The OncoSim-Breast Cancer Microsimulation Model
Abstract
:1. Introduction
2. Materials and Methods
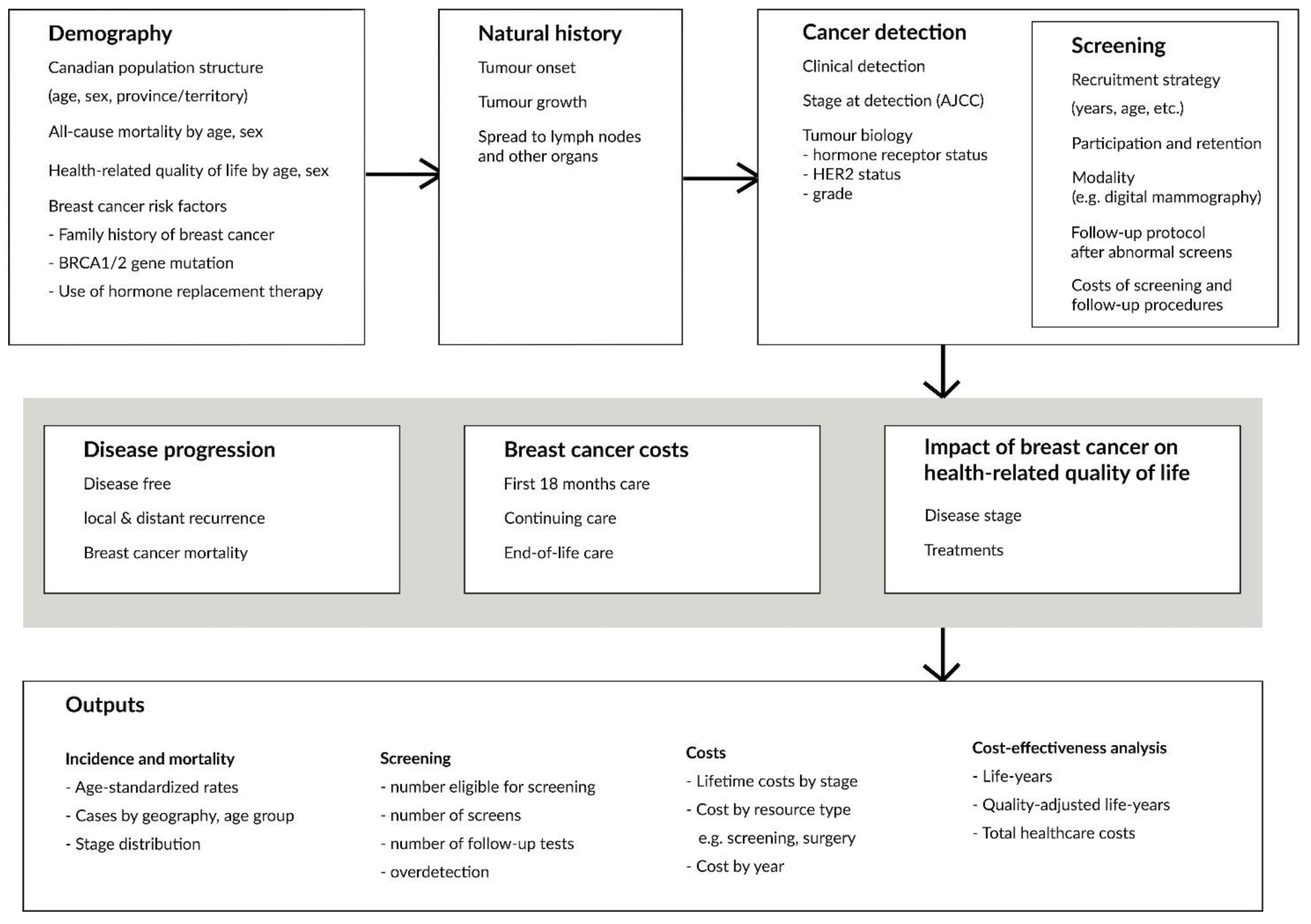
2.1. OncoSim-Breast
2.2. Demography
2.3. Natural History
2.4. Cancer Detection, Staging, and Tumour Biology
2.5. Disease Progression
2.6. Screening
2.7. Breast Cancer Costs
2.8. Health-Related Quality of Life
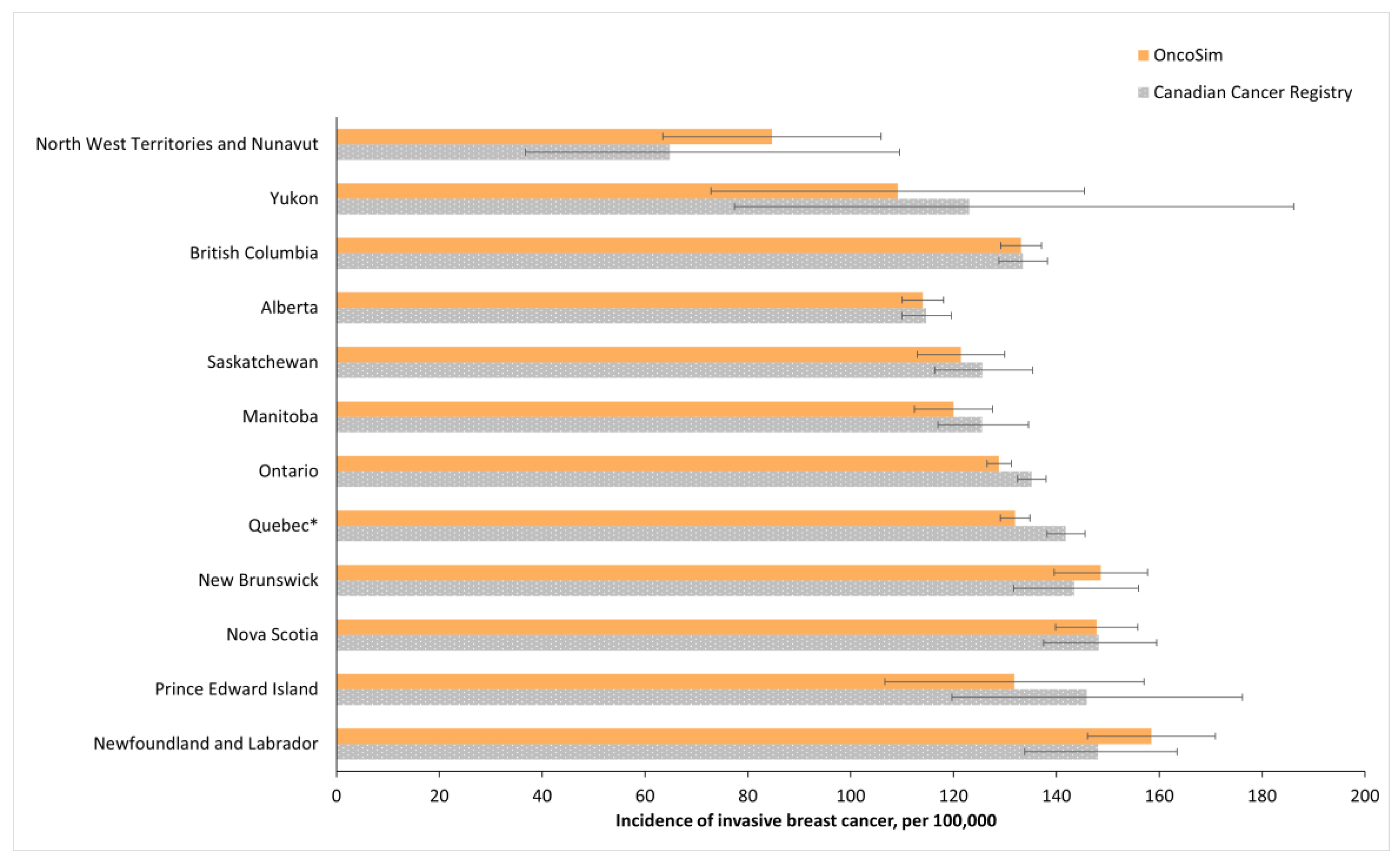
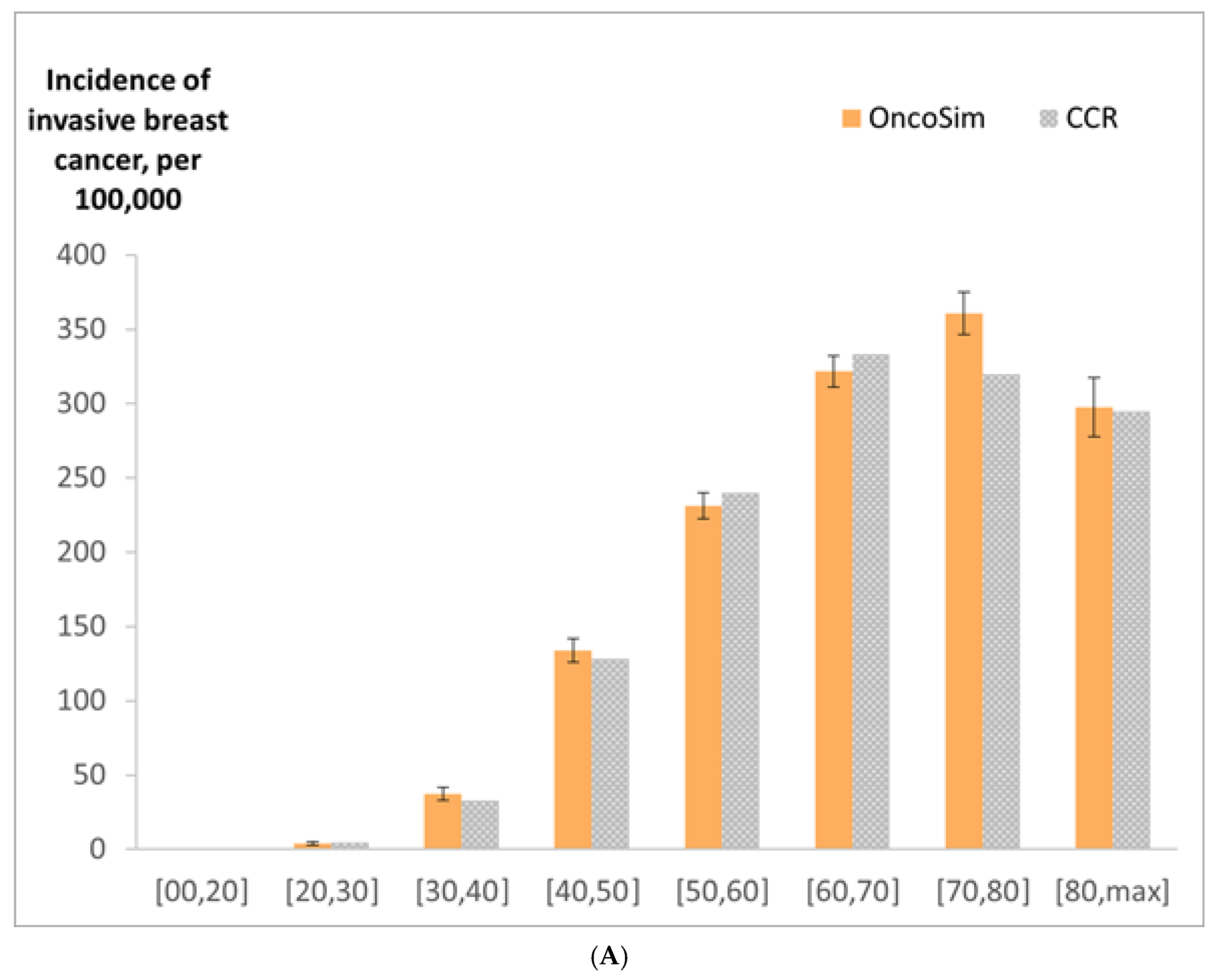
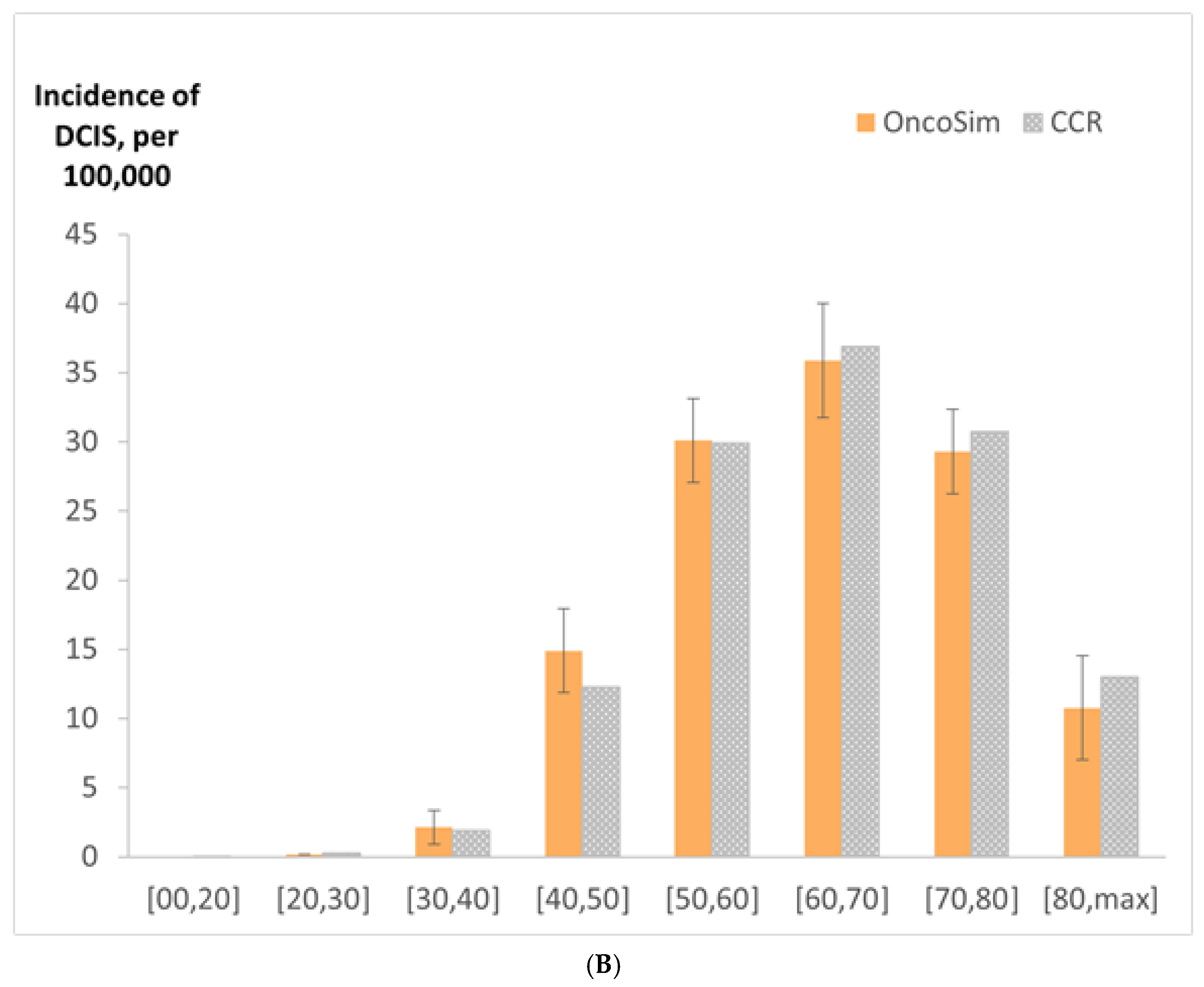
2.9. Model Validation
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, A.B.; To, T.; Baines, C.J.; Wall, C. The Canadian National Breast Screening Study-1: Breast cancer mortality after 11 to 16 years of follow-up: A randomized screening trial of mammography in women age 40 to 49 years. Ann. Intern. Med. 2002, 137, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyström, L.; Bjurstam, N.; Jonsson, H.; Zackrisson, S.; Frisell, J. Reduced breast cancer mortality after 20+ years of follow-up in the Swedish randomized controlled mammography trials in Malmö, Stockholm, and Göteborg. J. Med. Screen. 2017, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Bretveld, R.; Siesling, S.; Tilanus-Linthorst, M.M.A. Influence of tumour stage at breast cancer detection on survival in modern times: Population based study in 173,797 patients. BMJ 2015, 351, h4901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagoz, O.; Berry, D.A.; de Koning, H.J.; Feuer, E.J.; Lee, S.J.; Plevritis, S.K.; Schechter, C.B.; Stout, N.K.; Trentham-Dietz, A.; Mandelblatt, J.S. Introduction to the Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models. Med. Decis. Mak. 2018, 38, 3S–8S. [Google Scholar] [CrossRef]
