Pulmonary Resection after Radiosurgery and Neoadjuvant Immunochemotherapy for NSCLC Patients with Synchronous Brain Metastasis—A Case Series of Three Patients
Abstract
:Simple Summary
Abstract
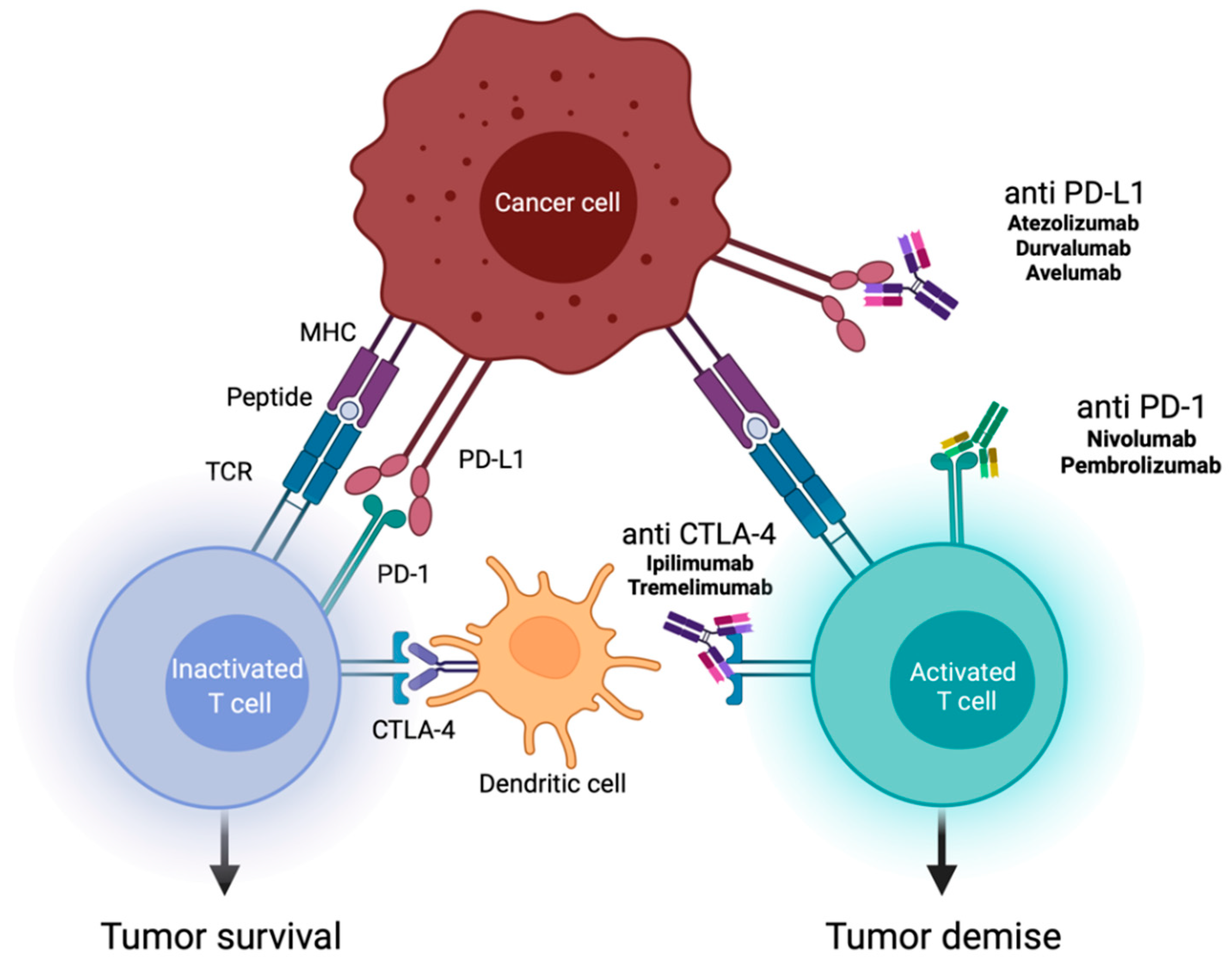
1. Introduction
2. Materials and Methods
3. Results
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Govindan, R.; Grannis, F.W., Jr.; et al. Non–Small Cell Lung Cancer, Version 2.2013. J. Natl. Compr. Cancer Netw. 2013, 11, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro-Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, E.; Peterson, J.; Brown, P.D.; Sheehan, J.P.; Quiñones-Hinojosa, A.; Zaorsky, N.G.; Trifiletti, D.M. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother. Oncol. 2019, 130, 104–112. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: Asco-Sno-Astro Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Taieb, J.; Moehler, M.; Boku, N.; Ajani, J.A.; Ruiz, E.Y.; Ryu, M.-H.; Guenther, S.; Chand, V.; Bang, Y.-J. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: Current status and future perspectives. Cancer Treat. Rev. 2018, 66, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Created with Biorender.com. Adapted from “Checkpointinhibitor Pathways”, by Biorender.Com (2022). Available online: https://App.Biorender.Com/Biorender-Templates (accessed on 28 January 2022).
