Refractory Splenic Marginal Zone Lymphoma Responsive to Combination Venetoclax and Bortezomib (Velcade) (V2) Therapy
Abstract
:1. Introduction
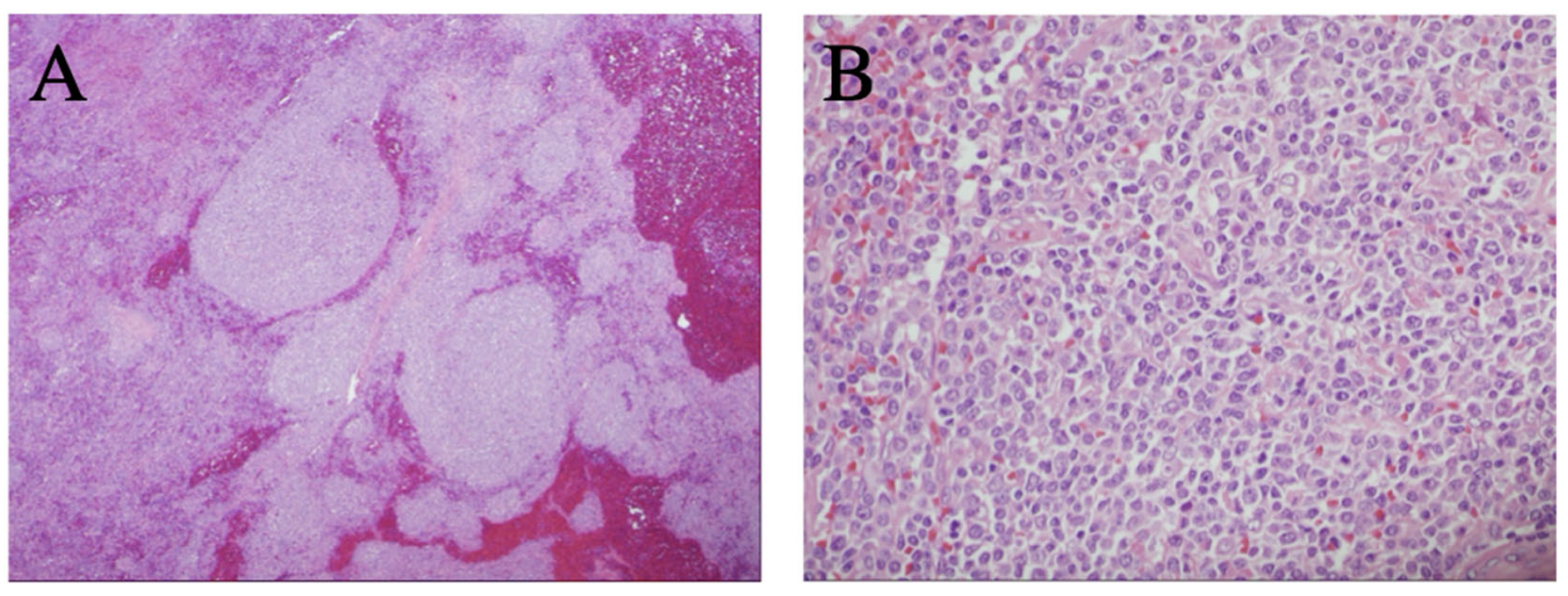
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dos Santos, T.S.; Tavares, R.S.; de Farias, D.L.C. Splenic marginal zone lymphoma: A literature review of diagnostic and therapeutic challenges. Rev. Bras. Hematol. Hemoter. 2017, 39, 146–154. [Google Scholar] [CrossRef]
- Camacho, F.I.; Mollejo, M.; Mateo, M.-S.; Algara, P.; Navas, C.; Hernández, J.-M.; Santoja, C.; Solé, F.; Sánchez-Beato, M.; Piris, M.A. Progression to Large B-Cell Lymphoma in Splenic Marginal Zone Lymphoma: A Description of a Series of 12 Cases. Am. J. Surg. Pathol. 2001, 25, 1268–1276. [Google Scholar] [CrossRef]
- Conconi, A.; Franceschetti, S.; von Hohenstaufen, K.A.; Margiotta-Casaluci, G.; Stathis, A.; Moccia, A.A.; Bertoni, F.; Ramponi, A.; Mazzucchelli, L.; Cavalli, F.; et al. Histologic transformation in marginal zone lymphomas. Ann. Oncol. 2015, 26, 2329–2335. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, stag-ing, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Thieblemont, C.; Felman, P.; Callet-Bauchu, E.; Traverse-Glehen, A.; Salles, G.; Coiffier, B.; Berger, F. Splenic marginal-zone lymphoma: A distinct clinical and pathological entity. Lancet Oncol. 2003, 4, 95–103. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Habermann, T.M. Epidemiology of marginal zone lymphoma. Ann. Lymphoma 2021, 5, 1. [Google Scholar] [CrossRef]
- Matutes, E.; Oscier, D.; Montalban, C.; Berger, F.; Callet-Bauchu, E.; Dogan, A.; Felman, P.; Franco, V.; Iannitto, E.; Mollejo, M.; et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia 2008, 22, 487–495. [Google Scholar] [CrossRef]
- Vallisa, D.; Bernuzzi, P.; Arcaini, L.; Sacchi, S.; Callea, V.; Marasca, R.; Lazzaro, A.; Trabacchi, E.; Anselmi, E.; Arcari, A.L.; et al. Role of Anti-Hepatitis C Virus (HCV) Treatment in HCV-Related, Low-Grade, B-Cell, Non-Hodgkin’s Lymphoma: A Multicenter Italian Experience. J. Clin. Oncol. 2005, 23, 468–473. [Google Scholar] [CrossRef]
- Tarella, C.; Arcaini, L.; Baldini, L.; Barosi, G.; Billio, A.; Marchetti, M.; Rambaldi, A.; Vitolo, U.; Zinzani, P.L.; Tura, S. Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation Guidelines for the Management of Indolent, Nonfollicular B-Cell Lymphoma (Marginal Zone, Lymphoplasmacytic, and Small Lymphocytic Lymphoma). Clin. Lymphoma Myeloma Leuk. 2015, 15, 75–85. [Google Scholar] [CrossRef]
- Kalpadakis, C.; Pangalis, G.A.; Angelopoulou, M.K.; Sachanas, S.; Kontopidou, F.N.; Yiakoumis, X.; Kokoris, S.I.; Dimitriadou, E.M.; Dimopoulou, M.N.; Moschogiannis, M.; et al. Treatment of Splenic Marginal Zone Lymphoma With Rituximab Monotherapy: Progress Report and Comparison With Splenectomy. Oncology 2013, 18, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenglet, J.; Traullé, C.; Mounier, N.; Benet, C.; Munoz-Bongrand, N.; Amorin, S.; Noguera, M.-E.; Traverse-Glehen, A.; Ffrench, M.; Baseggio, L.; et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk. Lymphoma 2014, 55, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Schechter, G.P. Treatment of Splenic Marginal Zone Lymphoma: Splenectomy Versus Rituximab. Semin. Hematol. 2010, 47, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kalpadakis, C.; Pangalis, G.A.; Vassilakopoulos, T.P.; Sachanas, S.; Angelopoulou, M.K. Treatment of splenic marginal zone lymphoma: Should splenectomy be abandoned? Leuk Lymphoma. 2014, 55, 1463–1470. [Google Scholar] [CrossRef]
