STR Profiling Reveals Tumor Genome Instability in Primary Mediastinal B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
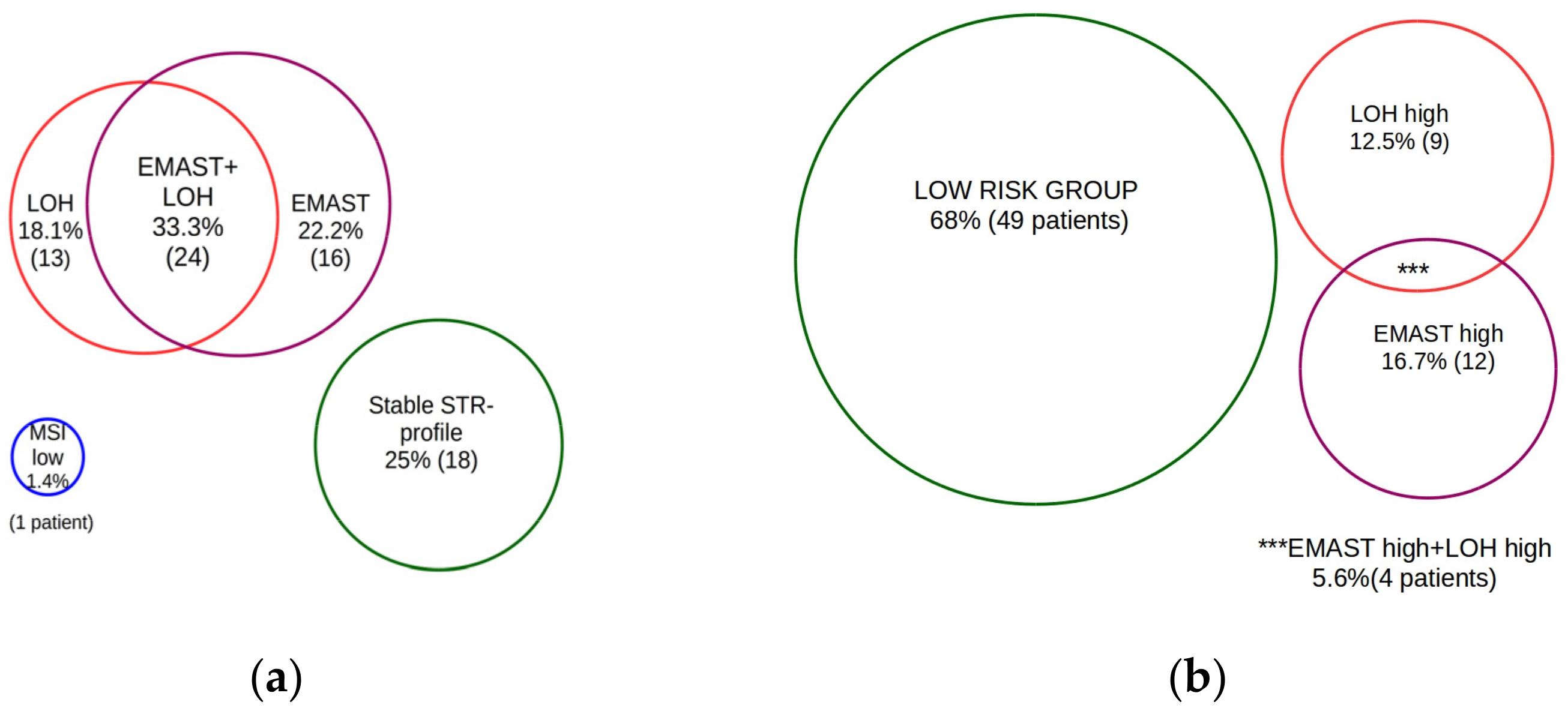
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martelli, M.; Ferreri, A.; Di Rocco, A.; Ansuinelli, M.; Johnson, P.W.M. Primary mediastinal large B-cell lymphoma. Crit. Rev. Oncol. Hematol. 2017, 113, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunleavy, K. Primary mediastinal B-cell lymphoma: Biology and evolving therapeutic strategies. Hematol. Am. Soc. Hematol. Educ. Progr. 2017, 2017, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Ehrentraut, S.; Nagel, S.; Eberth, S.; Pommerenke, C.; Dirks, W.G.; Geffers, R.; Kalavalapalli, S.; Kaufmann, M.; Meyer, C.; et al. Genomic Landscape of Primary Mediastinal B-Cell Lymphoma Cell Lines. PLoS ONE 2015, 10, e0139663. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Leroy, K.; Yu, X.; Gaulard, P.; Gascoyne, R.D.; Chan, W.C.; Zhao, T.; Haioun, C.; Greiner, T.C.; et al. Molecular Diagnosis of Primary Mediastinal B Cell Lymphoma Identifies a Clinically Favorable Subgroup of Diffuse Large B Cell Lymphoma Related to Hodgkin Lymphoma. J. Exp. Med. 2003, 198, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]
- Marginean, E.C.; Melosky, B. Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer with Microsatellite Instability? Part II—The Challenge of Programmed Death Ligand-1 Testing and Its Role in Microsatellite Instability-High Colorectal Cancer. Arch. Pathol. Lab. Med. 2018, 142, 26–34. [Google Scholar] [CrossRef]
- PPayandeh, Z.; Khalili, S.; Somi, M.H.; Mard-Soltani, M.; Baghbanzadeh, A.; Hajiasgharzadeh, K.; Samadi, N.; Baradaran, B. PD-1/PD-L1-dependent immune response in colorectal cancer. J. Cell. Physiol. 2020, 235, 5461–5475. [Google Scholar] [CrossRef]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.J.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef]
- Evrard, C.; Tachon, G.; Randrian, V.; Karayan-Tapon, L.; Tougeron, D. Microsatellite Instability: Diagnosis, Heterogeneity, Discordance, and Clinical Impact in Colorectal Cancer. Cancers 2019, 11, 1567. [Google Scholar] [CrossRef] [Green Version]
- Sarshekeh, A.M.; Overman, M.J.; Kopetz, S. Nivolumab in the treatment of microsatellite instability high metastatic colorectal cancer. Future Oncol. 2018, 14, 1869–1874. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Ribrag, V.; Moskowitz, C.H.; Michot, J.M.; Kuruvilla, J.; Balakumaran, A.; Zhang, Y.; Chlosta, S.; Shipp, M.A.; Armand, P. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017, 130, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 3291–3299. [Google Scholar] [CrossRef]
- Lees, C.; Keane, C.; Gandhi, M.K.; Gunawardana, J. Biology and therapy of primary mediastinal B-cell lymphoma: Current status and future directions. Br. J. Haematol. 2019, 185, 25–41. [Google Scholar] [CrossRef]
