Intravascular Large B-Cell Lymphoma: A Review with a Focus on the Prognostic Value of Skin Involvement
Abstract
:1. Epidemiology and Aetiology
2. Extracutaneous Clinical Presentation
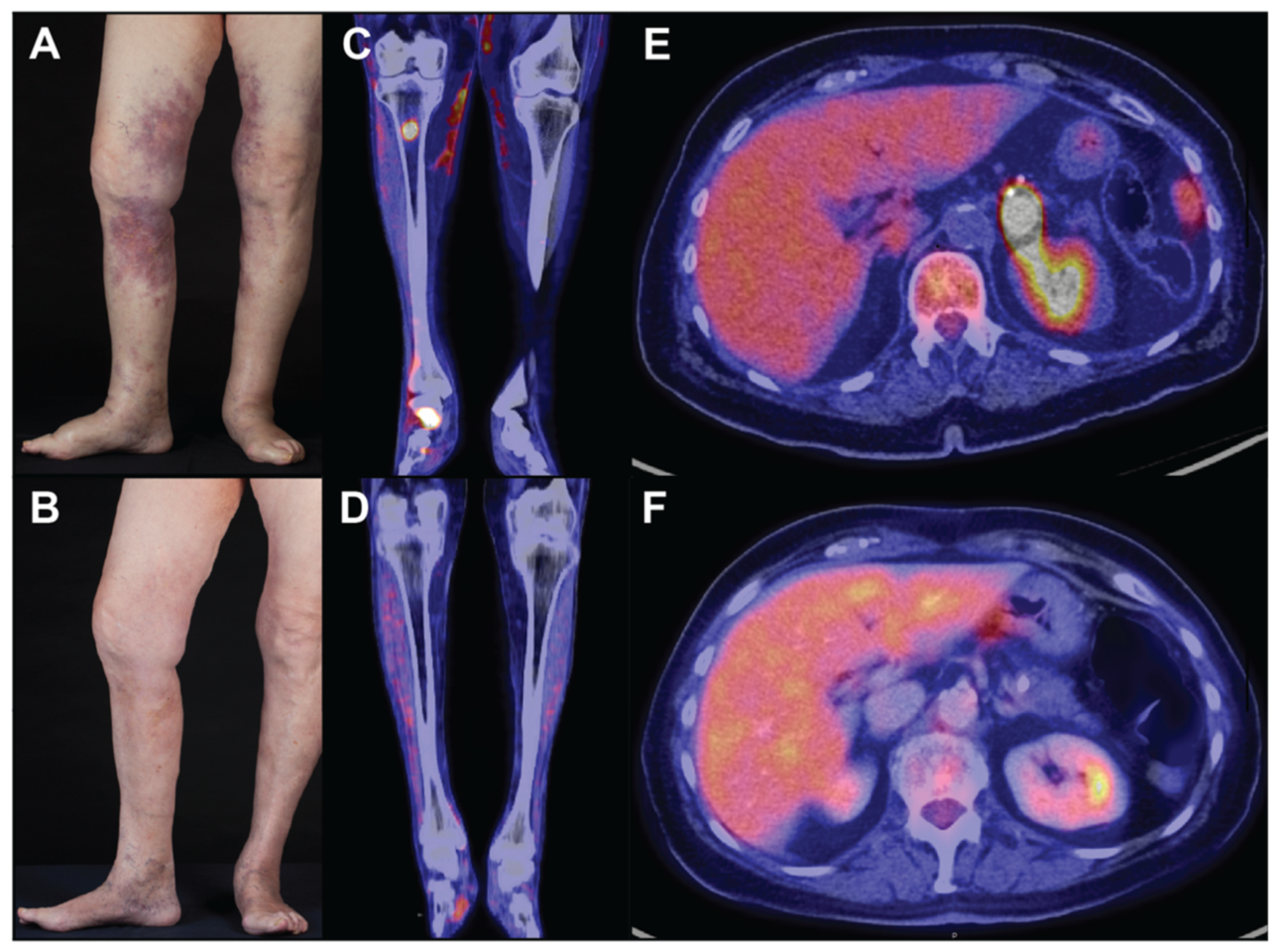
3. Skin Findings
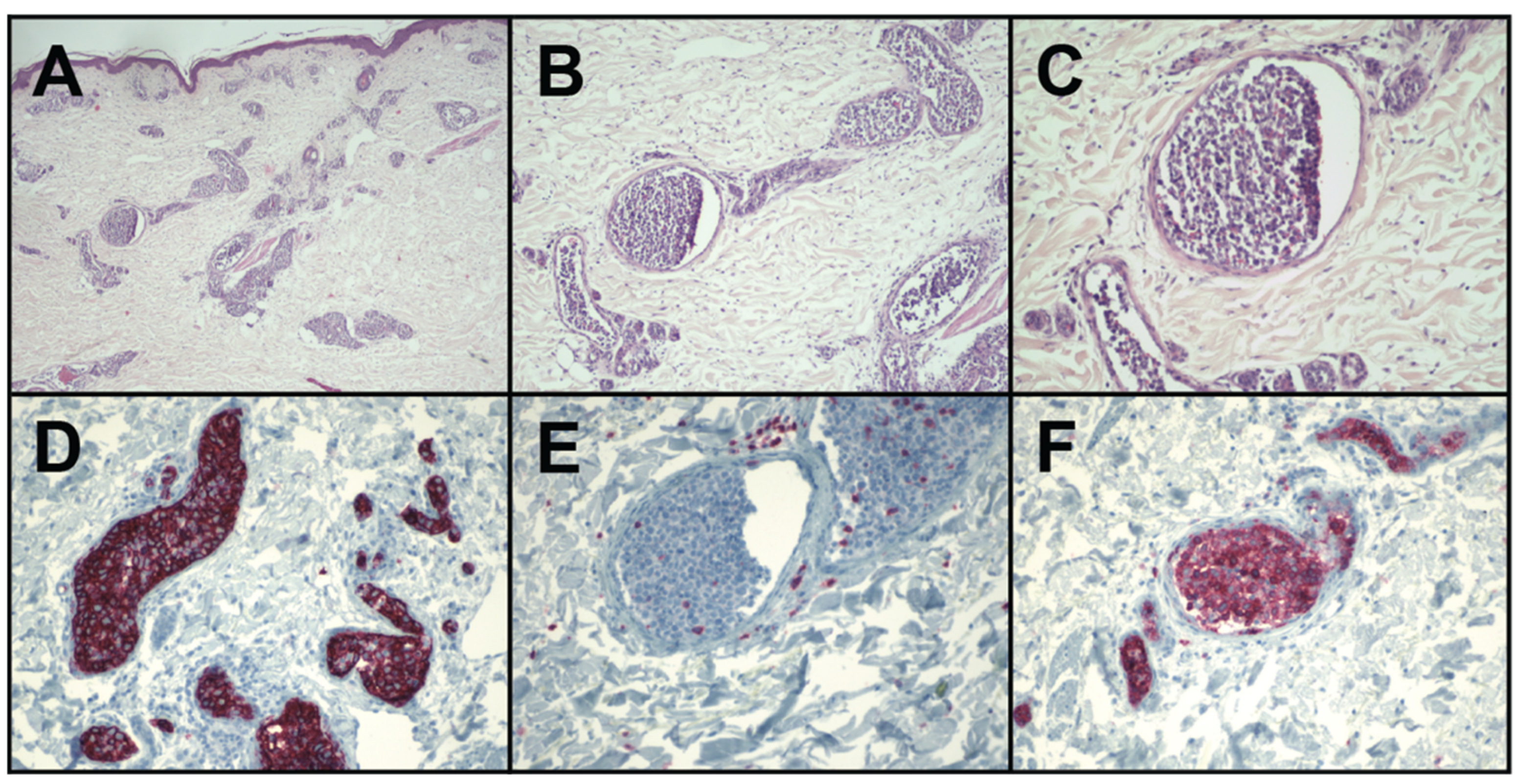
4. Diagnosis and Pathological Findings
5. Pretreatment Evaluations and Treatment
6. Treatment and Follow-Up
7. Prognostication
- Age > 60;
- Ann-Arbor stage III/IV disease;
- Elevated LDH level;
- Eastern Cooperative Oncology Group (ECOG) performance status [60] ≥ 2;
- >One extranodal site of disease.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANCA | Anti-neutrophil cytoplasmic antibody |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-6 | B-cell lymphoma 6 |
| CCI | Charlson Comorbidity Index |
| CD | Cluster of differentiation |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| DNA | Deoxyribonucleic acid |
| ECOG | Eastern Cooperative Oncology Group |
| HIV | Human immunodeficiency virus |
| IPI | International Prognostic Index |
| IVLBCL | Intravascular large B-cell lymphoma |
| LDH | Lactate dehydrogenase |
| LEF-1 | Lymphoid enhancer-binding factor 1 |
| MUM1 | Multiple myeloma 1 |
| NHL | Non-Hodgkin’s lymphoma |
| NK cell | Natural killer cell |
| PD-L1 | Programmed death-ligand 1 |
| PD-L2 | Programmed death-ligand 2 |
| PET-CT | Positron emission tomography-computed tomography |
| sIL-2R | Soluble IL-2 receptor |
| SUVmax | Maximal standardised uptake value |
| 18F-FDG | Fluorodeoxyglucose (18F) |
| 3Y-OS | Three-year overall survival |
| 5Y-OS | Five-year overall survival |
References
- Shimada, K.; Matsue, K.; Yamamoto, K.; Murase, T.; Ichikawa, N.; Okamoto, M.; Niitsu, N.; Kosugi, H.; Tsukamoto, N.; Miwa, H.; et al. Retrospective Analysis of Intravascular Large B-Cell Lymphoma Treated with Rituximab-Containing Chemotherapy as Reported by the IVL Study Group in Japan. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Yamaguchi, M.; Suzuki, R.; Okamoto, M.; Sato, Y.; Tamaru, J.; Kojima, M.; Miura, I.; Mori, N.; Yoshino, T.; et al. Intravascular Large B-Cell Lymphoma (IVLBCL): A Clinicopathologic Study of 96 Cases with Special Reference to the Immunophenotypic Heterogeneity of CD5. Blood 2007, 109, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreri, A.J.M.; Campo, E.; Seymour, J.F.; Willemze, R.; Ilariucci, F.; Ambrosetti, A.; Zucca, E.; Rossi, G.; López-Guillermo, A.; Pavlovsky, M.A.; et al. Intravascular Lymphoma: Clinical Presentation, Natural History, Management and Prognostic Factors in a Series of 38 Cases, with Special Emphasis on the ‘Cutaneous Variant’1. Br. J. Haematol. 