Not All Canadian Cancer Patients Are Equal—Disparities in Public Cancer Drug Funding across Canada
Abstract
:1. Introduction
2. Methods
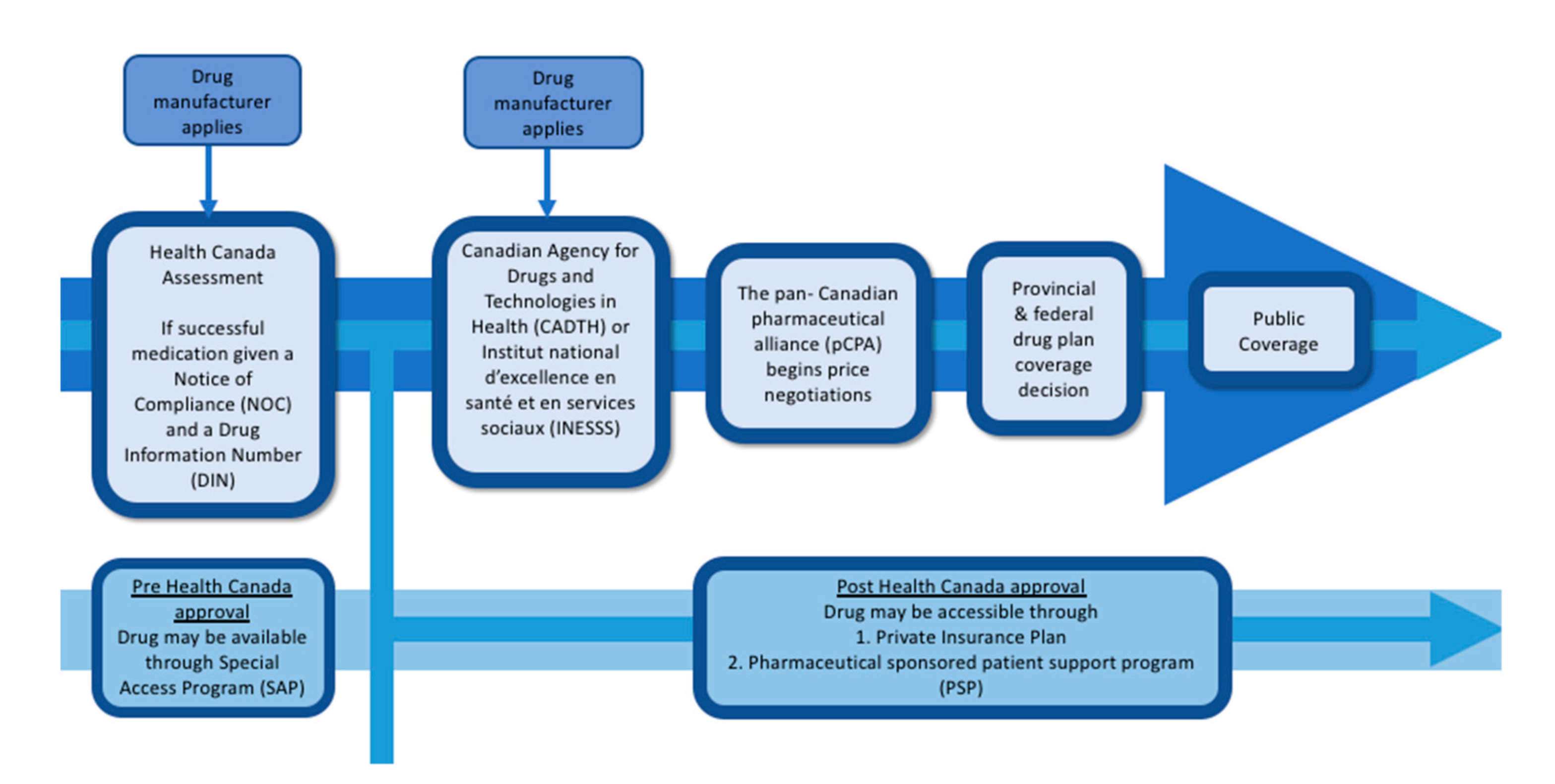
3. Drug Assessment and Approval to Public Formulary
4. Variation in Public Coverage of Take-Home Cancer Drugs
5. The Role of Patient Support Programs (PSPs)
| Jurisdiction | Oral Cancer Drugs | IV Cancer Drug | Supportive Care Drugs |
|---|---|---|---|
| Alberta | Covered, if on formulary | Covered, if on formulary | Not covered |
| British Colombia | Covered, if on formulary | Covered, if on formulary | Not covered |
| Manitoba | Covered, if on formulary | Covered, if on formulary | Exception Status |
| New Brunswick | Exception Status | Covered, if on formulary | Covered, if on formulary |
| Newfoundland and Labrador | Exception Status | Covered, if on formulary | Exception Status |
| Nova Scotia | Exception Status | Covered, if on formulary | Exception Status |
| Prince Edward Island | Limited coverage | Covered, if on formulary | Limited coverage |
| Ontario | Exception Status | Covered, if on formulary | Exception status |
| Saskatchewan | Covered, if on formulary | Covered, if on formulary | Covered, if on formulary |
| Quebec | Exception Status | Covered, if on formulary | Exception Status |
| Yukon | Exception Status | Covered, if on formulary | Exception Status |
| Non-insured Health Benefits, Northwest Territories Extended Health Benefits | Exception Status | Covered, if on formulary | Exception status |
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, D.; Stafinski, T.; Stuart, G. Access to Drugs for Cancer. Can. J. Public Health 2005, 96, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; North, S. Patterns of cost-related medication underuse among Canadian adults with cancer: A cross-sectional study using survey data. CMAJ Open 2021, 9, E474–E481. [Google Scholar] [CrossRef] [PubMed]
- Demers, V.; Melo, M.; Jackevicius, C.; Cox, J.; Kalavrouziotis, D.; Rinfret, S.; Humphries, K.H.; Johansen, H.; Tu, J.V.; Pilote, L. Comparison of provincial prescription drug plans and the impact on patients’ annual drug expenditures. Can. Med. Assoc. J. 2008, 178, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, C.J.; Fitch, M.I.; Loree, J.M.; Carlson, L.E.; Turner, D.; Cheung, W.Y.; Gopaul, D.; Ellis, J.; Ringash, J.; Mathews, M.; et al. Patient and Family Financial Burden Associated with Cancer Treatment in Canada: A National Study. Support. Care Cancer 2021, 29, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Lung Cancer Canada. Faces of Lung Cancer; Lung Cancer Canada: Toronto, ON, Canada, 2019. [Google Scholar]
- Patented Medicine Prices Review Board. Alignment among Public Formularies in Canada. Part 2: Oncology Medicines; Patented Medicine Prices Review Board: Ottawa, ON, Canada, 2021. [Google Scholar]
- Government of Canada. How Drugs Are Reviewed in Canada. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/fact-sheets/drugs-reviewed-canada.html (accessed on 17 October 2021).