- Coldman, A.; Flanagan, W.; Nadeau, C.; Wolfson, M.; Fitzgerald, N.; Memon, S.; Gauvreau, C.; Miller, A.; Earle, C. Projected effect of fecal immunochemical test threshold for colorectal cancer screening on outcomes and costs for Canada using the OncoSim microsimulation model. J. Cancer Policy 2017, 13, 38–46. [Google Scholar] [CrossRef]
- Coldman, A.; Pader, J.; Gauvreau, C.; Memon, S.; Fitzgerald, N.; Flanagan, W.; Nadeau, C.; Earle, C.; Wolfson, M.; Miller, A.; et al. Simulating results from trials of sigmoidoscopy screening using the OncoSim microsimulation model. J. Cancer Policy 2018, 15, 52–58. [Google Scholar] [CrossRef]
- Coldman, A.; Phillips, N.; Brisson, J.; Flanagan, W.; Wolfson, M.; Nadeau, C.; Fitzgerald, N.; Miller, A. Using the Cancer Risk Management Model to evaluate colorectal cancer screening options for Canada. Curr. Oncol. 2015, 22, e41–e50. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.; Gauvreau, C.; Flanagan, W.; Memon, S.; Fitzgerald, N.; Goffin, J.; Miller, A. Costs and Cost-Effectiveness of Smoking Cessation within an Organized CT Lung Cancer (LC) Screening Program. J. Thorac. Oncol. 2017, 12, S1757–S1758. [Google Scholar] [CrossRef]
- Evans, W.; Gauvreau, C.; Memon, S.; Goffin, J.; Lacombe, J.; Wolfson, M.; Fitzgerald, N.; Miller, A. Potential Health and Economic Consequences of Organized vs Opportunistic Lung Cancer Screening in Canada. J. Thorac. Oncol. 2017, 12, S309. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.K.; Flanagan, W.M.; Miller, A.B.; Goffin, J.R.; Memon, S.; Fitzgerald, N.; Wolfson, M. Implementing low-dose computed tomography screening for lung cancer in Canada: Implications of alternative at-risk populations, screening frequency, and duration. Curr. Oncol. 2016, 23, e179–e187. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, N.R.; Flanagan, W.M.; Evans, W.K.; Miller, A.B.; Canadian Partnership against Cancer Cancer Risk Management Lung Cancer Woring Grooup. Eligibility for low-dose computerized tomography screening among asbestos-exposed individuals. Scand. J. Work Environ. Health 2015, 41, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, W.M.; Evans, W.K.; Fitzgerald, N.R.; Goffin, J.R.; Miller, A.B.; Wolfson, M.C. Performance of the cancer risk management model lung cancer screening module. Health Rep. 2015, 26, 11–18. [Google Scholar] [PubMed]
- Gauvreau, C.L.; Fitzgerald, N.R.; Memon, S.; Flanagan, W.M.; Nadeau, C.; Asakawa, K.; Garner, R.; Miller, A.B.; Evans, W.K.; Popadiuk, C.M.; et al. The OncoSim model: Development and use for better decision-making in Canadian cancer control. Curr. Oncol. 2017, 24, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffin, J.R.; Flanagan, W.M.; Miller, A.B.; Fitzgerald, N.R.; Memon, S.; Wolfson, M.C.; Evans, W.K. Biennial lung cancer screening in Canada with smoking cessation—Outcomes and cost-effectiveness. Lung Cancer 2016, 101, 98–103. [Google Scholar] [CrossRef]
- Goffin, J.R.; Flanagan, W.M.; Miller, A.B.; Fitzgerald, N.R.; Memon, S.; Wolfson, M.C.; Evans, W.K. Cost-effectiveness of Lung Cancer Screening in Canada. JAMA Oncol. 2015, 1, 807–813. [Google Scholar] [CrossRef]