- Shen, H.; Cao, Y.; Li, X.; Tan, Y.; Chen, J.; Yang, Z.; Kong, Y.; Yuan, Y. Surgical Intervention Improves Survival for Metastatic Non-Small Cell Lung Cancer Patients. Medicine 2016, 95, e3800. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, C.; Kim, T.H.; Hong, J.B.; Park, C.H.; Chang, Y.S.; Kim, H.J.; Ahn, C.M.; Byun, M.K. Efficacy of Surgical Treatment for Brain Metastasis in Patients with Non-Small Cell Lung Cancer. Yonsei Med. J. 2015, 56, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-W.; Cho, H.-J.; Lee, W.-H.; Sonntag, W.E. Whole Brain Radiation-Induced Cognitive Impairment: Pathophysiological Mechanisms and Therapeutic Targets. Biomol. Ther. 2012, 20, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steindl, A.; Ms, S.Y.; Ms, K.G.; Seiwald, M.; Gatterbauer, B.; Dieckmann, K.; Frischer, J.M.; Klikovits, T.; Zöchbauer-Müller, S.; Grisold, A.; et al. Neurological symptom burden impacts survival prognosis in patients with newly diagnosed non–small cell lung cancer brain metastases. Cancer 2020, 126, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, K.E.; Rucker, A.J.; Ready, N.E.; D’Amico, T.A.; Antonia, S.J. The immunotherapeutic landscape in non–small cell lung cancer and its surgical horizons. J. Thorac. Cardiovasc. Surg. 2020, 159, 1616–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- Reuss, J.; Smith, K.N.; Anagnostou, V.; Zhang, J.; Zahurak, M.; Caushi, J.; Chan, H.Y.; Guo, H.; Hellmann, M.D.; Pardoll, D.M.; et al. Neoadjuvant nivolumab in resectable non-small cell lung cancer: Extended follow-up and molecular markers of response. J. Clin. Oncol. 2019, 37, 8524. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.; Lin, H.; Pataer, A.; Godoy, M.; Carter, B.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Li, N.; Ying, J.; Tao, X.; Zhang, F.; Zhao, Z.; Ling, Y.; Gao, Y.; Zhao, J.; Xue, Q.; Mao, Y.; et al. Efficacy and safety of neoadjuvant PD-1 blockade with sintilimab in resectable squamous non-small cell lung cancer (sqNSCLC). J. Clin. Oncol. 2019, 37, 8531. [Google Scholar] [CrossRef]
- Huynh, C.; Walsh, L.A.; Spicer, J.D. Surgery after neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 563–580. [Google Scholar] [CrossRef]
- Jones, D.R. Neoadjuvant immunochemotherapy in surgically resectable non-small-cell lung cancer: Surgical expertise required. Eur. J. Cardio-Thorac. Surg. 2021, 60, 88–90. [Google Scholar] [CrossRef]
- Yang, Z.-R.; Liu, M.-N.; Yu, J.-H.; Yang, Y.-H.; Chen, T.-X.; Han, Y.-C.; Zhu, L.; Zhao, J.-K.; Fu, X.-L.; Cai, X.-W. Treatment of stage III non-small cell lung cancer in the era of immunotherapy: Pathological complete response to neoadjuvant pembrolizumab and chemotherapy. Transl. Lung Cancer Res. 2020, 9, 2059–2073. [Google Scholar] [CrossRef]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic Versus Video-Assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-Analysis. Ann. Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, G.; Maniaci, A.; Bianchi, G.; Cammaroto, G.; Iannella, G.; Catalano, A.; Sgarzani, R.; De Vito, A.; Capaccio, P.; Pelucchi, S.; et al. Neck dissection and trans oral robotic surgery for oropharyngeal squamous cell carcinoma. Auris Nasus Larynx 2021, 49, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC Lung Cancer Staging Project: A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Kim, Y.-H.; Kwon, M.; Ryu, I.S.; Jung, G.-E.; Kim, S.-T.; Roh, J.-L.; Choi, S.-H.; Kim, S.Y.; Nam, S.-Y. Short-Term Treatment Results for Unilateral Vocal Fold Palsy Induced by Mediastinal Lesions. J. Voice 2014, 28, 809–815. [Google Scholar] [CrossRef]
- Cocuzza, S.; Di Luca, M.; Maniaci, A.; Russo, M.; Di Mauro, P.; Migliore, M.; Serra, A.; Spinato, G. Precision treatment of post pneumonectomy unilateral laryngeal paralysis due to cancer. Future Oncol. 2020, 16, 45–53. [Google Scholar] [CrossRef]
- Chen, L.; Douglass, J.; Kleinberg, L.; Ye, X.; Marciscano, A.E.; Forde, P.M.; Brahmer, J.; Lipson, E.; Sharfman, W.; Hammers, H.; et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int. J. Radiat. Oncol. 2018, 100, 916–925. [Google Scholar] [CrossRef]
- Simard, J.L.; Smith, M.; Chandra, S. Pseudoprogression of Melanoma Brain Metastases. Curr. Oncol. Rep. 2018, 20, 91. [Google Scholar] [CrossRef]
- Rauch, M.; Tausch, D.; Stera, S.; Blanck, O.; Wolff, R.; Meissner, M.; Urban, H.; Hattingen, E. MRI characteristics in treatment for cerebral melanoma metastasis using stereotactic radiosurgery and concomitant checkpoint inhibitors or targeted therapeutics. J. Neuro-Oncol. 2021, 153, 79–87. [Google Scholar] [CrossRef]
- Urban, H.; Steidl, E.; Hattingen, E.; Filipski, K.; Meissner, M.; Sebastian, M.; Koch, A.; Strzelczyk, A.; Forster, M.-T.; Baumgarten, P.; et al. Immune Checkpoint Inhibitor-Induced Cerebral Pseudoprogression: Patterns and Categorization. Front. Immunol. 2022, 12, 798811. [Google Scholar] [CrossRef]
- Urban, H.; Willems, L.M.; Ronellenfitsch, M.W.; Rosenow, F.; Steinbach, J.P.; Strzelczyk, A. Increased occurrence of status epilepticus in patients with brain metastases and checkpoint inhibition. OncoImmunology 2020, 9, 1851517. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, R.; Li, D.; Li, N.; Zhu, X. Prognostic factors of oligometastatic non-small cell lung cancer: A meta-analysis. J. Thorac. Dis. 2018, 10, 3701–3713. [Google Scholar] [CrossRef] [PubMed]




| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 56 | 53 | 58 |
| Sex | m | m | f |
| Nicotine consumption | 120 PYs | 60 PYs | 60 PYs |
| Disease | NSCLC adenocarcinoma | NSCLC adenocarcinoma | NSCLC adenocarcinoma |
| Pathology | TTF-1 positive, EGFR wt, ALK negative, BRAF wt, ROS-1 negative, K-RAS wt, PIK3CA positive, TP53 positive, HER2 wt, PD-L1: TPS 90% (high) | TTF-1 negative, EGFR wt, ALK negative, BRAF wt, ROS-1 negative, K-RAS mutation exon 2: codon 12, PD-L1: TPS 20% (moderate) | TTF-1 positive, EGFR wt, ALK negative, BRAF: not enough tissue for analysis, ROS-1 negative, K-RAS exon 2 mutation in codon 12, PD-L1: TPS 2% (moderate) |
| Clinical tumor stage | cT2 cN0 pM1b (BRA), UICC IVA | cT2b cN2 pM1b (BRA), UICC IVA | cT2 cN3 cM1c (BRA), UICC IVB |