- Watson, M.M.; Lea, D.; Gudlaugsson, E.; Skaland, I.; Hagland, H.R.; Søreide, K. Prevalence of PD-L1 expression is associated with EMAST, density of peritumoral T-cells and recurrence-free survival in operable non-metastatic colorectal cancer. Cancer Immunol. Immunother. 2020, 69, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Torshizi Esfahani, A.; Seyedna, S.Y.; Nazemalhosseini Mojarad, E.; Majd, A.; Asadzadeh Aghdaei, H. MSI-L/EMAST is a predictive biomarker for metastasis in colorectal cancer patients. J. Cell. Physiol. 2019, 234, 13128–13136. [Google Scholar] [CrossRef]
- Carethers, J.; Koi, M.; Tseng-Rogenski, S. EMAST is a Form of Microsatellite Instability That is Initiated by Inflammation and Modulates Colorectal Cancer Progression. Genes 2015, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Pawlik, T.M.; Raut, C.P.; Rodriguez-Bigas, M.A. Colorectal Carcinogenesis: MSI-H Versus MSI-L. Dis. Markers 2004, 20, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef]
- Luqmani, Y.; Mathew, M. Allelic variation of BAT-25 and BAT-26 mononucleotide repeat loci in tumours from a group of young women with breast cancer. Int. J. Oncol. 2004, 25, 771–775. [Google Scholar] [CrossRef]
- Sychevskaya, K.A.; Risinskaya, N.V.; Kravchenko, S.K.; Nikulina, E.E.; Misyurina, A.E.; Magomedova, A.U.; Sudarikov, A.B. Pitfalls in mononucleotide microsatellite repeats instability assessing (MSI) in the patients with B-cell lymphomas. Klin. Lab. Diagn. 2021, 66, 181–186. [Google Scholar] [CrossRef]
- Chen, M.H.; Chang, S.C.; Lin, P.C.; Yang, S.H.; Lin, C.C.; Lan, Y.T.; Lin, H.H.; Lin, C.H.; Lai, J.I.; Liang, W.Y.; et al. Combined Microsatellite Instability and Elevated Microsatellite Alterations at Selected Tetranucleotide Repeats (EMAST) Might Be a More Promising Immune Biomarker in Colorectal Cancer. Oncologist 2019, 24, 1534–1542. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Schmitz, N.; Ou, F.S.; Dixon, J.G.; Cunningham, D.; Pfreundschuh, M.; Seymour, J.F.; Jaeger, U.; Habermann, T.M.; Haioun, C.; et al. Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Diffuse Large B-Cell Lymphoma: An Individual Patient–Level Analysis of Multiple Randomized Trials (SEAL). J. Clin. Oncol. 2018, 36, 2593–2602. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Mishima, S.; Taniguchi, H.; Akagi, K.; Baba, E.; Fujiwara, Y.; Hirasawa, A.; Ikeda, M.; Maeda, O.; Muro, K.; Nishihara, H.; et al. Japan Society of Clinical Oncology provisional clinical opinion for the diagnosis and use of immunotherapy in patients with deficient DNA mismatch repair tumors, cooperated by Japanese Society of Medical Oncology, First Edition. Int. J. Clin. Oncol. 2020, 25, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-Y.; Zhang, D.; Wu, S.; Xu, M.; Zhou, X.; Lu, X.J.; Ji, J. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms, predictive factors, and future perspectives. Biomark. Res. 2020, 8, 35. [Google Scholar] [CrossRef]