2004, 127, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; Pandey, Y.; Middleton, D.; Broadfoot, B.; Sasapu, A.; Veeraputhiran, M. Intravascular Large B-Cell Lymphoma: A Diagnostic Dilemma. Cureus 2021, 13, e16459. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Kruyswijk, M.R.; De Bruin, C.D.; Meijer, C.J.; Van Berkel, W. Angiotropic (Intravascular) Large Cell Lymphoma of the Skin Previously Classified as Malignant Angioendotheliomatosis. Br. J. Dermatol. 1987, 116, 393–399. [Google Scholar] [CrossRef]
- Petroff, N.; Koger, O.W.; Fleming, M.G.; Fishleder, A.; Bergfeld, W.F.; Tuthill, R.; Tubbs, R. Malignant Angioendotheliomatosis: An Angiotropic Lymphoma. J. Am. Acad. Dermatol. 1989, 21, 727–733. [Google Scholar] [CrossRef]
- Vieites, B.; Fraga, M.; Lopez-Presas, E.; Pintos, E.; Garcia-Rivero, A.; Forteza, J. Detection of t(14;18) Translocation in a Case of Intravascular Large B-Cell Lymphoma: A Germinal Centre Cell Origin in a Subset of These Lymphomas? Histopathology 2005, 46, 466–468. [Google Scholar] [CrossRef]
- Khoury, H.; Lestou, V.S.; Gascoyne, R.D.; Bruyere, H.; Li, C.H.; Nantel, S.H.; Dalal, B.I.; Naiman, S.C.; Horsman, D.E. Multicolor Karyotyping and Clinicopathological Analysis of Three Intravascular Lymphoma Cases. Mod. Pathol. 2003, 16, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Yoshida, K.; Suzuki, Y.; Iriyama, C.; Inoue, Y.; Sanada, M.; Kataoka, K.; Yuge, M.; Takagi, Y.; Kusumoto, S.; et al. Frequent Genetic Alterations in Immune Checkpoint-Related Genes in Intravascular Large B-Cell Lymphoma. Blood 2021, 137, 1491–1502. [Google Scholar] [CrossRef]
- Miura, T.; Saito, S.; Saito, R.; Iwasaki, T.; Mezaki, N.; Sato, T.; Ajioka, Y.; Kakita, A.; Mashima, T. Long Spinal Cord Lesions Caused by Venous Congestive Myelopathy Associated with Intravascular Large B-Cell Lymphoma. Intern. Med. 2021, 60, 3809–3816. [Google Scholar] [CrossRef]
- Belli, E.; Milano, C.; Pesaresi, I.; Trivelli, I.; Tavoni, A.; Ciancia, E.; Alì, G.; Zampa, V.; Pizzanelli, C.; Siciliano, G.; et al. A Case of Intravascular Large B Cell Lymphoma with Brain Involvement Mimicking Progressive Multifocal Leukoencephalopathy. Int. J. Neurosci. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Pelletti, G.; Barzon, L.; Contran, M.; Emmi, A.; Arminio, A.; Macchi, V.; De Caro, R. Intravascular Large B-Cell Lymphoma Affecting Multiple Cranial Nerves: A Histopathological Study. Neuropathology 2021, 41, 396–405. [Google Scholar] [CrossRef]
- Gill, S.; Melosky, B.; Haley, L.; ChanYan, C. Use of Random Skin Biopsy to Diagnose Intravascular Lymphoma Presenting as Fever of Unknown Origin. Am. J. Med. 2003, 114, 56–58. [Google Scholar] [CrossRef]