| Location of primary tumor | Right lower pulmonary lobe | Right upper pulmonary lobe | Left lower pulmonary lobe |
| Initial neurological findings | Mnestic deficits, mild motoric aphasia (word-finding difficulty), and progressive numbness of the left side of the face | Oculomotor nerve deficits with ptosis, mydriasis, and abduction of the right eyeball | Impairment of fine motor skills, dizziness, apraxia, and weakness in the right arm |
| Location of BM | Right frontal lobe | Right temporal lobe | Left pre-central cortex and gyrus postcentralis |
| Karnofsky performance index before therapy | 90% | 90% | 90% |
| Treatment of BM | Neurosurgery and SRS | Neurosurgery and SRS | SRS |
| BM radiation dose | 21 Gy | Synchronous BM: 21 Gy, metachronous BM: 20 Gy | 19 Gy |
| Neoadjuvant therapy | Cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) | Cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) (+2 cycles of pembrolizumab as maintenance therapy) | Cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) |
| Steroid application | Dexamethasone 4 mg/d for 3 months during neoadjuvant therapy | Dexamethasone 4 mg/d for 3 weeks after SRS | Dexamethasone 8 mg/d for 3 weeks before neoadjuvant therapy |
| Pulmonary function testing before pulmonary resection | FVC: 3.51 L (58%) FEV1: 2.36 L (51%) Tiffeneau index: 88% | FVC: 4.33 L (87%) FEV1: 2.46 L (65%) Tiffeneau index: 57% | FVC: 2.32 L (86%) FEV1: 1.49 L (68%) Tiffeneau index: 64% |
| Pulmonary resection | Posterolateral thoracotomy, subsegment S1-resection, right lower lobe resection, systematic lymph node dissection | Posterolateral thoracotomy, extrapleural extended upper lobectomy, wedge resections (S1, S5, S6), systematic lymph node dissection | Posterolateral thoracotomy, extended left lower lobe resection, wedge resection S1, systematic lymph node dissection |
| Tumor stage after lung resection | ypT0 ypN0 (0/42), L0, V0, R0, Gx, pM1b (BRA), UICC IVA | ypT2b ypN0 (0/22), L0, V0, Pn0, R0, Gx, pM1b (BRA), UICC IVA | ypT2a ypN3 (5/23), L1, V1, Pn0, R0, cM1c (BRA), UICC IVB |
| Maintenance therapy | 200 mg per cycle (ongoing) | 200 mg per cycle (discontinued after 2 cycles because of progressive disease) | 200 mg per cycle (finished after 24 months) |
| Current Karnofsky performance index | 100% | 0% | 100% |
| Trial | Institution | Therapy | Inclusion Criteria | Tumor Histology | Local Treatment of Primary Tumor | Local Treatment of BM | No. of Patients | Study Start Date | Recruiting |
|---|---|---|---|---|---|---|---|---|---|
| NCT05012254 (NIVIPI-Brain) | Spanish Lung Cancer Group | Nivolumab plus Ipilimumab and 2 cycles of platinum-based chemotherapy | Synchronous and metachronous BM—6 months after other chemotherapy treatment has finished, asymptomatic, or oligosymptomatic BM | NSCLC | - | BM must not be suitable for resection or focal RT | 71 planned | November 2021 | yes |
| NCT03526900 (ATEZO-BRAIN) | Spanish Lung Cancer Group | Atezolizumab and platinum-based chemotherapy | Synchronous and metachronous asymptomatic BM | Nonsquamous NSCLC | - | WBRT or SRS only in case of brain progression | 43 | July 2018 | active, not recruiting |