- Clement, F.; Memedovich, K.A. Drug coverage in Canada: Gaps and opportunities. J. Psychiatry Neurosci. 2018, 43, 148–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government of Canada. Non-Insured Health Benefits: Drug Benefit List. Available online: https://www.sac-isc.gc.ca/eng/1572888328565/1572888420703 (accessed on 29 January 2022).
- Government of Canada. Public Service Health Care Plan Summary. Available online: https://www.canada.ca/en/treasury-board-secretariat/services/benefit-plans/health-care-plan/public-service-health-care-plan-glance.html (accessed on 29 January 2022).
- Keech, J.; Dai, W.F.; Trudeau, M.; Mercer, R.E.; Naipaul, R.; Wright, F.C.; Ferguson, S.E.; Darling, G.; Gavura, S.; Eisen, A.; et al. Impact of rarity on Canadian oncology health technology assessment and funding. Int. J. Technol. Assess. Health Care 2020, 36, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, A.; Penner, N.; Chan, K.; Sabharwal, M.; Grill, A. Understanding the Reasons for Provincial Discordance in Cancer drug Funding—A Survey of Policymakers. Curr. Oncol. 2018, 25, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenei, K.; Peacock, S.; Burgess, M.; Mitton, C. Describing Sources of Uncertainty in Cancer Drug Formulary Priority Setting across Canada. Curr. Oncol. 2021, 28, 236. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, A.; Mai, H.; Penner, N.; Amir, E.; Laupacis, A.; Sabharwal, M.; Chan, K.K.W. Impact of the Pan-Canadian Oncology Drug Review on Provincial Concordance with Respect to Cancer Drug Funding Decisions and Time to Funding. Curr. Oncol. 2017, 24, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotfrit, J.; Shin, J.J.; Mallick, R.; Stewart, D.J.; Wheatley-Price, P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncologist 2019, 25, e130–e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Trinkaus, M.; Hogeveen, S.; Mamdani, M.; Berry, S.R.; Jang, R.W.; Hoch, J.S.; Simmons, C. Overcoming Obstacles in Accessing Unfunded Oral Chemotherapy: Physician Experience and Challenges. J. Oncol. Pract. 2013, 9, 188–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.W. Benefits Outweigh Costs in Universal Healthcare: Business Case for Reimbursement of Take-Home Cancer Medicines in Ontario and Atlantic Canada. Am. J. Med. Med. Sci. 2014, 2014, 126–138. [Google Scholar] [CrossRef]
- Lamb-Palmer, D.; Loschmann, C.; Henricks, P.; Shen, J.; Downson, J.; Mohideen, S. Uncovering the Hidden Costs of Take-Home Cancer Drugs; Canadian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]
- Bick, R. Can Certainty Coalition Equal Access to Take-Home Cancer Drugs Provide Equal Access to Take-Home Cancer Drugs. Available online: https://assets.nationbuilder.com/cancertainty/pages/34/attachments/original/1645544202/CanCertainty_Coalition_Budget_Proposal_2022_%28FULL_FINAL%29.pdf (accessed on 11 February 2022).