- Jeong, A.-R.; Ball, E.D.; Goodman, A.M. Predicting Responses to Checkpoint Inhibitors in Lymphoma: Are We Up to the Standards of Solid Tumors? Clin. Med. Insights Oncol. 2020, 14, 1179554920976366. [Google Scholar] [CrossRef]
- Gamberi, B.; Gaidano, G.; Parsa, N.; Carbone, A.; Roncella, S.; Knowles, D.M.; Louie, D.C.; Shibata, D.; Chaganti, R.S.; Dalla-Favera, R. Microsatellite instability is rare in B-cell non-Hodgkin’s lymphomas. Blood 1997, 89, 975–979. [Google Scholar]
- Miyashita, K.; Fujii, K.; Yamada, Y.; Hattori, H.; Taguchi, K.; Yamanaka, T.; Yoshida, M.A.; Okamura, J.; Oda, S.; Muta, K.; et al. Frequent microsatellite instability in non-Hodgkin lymphomas irresponsive to chemotherapy. Leuk. Res. 2008, 32, 1183–1195. [Google Scholar] [CrossRef]
- Miyashita, K.; Fujii, K.; Taguchi, K.; Shimokawa, M.; Yoshida, M.A.; Abe, Y.; Okamura, J.; Oda, S.; Uike, N. A specific mode of microsatellite instability is a crucial biomarker in adult T-cell leukaemia/lymphoma patients. J. Cancer Res. Clin. Oncol. 2017, 143, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Li, J.; Xue, T.; Yu, B.; Li, X.; Zhou, X. Microsatellite instability and its associations with the clinicopathologic characteristics of diffuse large B-cell lymphoma. Cancer Med. 2020, 9, 2330–2342. [Google Scholar] [CrossRef]
- Sychevskaya, K.A.; Kravchenko, S.K.; Risinskaya, N.V.; Misyurina, A.E.; Nikulina, E.E.; Babaeva, F.E.; Sudarikov, A.B. Microsatellite instability (MSI, EMAST) in the pathogenesis of follicular lymphoma. Oncohematology 2021, 16, 56–69. [Google Scholar] [CrossRef]
- Van Oers, J.M.M.; Edwards, Y.; Chahwan, R.; Zhang, W.; Smith, C.; Pechuan, X.; Schaetzlein, S.; Jin, B.; Wang, Y.; Bergman, A.; et al. The MutSβ complex is a modulator of p53-driven tumorigenesis through its functions in both DNA double-strand break repair and mismatch repair. Oncogene 2014, 33, 3939–3946. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Thienpont, B.; Yesilyurt, B.T.; Moisse, M.; Reumers, J.; Coenegrachts, L.; Sagaert, X.; Schrauwen, S.; Smeets, D.; Matthijs, G.; et al. Mismatch repair deficiency endows tumors with a unique mutation signature and sensitivity to DNA double-strand breaks. eLife 2014, 3, e02725. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Vnencak-Jones, C.L.; Cates, J.M.; Shi, C. Deciphering Elevated Microsatellite Alterations at Selected Tetra/Pentanucleotide Repeats, Microsatellite Instability, and Loss of Heterozygosity in Colorectal Cancers. J. Mol. Diagn. 2018, 20, 366–372. [Google Scholar] [CrossRef]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Zhang, S.; Xiong, L.; Xi, S.; Tao, R.; Chen, C.; Li, J.; Chen, J.; Li, C. Investigation of an Alternative Marker for Hypermutability Evaluation in Different Tumors. Genes 2021, 12, 197. [Google Scholar] [CrossRef]