- Ponzoni, M.; Ferreri, A.J.M.; Campo, E.; Facchetti, F.; Mazzucchelli, L.; Yoshino, T.; Murase, T.; Pileri, S.A.; Doglioni, C.; Zucca, E.; et al. Definition, Diagnosis, and Management of Intravascular Large B-Cell Lymphoma: Proposals and Perspectives from an International Consensus Meeting. J. Clin. Oncol. 2007, 25, 3168–3173. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nakamura, S.; Kawauchi, K.; Matsuzaki, H.; Sakai, C.; Inaba, T.; Nasu, K.; Tashiro, K.; Suchi, T.; Saito, H. An Asian Variant of Intravascular Large B-Cell Lymphoma: Clinical, Pathological and Cytogenetic Approaches to Diffuse Large B-Cell Lymphoma Associated with Haemophagocytic Syndrome. Br. J. Haematol. 2000, 111, 826–834. [Google Scholar] [CrossRef]
- Cobcroft, R. Images in Haematology. Diagnosis of Angiotropic Large B-Cell Lymphoma from a Peripheral Blood Film. Br. J. Haematol. 1999, 104, 429. [Google Scholar] [CrossRef]
- Dou, L.; Wu, C.; Zeng, Z.; Zhu, J.; Su, L.; Wang, T. Hemophagocytic Syndrome and Neurological Involvement in a Case of Intravascular Large B-Cell Lymphoma. J. Int. Med. Res. 2021, 49, 3000605211006644. [Google Scholar] [CrossRef]
- Storandt, M.H.; Koponen, M.A. Intravascular Large B-Cell Lymphoma Presenting with Fever and Refractory Acidosis. Autops. Case Rep. 2021, 11, e2021324. [Google Scholar] [CrossRef]
- Akkour, K.; Alhulwah, M.; Alhalal, H.; Alqahtani, N.; Arafah, M. Primary Extranodal Diffuse Large B-Cell Lymphoma of the Uterine Cervix. Malays. J. Pathol. 2021, 43, 327–331. [Google Scholar]
- Hakroush, S.; Lehnig, L.-Y.; Wallbach, M.; Schanz, J.; Koziolek, M.J. Renal Involvement of Intravascular Large B-Cell Lymphoma: A Challenging Diagnosis. J. Nephrol. 2021. [CrossRef]
- Rallabandi, H.B.; Thirukovela, J.; Swain, M.; Meeramira, D.; Gowrishankar, S. Intravascular Large B Cell Lymphoma of Prostate, a Rare Entity. Indian J. Pathol. Microbiol. 2021, 64, 575–578. [Google Scholar] [CrossRef] [PubMed]
- al-Hazzaa, S.A.; Green, W.R.; Mann, R.B. Uveal Involvement in Systemic Angiotropic Large Cell Lymphoma. Microscopic and Immunohistochemical Studies. Ophthalmology 1993, 100, 961–965. [Google Scholar] [CrossRef]
- Brunet, V.; Marouan, S.; Routy, J.-P.; Hashem, M.A.; Bernier, V.; Simard, R.; Petrella, T.; Lamarre, L.; Théorêt, G.; Carrier, C.; et al. Retrospective Study of Intravascular Large B-Cell Lymphoma Cases Diagnosed in Quebec: A Retrospective Study of 29 Case Reports. Medicine 2017, 96, e5985. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, H.; Morishita, Y.; Saito, S.; Shimada, K.; Ozeki, K.; Kohno, A.; Kato, Y.; Nagasaka, T. Usefulness of Bone Marrow Aspiration for Definite Diagnosis of Asian Variant of Intravascular Lymphoma: Four Autopsied Cases. Leuk. Lymphoma 2004, 45, 1611–1616. [Google Scholar] [CrossRef]