- Sorin, M.; Franco, E.L.; Quesnel-Vallée, A. Inter- and Intraprovincial Inequities in Public Coverage of Cancer Drug Programs across Canada: A Plea for the Establishment of a Pan-Canadian Pharmacare Program. Curr. Oncol. 2019, 26, 266–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitch, M.I.; Longo, C.J.; Chan, R.J. Cancer patients’ perspectives on financial burden in a universal healthcare system: Analysis of qualitative data from participants from 20 provincial cancer centers in Canada. Patient Educ. Couns. 2021, 104, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Nova Scotia Department of Health and Wellness. Family Pharmacare-Calculator. Available online: https://novascotia.ca/dhw/pharmacare/family-calculator.asp (accessed on 4 March 2022).
- Nova Scotia Health. Fund to Help Patients with Cost of Take Home Cancer Drugs. Available online: https://www.nshealth.ca/news/fund-help-patients-cost-take-home-cancer-drugs (accessed on 4 March 2022).
- Saskatchewan Cancer Agency. Saskatchewan Cancer Agency Drug Formulary. Available online: http://www.saskcancer.ca/images/pdfs/health_professionals/drug_formulary/drug_formulary/SCA_Drug_Formulary_-_2021-11-01.pdf (accessed on 1 November 2021).
- Ontario Drug Formulary: Pegfilgrastim. Available online: https://www.formulary.health.gov.on.ca/formulary/detail.xhtml?drugId=02497395 (accessed on 29 November 2021).
- Outpatient Cancer Drug Benefit Program. Alberta Health Services. Available online: https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1025651 (accessed on 18 September 2021).
- Leaver, P.J.; Jang, H.S.-I.; Vernon, S.T.; Fernando, S.L. Immune checkpoint inhibitor-mediated myasthenia gravis with focal subclinical myocarditis progressing to symptomatic cardiac disease. BMJ Case Rep. 2020, 13, e232920. [Google Scholar] [CrossRef]
- Government of Manitoba. List of Oral Oncology Drugs Dispensed at CancerCare Manitoba. Available online: https://www.gov.mb.ca/health/pharmacare/profdocs/oral_oncology_drugs_list.pdf (accessed on 1 October 2021).
- New Brunswick Drug Formulary. Available online: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/NBDrugPlan/NewBrunswickDrugPlansFormulary.pdf (accessed on 1 November 2021).
- Newfoundland and Labrador Health and Community Services. Criteria for the Coverage of Special Authorization Drugs. Available online: https://www.gov.nl.ca/hcs/files/Criteria-Feb-2021.pdf (accessed on 1 November 2021).
- Nova Scotia Department of Health. Criteria for Coverage of Exception Status Coverage. Available online: https://novascotia.ca/dhw/pharmacare/documents/Criteria-for-Exception-Status-Coverage.pdf (accessed on 1 November 2021).
- Ontario Ministry of Health. Exceptional Access Program Reimbursement Criteria for Frequently Requested Drugs. Available online: https://health.gov.on.ca/en/pro/programs/drugs/docs/frequently_requested_drugs.pdf (accessed on 29 January 2022).
- Yukon Drug Programs. Yukon Public Formulary Search. Available online: https://ihs.gov.yk.ca/drugs/f?p=161:9000 (accessed on 1 November 2021).