- Bennett, M.; Yegena, S.; Dave, H.P.; Schechter, G.P. Re: Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol. Oncol. 2008, 26, 114. [Google Scholar] [CrossRef] [PubMed]
- Else, M.; De La Cruz, F.; Batty, P.; Dearden, C.E.; Catovsky, D.; Matutes, E.; Marín-Niebla, A.; La Cruz, F.; Ríos, E. Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br. J. Haematol. 2012, 159, 322–328. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Catovsky, D.; Schlette, E.; O’Brien, S.; Wierda, W.G.; Kantarjian, H. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer 2006, 107, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kalpadakis, C.; Pangalis, G.A.; Angelopoulou, M.K.; Vassilakopoulos, T.P. Treatment of splenic marginal zone lymphoma. Best Pr. Res. Clin. Haematol. 2016, 30, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Sharma, K.; Yegena, S.; Gavish, I.; Dave, H.P.; Schechter, G.P. Rituximab monotherapy for splenic marginal zone lymphoma. Haematologica 2005, 90, 856–858. [Google Scholar]
- Iannitto, E.; Bellei, M.; Amorim, S.; Ferreri, A.J.M.; Marcheselli, L.; Cesaretti, M.; Haioun, C.; Mancuso, S.; Bouabdallah, K.; Gressin, R.; et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: Results from the phase II BRISMA/IELSG36 study. Br. J. Haematol. 2018, 183, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Niemann, C.U.; Wiestner, A. B-cell receptor signaling as a driver of lymphoma development and evolution. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2013; Volume 23, pp. 410–421. [Google Scholar]
- Noy, A.; De Vos, S.; Thieblemont, C.; Martin, P.; Flowers, C.R.; Morschhauser, F.; Collins, G.; Ma, S.; Coleman, M.; Peles, S.; et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017, 129, 2224–2232. [Google Scholar] [CrossRef]
- Noy, A.; De Vos, S.; Coleman, M.; Martin, P.; Flowers, C.R.; Thieblemont, C.; Morschhauser, F.; Collins, G.P.; Ma, S.; Peles, S.; et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: Long-term follow-up and biomarker analysis. Blood Adv. 2020, 24, 5773–5784. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, Y.; Watkins, A.J.; Kocialkowski, S.; Zeng, N.; Hamoudi, R.; Isaacson, P.G.; de Leval, L.; Wotherspoon, A.; Du, M.-Q. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 2012, 97, 595–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patriarca, A.; Gaidano, G. Investigational drugs for the treatment of diffuse large B-cell lymphoma. Expert Opin. Investig. Drugs 2021, 30, 25–38. [Google Scholar] [CrossRef] [PubMed]
- De Vos, S.; Goy, A.; Dakhil, S.R.; Saleh, M.N.; McLaughlin, P.; Belt, R.; Boral, A.L. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J. Clin. Oncol. 2009, 27, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199. [Google Scholar] [CrossRef]