- Kojima, K.; Kaneda, K.; Yasukawa, M.; Tanaka, K.; Inoue, T.; Yamashita, T.; Dansako, H.; Sakugawa, S.T.; Kozuka, T.; Hara, M.; et al. Specificity of Polymerase Chain Reaction-Based Clonality Analysis of Immunoglobulin Heavy Chain Gene Rearrangement for the Detection of Bone Marrow Infiltrate in B-Cell Lymphoma-Associated Haemophagocytic Syndrome. Br. J. Haematol. 2002, 119, 616–621. [Google Scholar] [CrossRef]
- Shimada, K.; Murase, T.; Matsue, K.; Okamoto, M.; Ichikawa, N.; Tsukamoto, N.; Niitsu, N.; Miwa, H.; Asaoku, H.; Kosugi, H.; et al. Central Nervous System Involvement in Intravascular Large B-Cell Lymphoma: A Retrospective Analysis of 109 Patients. Cancer Sci. 2010, 101, 1480–1486. [Google Scholar] [CrossRef]
- Chapin, J.E.; Davis, L.E.; Kornfeld, M.; Mandler, R.N. Neurologic Manifestations of Intravascular Lymphomatosis. Acta Neurol. Scand. 1995, 91, 494–499. [Google Scholar] [CrossRef]
- Röglin, J.; Böer, A. Skin Manifestations of Intravascular Lymphoma Mimic Inflammatory Diseases of the Skin. Br. J. Dermatol. 2007, 157, 16–25. [Google Scholar] [CrossRef]
- Kiyohara, T.; Kumakiri, M.; Kobayashi, H.; Shimizu, T.; Ohkawara, A.; Ohnuki, M. A Case of Intravascular Large B-Cell Lymphoma Mimicking Erythema Nodosum: The Importance of Multiple Skin Biopsies. J. Cutan. Pathol. 2000, 27, 413–418. [Google Scholar] [CrossRef]
- Asada, N.; Odawara, J.; Kimura, S.; Aoki, T.; Yamakura, M.; Takeuchi, M.; Seki, R.; Tanaka, A.; Matsue, K. Use of Random Skin Biopsy for Diagnosis of Intravascular Large B-Cell Lymphoma. Mayo Clin. Proc. 2007, 82, 1525–1527. [Google Scholar] [CrossRef]
- Le, E.N.; Gerstenblith, M.R.; Gelber, A.C.; Manno, R.L.; Ranasinghe, P.D.; Sweren, R.J.; McGirt, L.Y. The Use of Blind Skin Biopsy in the Diagnosis of Intravascular B-Cell Lymphoma. J. Am. Acad. Dermatol. 2008, 59, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Matsue, K.; Abe, Y.; Kitadate, A.; Miura, D.; Narita, K.; Kobayashi, H.; Takeuchi, M.; Enzan, N.; Tanaka, A.; Takeuchi, K. Sensitivity and Specificity of Incisional Random Skin Biopsy for Diagnosis of Intravascular Large B-Cell Lymphoma. Blood 2019, 133, 1257–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozenbaum, D.; Tung, J.; Xue, Y.; Hoang, M.P.; Kroshinsky, D. Skin Biopsy in the Diagnosis of Intravascular Lymphoma: A Retrospective Diagnostic Accuracy Study. J. Am. Acad. Dermatol. 2021, 85, 665–670. [Google Scholar] [CrossRef]