- Lacombe, J.; Gauvreau, C.; Memon, S.; Popadiuk, C.; Flanagan, W.; Nadeau, C.; Coldman, A.J.; Wolfson, M.; Miller, M. Exploring the health outcomes of various pan-Canadian cervical cancer screening programs using microsimulation modeling. Am. J. Epidemiol. 2016, 184, 79. [Google Scholar]
- Miller, A.B.; Gribble, S.; Nadeau, C.; Asakawa, K.; Flanagan, W.M.; Wolfson, M.; Coldman, A.; Evans, W.K.; Fitzgerald, N.; Lockwood, G.; et al. Evaluation of the natural history of cancer of the cervix, implications for prevention. The Cancer Risk Management Model (CRMM)—Human papillomavirus and cervical components. J. Cancer Policy 2015, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Popadiuk, C.; Coldman, A.; Memon, S.; Fitzgerald, N.; Gribble, S.; Lockwood, G.; Wolfson, M.; Miller, A. Comparing the health and economic impacts of cervical cancer screening strategies using the Cancer Risk Management Model (CRMM). Gynecol. Oncol. 2016, 141, 63. [Google Scholar] [CrossRef]
- Popadiuk, C.; Gauvreau, C.; Bhavsar, M.; Nadeau, C.; Asakawa, K.; Flanagan, W.; Wolfson, M.; Coldman, A.; Memon, S.; Fitzgerald, N.; et al. Using the Cancer Risk Management Model to evaluate the health and economic impacts of cytology compared with human papillomavirus DNA testing for primary cervical cancer screening in Canada. Curr. Oncol. 2016, 23, S56–S63. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, D.A.; Flanagan, W.M.; Tanuseputro, P.; Bennett, C.J.; Tuna, M.; Kopec, J.A.; Wolfson, M.C.; Manuel, D.G. The Population Health Model (POHEM): An overview of rationale, methods and applications. Popul. Health Metr. 2015, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br. J. Cancer 2000, 83, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Baines, C.J.; To, T.; Wall, C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ 1992, 147, 1459–1476. [Google Scholar]
- Statistics Canada. National Population Health Survey. Available online: https://www150.statcan.gc.ca/n1/en/catalogue/82F0001X (accessed on 22 February 2022).
- National Cancer Institute Cancer Intervention and Surveillance Modeling Network. University of Wisconsin Breast Cancer Simulation Model. Available online: https://cisnet.flexkb.net/mp/pub/CISNET_ModelProfile_BREAST_UWISC_001_07232013_58567.pdf (accessed on 22 February 2022).
- Singletary, S.E. Rating the risk factors for breast cancer. Ann. Surg. 2003, 237, 474. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Rohan, T.E.; Manson, J.E.; Aragaki, A.K.; Kaunitz, A.M.; Stefanick, M.L.; Simon, M.S.; Johnson, K.C.; Wactawski-Wende, J.; O’sullivan, M.J.; et al. Breast cancer after use of estrogen plus progestin and estrogen alone: Analyses of data from 2 women’s health initiative randomized clinical trials. JAMA Oncol. 2015, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance Plan Schedule of Benefits and Fees. Available online: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/ (accessed on 22 February 2022).
- Ontario Breast Screening Program 2011 Report; Cancer Care Ontario: Toronto, ON, Canada, 2013.