| Parameters | PMBCL | LOH-Positive | LOH-Negative | P χ2 | EMAST-Positive | EMAST-Negative | P χ2 |
|---|---|---|---|---|---|---|---|
| n | 58 | 27 (47%) | 31 (53%) | 35 (60%) | 23 (40%) | ||
| Male:Female | 19:39 | 10:17 | 9:22 | 0.518 | 11:24 | 8:15 | 0.791 |
| Age, median | 33 (20–58) y | 32 (21–52) y | 33 (20–58) y | 31 (21–58) y | 33 (20–52) y | ||
| 1 LDH N ↑ N | 4 (7%) 54 (93%) | 2 (8%) 25 (92%) | 2 (7%) 29 (93%) | 0.887 | 4 (12%) 31 (88%) | 0 23 (100%) | 0.093 |
| 2 aa IPI 0 1 2 3 | 1 (2%) 2 (3%) 47 (81%) 8 (14%) | 1 (4%) 1 (4%) 20 (74%) 5 (18%) | 0 1 (3%) 27 (87%) 3 (10%) | 0.207 | 1 (3%) 2 (6%) 27 (77%) 5 (14%) | 0 0 20 (87%) 3 (17%) | 0.352 |
| Extramediastinal disease | 9 (15%) | 6 (22%) | 3 (10%) | 0.189 | 6 (17%) | 3 (13%) | 0.694 |
| Bulky disease | 57 (98%) | 27 (100%) | 30 (96%) | 0.347 | 34 (97%) | 23 (100%) | 0.414 |
| Involvement pleura/pericardium | 41 (71%) | 20 (74%) | 21 (67%) | 0.598 | 24 (68%) | 17 (74%) | 0.662 |
| 3 CR refractory disease | 52 (90%) 6 (10%) | 23 (85%) 4 (15%) | 29 (93%) 2 (7%) | 0.297 | 30 (86%) 5 (14%) | 22 (97%) 1 (3%) | 0.225 |
| Patient | LOH | EMAST | STR Aberrations |
|---|---|---|---|
| #3 | no | 2p | EMAST |
| #6 | 13q | 2p | LOH + EMAST |
| #7 | 5q | 1q, 6q, 7q | LOH + EMAST high |
| #17 | 2p | no | LOH |
| #47 | no | 4q, 13q | EMAST |
| #51 | 12p | 6q | LOH + EMAST |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risinskaya, N.; Mangasarova, Y.; Nikulina, E.; Kozhevnikova, Y.; Chabaeva, J.; Yushkova, A.; Magomedova, A.; Kulikov, S.; Julhakyan, H.; Kravchenko, S.; et al. STR Profiling Reveals Tumor Genome Instability in Primary Mediastinal B-Cell Lymphoma. Curr. Oncol. 2022, 29, 3449-3459. https://doi.org/10.3390/curroncol29050278
Risinskaya N, Mangasarova Y, Nikulina E, Kozhevnikova Y, Chabaeva J, Yushkova A, Magomedova A, Kulikov S, Julhakyan H, Kravchenko S, et al. STR Profiling Reveals Tumor Genome Instability in Primary Mediastinal B-Cell Lymphoma. Current Oncology. 2022; 29(5):3449-3459. https://doi.org/10.3390/curroncol29050278
Chicago/Turabian StyleRisinskaya, Natalya, Yana Mangasarova, Elena Nikulina, Yana Kozhevnikova, Julia Chabaeva, Anna Yushkova, Aminat Magomedova, Sergey Kulikov, Hunan Julhakyan, Sergey Kravchenko, and et al. 2022. "STR Profiling Reveals Tumor Genome Instability in Primary Mediastinal B-Cell Lymphoma" Current Oncology 29, no. 5: 3449-3459. https://doi.org/10.3390/curroncol29050278
APA StyleRisinskaya, N., Mangasarova, Y., Nikulina, E., Kozhevnikova, Y., Chabaeva, J., Yushkova, A., Magomedova, A., Kulikov, S., Julhakyan, H., Kravchenko, S., & Sudarikov, A. (2022). STR Profiling Reveals Tumor Genome Instability in Primary Mediastinal B-Cell Lymphoma. Current Oncology, 29(5), 3449-3459. https://doi.org/10.3390/curroncol29050278