- Alhumidi, A. Cutaneous Intravascular NK/T-Cell Lymphoma Mimic Panniculitis Clinically, Case Report and Literature Brief Review. Diagn. Pathol. 2015, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, S.; Ma, H.; Shi, D.; Huang, C.; Lu, C.; Gao, T.; Wang, G. Intravascular NK/T-Cell Lymphoma: A Report of Five Cases with Cutaneous Manifestation from China. J. Cutan. Pathol. 2015, 42, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; et al. Preclinical Activity of the Type II CD20 Antibody GA101 (Obinutuzumab) Compared with Rituximab and Ofatumumab in Vitro and in Xenograft Models. Mol. Cancer Ther. 2013, 12, 2031–2042. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Reth, M. Oligomeric Organization of the B-Cell Antigen Receptor on Resting Cells. Nature 2010, 467, 465–469. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Zhou, H.; Zhou, X.; Shao, J. Analysis of Clinicopathological Features and Prognostic Factors of Non-Hodgkin’s Intravascular Large B-Cell Lymphoma. Oncol. Lett. 2020, 20, 43. [Google Scholar] [CrossRef]
- Sleater, J.P.; Segal, G.H.; Scott, M.D.; Masih, A.S. Intravascular (Angiotropic) Large Cell Lymphoma: Determination of Monoclonality by Polymerase Chain Reaction on Paraffin-Embedded Tissues. Mod. Pathol. 1994, 7, 593–598. [Google Scholar]
- Lakhani, S.R.; Hulman, G.; Hall, J.M.; Slack, D.N.; Sloane, J.P. Intravascular Malignant Lymphomatosis (Angiotropic Large-Cell Lymphoma). A Case Report with Evidence for T-Cell Lineage with Polymerase Chain Reaction Analysis. Histopathology 1994, 25, 283–286. [Google Scholar] [CrossRef]
- Sepp, N.; Schuler, G.; Romani, N.; Geissler, D.; Gattringer, C.; Burg, G.; Bartram, C.R.; Fritsch, P. “Intravascular Lymphomatosis” (Angioendotheliomatosis): Evidence for a T-Cell Origin in Two Cases. Hum. Pathol. 1990, 21, 1051–1058. [Google Scholar] [CrossRef]
- Sangueza, O.; Hyder, D.M.; Sangueza, P. Intravascular Lymphomatosis: Report of an Unusual Case with T Cell Phenotype Occurring in an Adolescent Male. J. Cutan. Pathol. 1992, 19, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Samols, M.A.; Su, A.; Ra, S.; Cappel, M.A.; Louissant, A.J.; Knudson, R.A.; Ketterling, R.P.; Said, J.; Binder, S.; Harris, N.L.; et al. Intralymphatic Cutaneous Anaplastic Large Cell Lymphoma/Lymphomatoid Papulosis: Expanding the Spectrum of CD30-Positive Lymphoproliferative Disorders. Am. J. Surg. Pathol. 2014, 38, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Falkenbach, E.; Fernández-Figueras, M.T.; Rodríguez-Peralto, J.L. Benign Atypical Intravascular CD30(+) T-Cell Proliferation: A Reactive Condition Mimicking Intravascular Lymphoma. Am. J. Dermatopathol. 2013, 35, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.L.; Stone, M.S.; Liu, V. Atypical Intravascular CD30+ T-Cell Proliferation Following Trauma in a Healthy 17-Year-Old Male: First Reported Case of a Potential Diagnostic Pitfall and Literature Review. J. Cutan. Pathol. 2009, 36, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Weingertner, N.; Mitcov, M.; Chenard, M.