- NIHB Program. Non-Insured Health Benefits Drug Benefit List. Available online: https://nihb.express-scripts.ca/NIHBProvider/benefits/pharmacy?page=drugbenefit-grid&benefit=pharmacy (accessed on 1 November 2021).
- CancerCare Manitoba. Manitoba Home Cancer Drug Program. Available online: https://www.cancercare.mb.ca/export/sites/default/Treatments/.galleries/files/home-cancer-drug-program-files/HCD-Program-patient-handout-April-19-2012.pdf (accessed on 29 January 2022).
- New Brunswick Government. New Brunswick Drug Plans Eligibility and Frequently Asked Questions. Available online: https://www2.gnb.ca/content/gnb/en/departments/health/MedicarePrescriptionDrugPlan/NBDrugPlan/questions.html (accessed on 1 November 2021).
- Newfoundland and Labrador Health and Community Services. Newfoundland and Labrador Perscription Drug Program Plan Overview. Available online: https://www.gov.nl.ca/hcs/prescription/nlpdp-plan-overview/ (accessed on 29 January 2022).
- Department of Health and Wellness Nova Scotia. Drug Assistance for Cancer Patients. Available online: https://novascotia.ca/dhw/pharmacare/cancer-assistance.asp (accessed on 29 January 2022).
- Health PEI. Prince Edward Island Catastrophic Drug Program. Available online: https://www.princeedwardisland.ca/en/information/health-pei/catastrophic-drug-program (accessed on 29 January 2022).
- Saskatchewan Provincial Government. Drug Cost Assistance. Available online: https://www.saskatchewan.ca/residents/health/prescription-drug-plans-and-health-coverage/extended-benefits-and-drug-plan/drug-cost-assistance (accessed on 29 January 2022).
- Barua, B.; Westcott, W.; Ngheim Vo, V. Timely Access to New Pharmaceuticals in Canada, the United States, and the European Union; Fraser Institute: Vancouver, BC, Canada, 2021. [Google Scholar]
- Pignatti, F.; Wilking, U.; Postmus, D.; Wilking, N.; Delgado, J.; Bergh, J. The value of anticancer drugs—A regulatory view. Nat. Rev. Clin. Oncol. 2021, 19, 207–215. [Google Scholar] [CrossRef] [PubMed]
- PriceWaterhouseCoopers. Six Drug Pricing Models have Emerged to Improve Product Access and Affordability. Available online: https://www.pwc.com/us/en/industries/health-industries/library/6-drug-pricing-models.html (accessed on 5 March 2022).
- Fitch, M.I.; Longo, C.J. Emerging Understanding About the Impact of Financial Toxicity Related to Cancer: Canadian Perspectives. Semin. Oncol. Nurs. 2021, 37, 151174. [Google Scholar] [CrossRef] [PubMed]
- Rae, E.; Chan, A.; Yeung, L.; Gavura, S.; Arias, J.; Kukreti, V.; Kaizer, L. Enhancing the delivery of take-home cancer therapies in Ontario. J. Clin. Oncol. 2014, 32 (Suppl. S30), 46. [Google Scholar] [CrossRef]
- Morgan, S.G.; Gagnon, M.-A.; Charbonneau, M.; Vadeboncoeur, A. Evaluating the effects of Quebec’s private-public drug insurance system. Can. Med. Assoc. J. 2017, 189, E1259–E1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, J.; Shearer, B.; Morgan, S.G. Prescription drug coverage in Canada: A review of the economic, policy and political considerations for universal pharmacare. J. Pharm. Policy Pract. 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacPhail, C.; Snow, S. Not All Canadian Cancer Patients Are Equal—Disparities in Public Cancer Drug Funding across Canada. Curr. Oncol. 2022, 29, 2064-2072. https://doi.org/10.3390/curroncol29030166
MacPhail C, Snow S. Not All Canadian Cancer Patients Are Equal—Disparities in Public Cancer Drug Funding across Canada. Current Oncology. 2022; 29(3):2064-2072. https://doi.org/10.3390/curroncol29030166
Chicago/Turabian StyleMacPhail, Ceilidh, and Stephanie Snow. 2022. "Not All Canadian Cancer Patients Are Equal—Disparities in Public Cancer Drug Funding across Canada" Current Oncology 29, no. 3: 2064-2072. https://doi.org/10.3390/curroncol29030166
APA StyleMacPhail, C., & Snow, S. (2022). Not All Canadian Cancer Patients Are Equal—Disparities in Public Cancer Drug Funding across Canada. Current Oncology, 29(3), 2064-2072. https://doi.org/10.3390/curroncol29030166