- Cheung, M.C.; Earle, C.C.; Rangrej, J.; Ho, T.H.; Liu, N.; Barbera, L.; Saskin, R.; Porter, J.; Seung, S.J.; Mittmann, N. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer 2015, 121, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Guertin, J.R.; Feeny, D.; Tarride, J.-E. Age- and sex-specific Canadian utility norms, based on the 2013–2014 Canadian Community Health Survey. CMAJ 2018, 190, E155–E161. [Google Scholar] [CrossRef] [Green Version]
- Boswell-Purdy, J.; Flanagan, W.M.; Roberge, H.; Le Petit, C.; White, K.J.; Berthelot, J.-M. Population health impact of cancer in Canada, 2001. Chronic Dis. Inj. Can. 2007, 28, 42–55. [Google Scholar]
- Alagoz, O.; Ergun, M.A.; Cevik, M.; Sprague, B.L.; Fryback, D.G.; Gangnon, R.E.; Hampton, J.M.; Stout, N.K.; Trentham-Dietz, A. The University of Wisconsin Breast Cancer Epidemiology Simulation Model: An Update. Med. Decis. Mak. 2018, 38, 99s–111s. [Google Scholar] [CrossRef]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, S.; Anagnostaki, L.; Jirström, K.; Landberg, G.; Manjer, J. Breast tumours following combined hormone replacement therapy express favourable prognostic factors. Int. J. Cancer 2007, 120, 2202–2207. [Google Scholar] [CrossRef]
- Flanagan, W.M.; McIntosh, C.N.; Le Petit, C.; Berthelot, J.-M. Deriving utility scores for co-morbid conditions: A test of the multiplicative model for combining individual condition scores. Popul. Health Metr. 2006, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Canada. Deaths, by Cause: Malignant Neoplasm of Breast (C50), Canada, 2018 (Table: 13-10-0142-01). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1310014201 (accessed on 22 February 2022).
