaski, H.; Moravek, M.B.; et al. The National Physicians Cooperative: Transforming fertility management in the cancer setting and beyond. Future Oncol. 2018, 14, 3059–3072. [Google Scholar] [CrossRef]
- Ataman, L.M.; Rodrigues, J.K.; Marinho, R.M.; Caetano, J.P.; Chehin, M.B.; da Motta, E.L.A.; Serafini, P.; Suzuki, N.; Furui, T.; Takae, S.; et al. Creating a Global Community of Practice for Oncofertility. JCO Glob. Oncol. 2020, 2, 317–330. [Google Scholar] [CrossRef]
| Decision Aid | Assessment Tool | Decision Tree | |
|---|---|---|---|
| Intended Use | Patients and parents | Health care providers | Health care providers |
| Developmental Stage Example | Multiple decision aids developed, and some validated [66,67,69] Rank the following statement from 1 (not important) to 4 (very important): having my own biological child after my cancer treatment is over. Adapted from [72] | No assessment tools developed, suggested by [53] Rank the following statement from 1 (low) to 4 (high): my patient’s understanding of female reproduction. Adapted from [53] | One decision tree developed, but none validated [78]  Adapted from [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazer, T.S.; Schulte, F. Barriers to Oncofertility Care among Female Adolescent Cancer Patients in Canada. Curr. Oncol. 2022, 29, 1583-1593. https://doi.org/10.3390/curroncol29030133
Glazer TS, Schulte F. Barriers to Oncofertility Care among Female Adolescent Cancer Patients in Canada. Current Oncology. 2022; 29(3):1583-1593. https://doi.org/10.3390/curroncol29030133
Chicago/Turabian StyleGlazer, Tali Sara, and Fiona Schulte. 2022. "Barriers to Oncofertility Care among Female Adolescent Cancer Patients in Canada" Current Oncology 29, no. 3: 1583-1593. https://doi.org/10.3390/curroncol29030133