-P.; Cribier, B. Intralymphatic CD30+ T-Cell Proliferation during DRESS: A Mimic of Intravascular Lymphoma. J. Cutan. Pathol. 2016, 43, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Keller, K.; John, H.; Dommann-Scherrer, C. Benign Atypical Intravascular CD30+ T-Cell Proliferation: A Recently Described Reactive Lymphoproliferative Process and Simulator of Intravascular Lymphoma: Report of a Case Associated with Lichen Sclerosus and Review of the Literature. Am. J. Clin. Pathol. 2014, 142, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Juweid, M.E.; Stroobants, S.; Hoekstra, O.S.; Mottaghy, F.M.; Dietlein, M.; Guermazi, A.; Wiseman, G.A.; Kostakoglu, L.; Scheidhauer, K.; Buck, A.; et al. Use of Positron Emission Tomography for Response Assessment of Lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J. Clin. Oncol. 2007, 25, 571–578. [Google Scholar] [CrossRef]
- Shimada, K.; Kosugi, H.; Shimada, S.; Narimatsu, H.; Koyama, Y.; Suzuki, N.; Yuge, M.; Nishibori, H.; Iwata, Y.; Nakamura, S.; et al. Evaluation of Organ Involvement in Intravascular Large B-Cell Lymphoma by 18F-Fluorodeoxyglucose Positron Emission Tomography. Int. J. Hematol. 2008, 88, 149–153. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Czuczman, M.S.; Weaver, R.; Alkuzweny, B.; Berlfein, J.; Grillo-López, A.J. Prolonged Clinical and Molecular Remission in Patients With Low-Grade or Follicular Non-Hodgkin’s Lymphoma Treated With Rituximab Plus CHOP Chemotherapy: 9-Year Follow-Up. J. Clin. Oncol. 2004, 22, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Bouzani, M.; Karmiris, T.; Rontogianni, D.; Delimpassi, S.; Apostolidis, J.; Mpakiri, M.; Nikiforakis, E. Disseminated Intravascular B-Cell Lymphoma: Clinicopathological Features and Outcome of Three Cases Treated with Anthracycline-Based Immunochemotherapy. Oncologist 2006, 11, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Dognini, G.P.; Govi, S.; Crocchiolo, R.; Bouzani, M.; Bollinger, C.R.; D’Incan, M.; Delaporte, E.; Hamadani, M.; Jardin, F.; et al. Can Rituximab Change the Usually Dismal Prognosis of Patients with Intravascular Large B-Cell Lymphoma? J. Clin. Oncol. 2008, 26, 5134–5136; author reply 5136. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Nishimura, M.; Yokota, A.; Munekata, S.; Kobayashi, T.; Saito, Y. Successful Treatment of Intravascular Malignant Lymphomatosis with High-Dose Chemotherapy and Autologous Peripheral Blood Stem Cell Transplantation. Bone Marrow Transplant. 2001, 27, 1101–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laufer, I.; Hanover, A.; Lis, E.; Yamada, Y.; Bilsky, M. Repeat Decompression Surgery for Recurrent Spinal Metastases. J. Neurosurg. Spine 2010, 13, 109–115. [Google Scholar] [CrossRef]