- Moss, S.M.; Cuckle, H.; Evans, A.; Johns, L.; Waller, M.; Bobrow, L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: A randomised controlled trial. Lancet 2006, 368, 2053–2060. [Google Scholar] [CrossRef]
- Moss, S.M.; Wale, C.; Smith, R.; Evans, A.; Cuckle, H.; Duffy, S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years’ follow-up: A randomised controlled trial. Lancet Oncol. 2015, 16, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Broek, J.J.V.D.; Van Ravesteyn, N.T.; Mandelblatt, J.S.; Huang, H.; Ergun, M.A.; Burnside, E.S.; Xu, C.; Li, Y.; Alagoz, O.; Lee, S.J.; et al. Comparing CISNET Breast Cancer Incidence and Mortality Predictions to Observed Clinical Trial Results of Mammography Screening from Ages 40 to 49. Med. Decis. Mak. 2018, 38, 140S–150S. [Google Scholar] [CrossRef]
- Moss, S.; Waller, M.; Anderson, T.J.; Cuckle, H. Randomised controlled trial of mammographic screening in women from age 40: Predicted mortality based on surrogate outcome measures. Br. J. Cancer 2005, 92, 955–960. [Google Scholar] [CrossRef]
- Yong, J.H.; Mainprize, J.G.; Yaffe, M.J.; Ruan, Y.; Poirier, A.E.; Coldman, A.; Nadeau, C.; Iragorri, N.; Hilsden, R.J.; Brenner, D.R. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J. Med. Screen 2021, 28, 100–107. [Google Scholar] [CrossRef]
- Kerlikowske, K. Epidemiology of ductal carcinoma in situ. J. Natl. Cancer Inst. 2010, 41, 139–141. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Wall, C.; Baines, C.J.; Miller, A.B. Is carcinoma in situ a precursor lesion of invasive breast cancer? IJC 2014, 135, 1646–1652. [Google Scholar]
- Nekhlyudov, L.; Habel, L.A.; Achacoso, N.; Jung, I.; Haque, R.; Collins, L.C.; Schnitt, S.J.; Quesenberry, C.P., Jr.; Fletcher, S.W. Ten-year risk of diagnostic mammograms and invasive breast procedures after breast-conserving surgery for DCIS. J. Natl. Cancer Inst. 2012, 104, 614–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, T.M.; Jarosek, S.; Habermann, E.B.; Arrington, A.; Abraham, A.; Morris, T.J.; Virnig, B.A. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J. Clin. Oncol. 2009, 27, 1362–1367. [Google Scholar] [CrossRef]
- Coldman, A.J.; Phillips, N. False-positive Screening Mammograms and Biopsies Among Women Participating in a Canadian Provincial Breast Screening Program. Can. J. Public Health 2012, 103, e420–e424. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.A.; Miglioretti, D.L.; Yankaskas, B.C.; Kerlikowske, K.; Rosenberg, R.; Rutter, C.M.; Geller, B.M.; Abraham, L.A.; Taplin, S.H.; Dignan, M.; et al. Individual and Combined Effects of Age, Breast Density, and Hormone Replacement Therapy Use on the Accuracy of Screening Mammography. Ann. Intern. Med. 2003, 138, 168–175. [Google Scholar] [CrossRef] [PubMed]


| Model Inputs | Estimates | Data Sources |
|---|---|---|
| Demography | ||
| Canadian population structure (age, sex, province/territory) | Statistics Canada Demography Division | |
| All-cause mortality by age, sex | Statistics Canada Demography Division | |
Breast cancer risk factors
| Supplemental File S1: Table S1 | Anglian Breast Cancer Study group [21] Canadian National Breast Screening Study (CNBSS) [22] National Population Health Survey (1994–2010) [23] |
| Natural history | ||
| Rate of occult tumour onset (oncogenesis) | Supplemental File S1: Figure S1 | Calibrated from the input parameters in the University of Wisconsin Breast Cancer Model [24] to match the incidence data in the cancer registry *. |
| Distribution of tumour type (DCIS vs. invasive) by age | Supplemental File S1: Table S2 | |
| Relative risk of developing occult tumour based on BRCA1/2 gene mutation and breast cancer family history | Supplemental File S1: Table S3 | Calibrated from Singletary SE (2003) [25] to match the incidence data in the cancer registry * |