| NCT04787185 (STRAIT-LUC) | Azienda Ospedaliero-Universitaria Careggi | Immunotherapy | Up to 10 BMs treatable with RS or HFSRT | NSCLC | - | RS or HFSRT | 50 planned | April 2020 | yes |
| NCT04964960 | University of Kentucky | Pembrolizumab and platinum-based chemotherapy | Asymptomatic BM (less than 10 lesions) | NSCLC | - | - | 45 planned | December 2021 | yes |
| NCT02978404 | Centre hospitalier de l’Université de Montréal | Nivolumab | BMs with a combined maximum disease volume of 10cc | NSCLC, SCLC, melanoma, or ccRCC | - | SRS during Nivolumab treatment | 26 | June 2017 | active, not recruiting |
| NCT02696993 | MD Anderson Cancer Center | Nivolumab (plus Ipilimumab) | At least one BM >= 0.3 cm amenable to radiation therapy | NSCLC | - | SRS or WBRT | 88 planned | December 2016 | yes |
| NCT04768075 | Guangdong Association of Clinical Trials | Camrelizumab and platinum-based chemotherapy | Symptomatic and asymptomatic BM | NSCLC | - | SRT or WBRT | 200 planned | March 2021 | not yet |
| NCT03965468 (CHESS) | European Thoracic Oncology Platform | Durvalumab and platinum-based chemotherapy | Up to 3 metastases including BM, must have at least 1 extracerebral metastasis | NSCLC | Resection or definitive radiotherapy | SBRT for extracranial metastasis, radiosurgery, or neurosurgery for BM | 47 planned | November 2019 | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, A.; Sponholz, S.; Trainer, S.; Stratmann, J.; Sebastian, M.; Rauch, M.; Wolff, R.; Steinbach, J.P.; Ronellenfitsch, M.W.; Urban, H. Pulmonary Resection after Radiosurgery and Neoadjuvant Immunochemotherapy for NSCLC Patients with Synchronous Brain Metastasis—A Case Series of Three Patients. Curr. Oncol. 2022, 29, 2225-2239. https://doi.org/10.3390/curroncol29040181
Koch A, Sponholz S, Trainer S, Stratmann J, Sebastian M, Rauch M, Wolff R, Steinbach JP, Ronellenfitsch MW, Urban H. Pulmonary Resection after Radiosurgery and Neoadjuvant Immunochemotherapy for NSCLC Patients with Synchronous Brain Metastasis—A Case Series of Three Patients. Current Oncology. 2022; 29(4):2225-2239. https://doi.org/10.3390/curroncol29040181
Chicago/Turabian StyleKoch, Agnes, Stefan Sponholz, Stephan Trainer, Jan Stratmann, Martin Sebastian, Maximilian Rauch, Robert Wolff, Joachim P. Steinbach, Michael W. Ronellenfitsch, and Hans Urban. 2022. "Pulmonary Resection after Radiosurgery and Neoadjuvant Immunochemotherapy for NSCLC Patients with Synchronous Brain Metastasis—A Case Series of Three Patients" Current Oncology 29, no. 4: 2225-2239. https://doi.org/10.3390/curroncol29040181
APA StyleKoch, A., Sponholz, S., Trainer, S., Stratmann, J., Sebastian, M., Rauch, M., Wolff, R., Steinbach, J. P., Ronellenfitsch, M. W., & Urban, H. (2022). Pulmonary Resection after Radiosurgery and Neoadjuvant Immunochemotherapy for NSCLC Patients with Synchronous Brain Metastasis—A Case Series of Three Patients. Current Oncology, 29(4), 2225-2239. https://doi.org/10.3390/curroncol29040181