- Bokstein, F.; Lossos, A.; Lossos, I.S.; Siegal, T. Central Nervous System Relapse of Systemic Non-Hodgkin’s Lymphoma: Results of Treatment Based on High-Dose Methotrexate Combination Chemotherapy. Leuk. Lymphoma 2002, 43, 587–593. [Google Scholar] [CrossRef]
- Reni, M.; Ferreri, A.J.; Guha-Thakurta, N.; Blay, J.Y.; Dell’Oro, S.; Biron, P.; Hochberg, F.H. Clinical Relevance of Consolidation Radiotherapy and Other Main Therapeutic Issues in Primary Central Nervous System Lymphomas Treated with Upfront High-Dose Methotrexate. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 419–425. [Google Scholar] [CrossRef]
- Shimada, K.; Yamaguchi, M.; Atsuta, Y.; Matsue, K.; Sato, K.; Kusumoto, S.; Nagai, H.; Takizawa, J.; Fukuhara, N.; Nagafuji, K.; et al. Favorable Outcomes of Newly Diagnosed Intravascular Large B-Cell Lymphoma Patients Treated with R-CHOP Combined with High-Dose Methotrexate Plus Intrathecal Chemotherapy: Results from a Multicenter Phase 2 Trial (PRIMEUR-IVL). Blood 2019, 134, 350. [Google Scholar] [CrossRef]
- A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The Revised International Prognostic Index (R-IPI) Is a Better Predictor of Outcome than the Standard IPI for Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Miura, K.; Konishi, J.; Miyake, T.; Masanori, M.; Hojo, A.; Masaki, Y.; Uno, M.; Ozaki, J.; Yoshida, C.; Niiya, D.; et al. A New Prognostic Model for Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Blood 2015, 126, 2680. [Google Scholar] [CrossRef]
- Goto, N.; Tsurumi, H.; Goto, H.; Shimomura, Y.I.; Kasahara, S.; Hara, T.; Yasuda, I.; Shimizu, M.; Murakami, N.; Yoshikawa, T.; et al. Serum Soluble Interleukin-2 Receptor (SIL-2R) Level Is Associated with the Outcome of Patients with Diffuse Large B Cell Lymphoma Treated with R-CHOP Regimens. Ann. Hematol. 2012, 91, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Fonkem, E.; Lok, E.; Robison, D.; Gautam, S.; Wong, E.T. The Natural History of Intravascular Lymphomatosis. Cancer Med. 2014, 3, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Suehara, Y.; Sakata-Yanagimoto, M.; Hattori, K.; Nanmoku, T.; Itoh, T.; Kaji, D.; Yamamoto, G.; Abe, Y.; Narita, K.; Takeuchi, M.; et al. Liquid Biopsy for the Identification of Intravascular Large B-Cell Lymphoma. Haematologica 2018, 103, e241–e244. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kohno, K.; Matsue, K.; Sakakibara, A.; Ishikawa, E.; Shimada, S.; Shimada, K.; Mabuchi, S.; Takahara, T.; Kato, S.; et al. PD-L1 (SP142) Expression in Neoplastic Cells Predicts a Poor Prognosis for Patients with Intravascular Large B-Cell Lymphoma Treated with Rituximab-Based Multi-Agent Chemotherapy. Cancer Med. 2020, 9, 4768–4776. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Yan, Y.; Zhang, Y.; Du, Y.; Sun, G. Prognostic and Clinicopathological Value of PD-L1 in Melanoma: A Meta-Analysis. Am. J. Med. Sci. 2020, 359, 339–346. [Google Scholar] [CrossRef]