| Relative risk of developing occult tumour based on hormone therapy use | Supplemental File S1: Table S4 | Calibrated to match the results of a study reporting the impact of hormone therapy use on breast cancer risk [26] |
| Tumour growth | Supplemental File S1: Table S5 | Calibrated from the Wisconsin Breast model’s parameters [24] to match stage-specific incidence data in the Canadian Cancer Registry (1992–2013) and Canadian Cancer Screening Database (2007–2008) |
| Tumour spread to other lymph nodes, hazard | Supplemental File S1: Tables S5 and S6 | |
| Metastasis hazard | Supplemental File S1: Tables S5 and S7 | Calibrated to match stage-specific incidence data in Canadian Cancer Registry (1992–2013) and Canadian Breast Cancer Screening Database (2007–2008). |
| Cancer detection | ||
| Probability of clinical detection by tumour size | Supplemental File S1: Table S8 | Calibrated from the input parameters in the University of Wisconsin Breast Cancer Model [24] to match the incidence data in the cancer registry *. |
| Stage distribution at detection | Supplemental File S1: Tables S9–S11 | Canadian Cancer Registry * |
| Breast tumour biology | Joint distribution of hormone receptor status, HER2neu status, and grade at detection, by tumour size, nodal involvement, metastatic status, and age of women at tumour detection (Supplementary File S2) | Canadian Cancer Registry * |
| Disease progression | ||
| Stage-specific recurrence and survival risks | Supplemental File S1: Tables S12–S16 | Unpublished data from British Columbia † |
| Province/territory-specific relative risk of breast cancer survival | Supplemental File S1: Table S17 | Canadian Cancer Registry * |
| Screening | ||
| Sensitivity and specificity of mammography | Supplemental File S1: Figure S5 and Table S18 | |
| Cost of follow-up procedures for abnormal screen results | Supplemental File S1: Table S19 | Ontario Breast Screening Program 2011, Canadian Breast Cancer Screening Database 2004–2008 and Ontario Health Insurance fee schedules [27,28] |
| Breast cancer costs | Supplemental File S1: Section 6 | Retrospective administrative database analysis using Ontario data, Ontario Health Insurance Program schedule of benefits, and end-of-life costing study of breast cancer patients [27,29] |
| Age-specific health state utilities–Canadian general population | Supplemental File S1: Table S21 | [30] |
| Breast cancer-specific preference score | Supplemental File S1: Table S22 | [31] |
| OncoSim | Age Trial | CISNET Models * | |
|---|---|---|---|
| Detection of invasive breast cancer | 16% more | 10% (95% CI: 0.95 to 1.21) [40] | N/A |
| Breast cancer death reduction at 10-year follow-up | 15% | 25% (95% CI, 3% to 42%) [37] | 15% (range, 13% to 17%) |
| Breast cancer death reduction at 17-year follow-up | 15% | 12% (95% CI: −4% to 26%) [38,39] | 13% (range, 10% to 17%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, J.H.E.; Nadeau, C.; Flanagan, W.M.; Coldman, A.J.; Asakawa, K.; Garner, R.; Fitzgerald, N.; Yaffe, M.J.; Miller, A.B. The OncoSim-Breast Cancer Microsimulation Model. Curr. Oncol. 2022, 29, 1619-1633. https://doi.org/10.3390/curroncol29030136
Yong JHE, Nadeau C, Flanagan WM, Coldman AJ, Asakawa K, Garner R, Fitzgerald N, Yaffe MJ, Miller AB. The OncoSim-Breast Cancer Microsimulation Model. Current Oncology. 2022; 29(3):1619-1633. https://doi.org/10.3390/curroncol29030136
Chicago/Turabian StyleYong, Jean H. E., Claude Nadeau, William M. Flanagan, Andrew J. Coldman, Keiko Asakawa, Rochelle Garner, Natalie Fitzgerald, Martin J. Yaffe, and Anthony B. Miller. 2022. "The OncoSim-Breast Cancer Microsimulation Model" Current Oncology 29, no. 3: 1619-1633. https://doi.org/10.3390/curroncol29030136
APA StyleYong, J. H. E., Nadeau, C., Flanagan, W. M., Coldman, A. J., Asakawa, K., Garner, R., Fitzgerald, N., Yaffe, M. J., & Miller, A. B. (2022). The OncoSim-Breast Cancer Microsimulation Model. Current Oncology, 29(3), 1619-1633. https://doi.org/10.3390/curroncol29030136