| Classic Variant | Hemophagocytic Syndrome-Associated Variant | |
|---|---|---|
| Neurological symptoms (CNS involvement) | 39–76%, rapidly progressing | 27% |
| Cutaneous symptoms | 17–39% | 5% |
| Bone marrow involvement | 32% | 75% |
| Splenic involvement | 26% | 67% |
| Hepatic involvement | 26% | 55% |
| Hemophagocytic syndrome | not present | present |
Common findings:
| B symptoms LDH ↑, sedimentation rate and C-reactive protein (CRP) ↑, anemia | |
| Differing findings: | ||
| 29% 18% | 76% 84% |
| Morphologic Feature | Differential Diagnosis |
|---|---|
| Erythematous nodule without epidermal involvement | Primary B-cell lymphoma of the skin Pseudolymphoma Cutaneous manifestation of Crohn’s disease |
| Livid patch/nodule with telangiectasia on lower extremity | Thrombophlebitis Chronic venous insufficiency Acroangiodermatitis (Pseudo–Kaposi sarcoma) Kaposi sarcoma Haemangioma |
| Painful subcutaneous plaques with erythema and induration | Lupus panniculitis Subcutaneous panniculitis-like T-cell lymphoma Vasculitis (medium-sized vessels) Angioendotheliomatosis Panniculitis of alpha-1-antitrypsin deficiency Eosinophilic fasciitis |
| Contusiform rash on lower legs | Erythema nodosum Traumatic panniculitis Haematoma |
| Migrating/Figurate erythema | Autoinflammatory syndromes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breakell, T.; Waibel, H.; Schliep, S.; Ferstl, B.; Erdmann, M.; Berking, C.; Heppt, M.V. Intravascular Large B-Cell Lymphoma: A Review with a Focus on the Prognostic Value of Skin Involvement. Curr. Oncol. 2022, 29, 2909-2919. https://doi.org/10.3390/curroncol29050237
Breakell T, Waibel H, Schliep S, Ferstl B, Erdmann M, Berking C, Heppt MV. Intravascular Large B-Cell Lymphoma: A Review with a Focus on the Prognostic Value of Skin Involvement. Current Oncology. 2022; 29(5):2909-2919. https://doi.org/10.3390/curroncol29050237
Chicago/Turabian StyleBreakell, Thomas, Heidi Waibel, Stefan Schliep, Barbara Ferstl, Michael Erdmann, Carola Berking, and Markus V. Heppt. 2022. "Intravascular Large B-Cell Lymphoma: A Review with a Focus on the Prognostic Value of Skin Involvement" Current Oncology 29, no. 5: 2909-2919. https://doi.org/10.3390/curroncol29050237
APA StyleBreakell, T., Waibel, H., Schliep, S., Ferstl, B., Erdmann, M., Berking, C., & Heppt, M. V. (2022). Intravascular Large B-Cell Lymphoma: A Review with a Focus on the Prognostic Value of Skin Involvement. Current Oncology, 29(5), 2909-2919. https://doi.org/10.3390/curroncol29050237