- Becnel, M.R.; Nastoupil, L.J.; Samaniego, F.; Davis, R.E.; You, M.J.; Green, M.; Hagemeister, F.B.; Fanale, M.A.; Fayad, L.E.; Westin, J.R.; et al. Lenalidomide plus rituximab (R2) in previously untreated marginal zone lymphoma: Subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br. J. Haematol. 2019, 185, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Andorsky, D.J.; Coleman, M.; Yacoub, A.; Melear, J.M.; Fanning, S.R.; Kolibaba, K.; Lansigan, K.; Reynolds, C.; Foon, K.F.; Li, J.; et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. JCO 2019, 37 (Suppl. 15), 7513. [Google Scholar] [CrossRef]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Hughes, M.E.; Landsburg, D.J.; Rubin, D.J.; Schuster, S.J.; Svoboda, J.; Gerson, J.N.; Namoglu, E.; Nasta, S.D. Treatment of Patients with Relapsed/Refractory Non-Hodgkin Lymphoma with Venetoclax: A Single-Center Evaluation of Off-Label Use. Clin. Lymphoma Myeloma Leuk. 2019, 19, 791–798. [Google Scholar] [CrossRef]
- Gerecitano, J.F.; Roberts, A.W.; Seymour, J.F. A phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Blood 2015, 126, 254. [Google Scholar] [CrossRef]
- de Vos, S.; Swinnen, L.J.; Wang, D.; Reid, E.; Fowler, N.; Cordero, J.; Dunbar, M.; Enschede, S.H.; Nolan, C.; Petrich, A.M.; et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: A phase Ib dose-finding study. Ann. Oncol. 2018, 29, 1932–1938. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Flinn, I.W.; Yuen, S.L.S.; Topp, M.S.; Rusconi, C.; Fleury, I.; Le Dû, K.; Arthur, C.; Pro, B.; Gritti, G.; et al. Venetoclax-rituximab with or without bendamustine vs. bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood 2020, 136, 2628–2637. [Google Scholar]
- Zelenetz, A.D.; Salles, G.; Mason, K.; Casulo, C.; Le Gouill, S.; Sehn, L.H.; Tilly, H.; Cartron, G.; Chamuleau, M.E.D.; Goy, A.; et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: Results from the CAVALLI phase 1b trial. Blood 2019, 133, 1964–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Salcedo, L.M.; Desai, V.; Dalia, S. Venetoclax: Evidence to date and clinical potential. Drugs Context 2019, 8, 212574. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P. Nodal Marginal Zone Lymphoma with Increased Large Cells: Myth Versus Entity. Arch. Pathol. Lab. Med. 2011, 135, 964–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasebø, E.; Berven, F.S.; Hovland, R.; Døskeland, S.O.; Bruserud, Ø.; Selheim, F.; Hernandez-Valladares, M. The Progression of Acute Myeloid Leukemia from First Diagnosis to Chemoresistant Relapse: A Comparison of Proteomic and Phosphoproteomic Profiles. Cancers 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Danilova, O.V.; Dumont, L.J.; Levy, N.B.; Lansigan, F.; Kinlaw, W.B.; Danilov, A.V.; Kaur, P. FASN and CD36 predict survival in rituximab-treated diffuse large B-cell lymphoma. J. Hematop. 2013, 6, 11–18. [Google Scholar] [CrossRef] [Green Version]

| Date | Specimen | Pathology | Antigen Profile | Additional Characterization |
|---|---|---|---|---|
| 1 February 2001 | Liver Bx | Nodular lymphoid infiltrates composed of a mixture of small and scattered large cells | Positive: CD20, kappa, BCL2, focal IgD and BCL6 (mostly on few larger cells). Negative: Lambda, CD5, CD10 and MUM1 | Ki67 < 15%; focal p53 expression (few large cells) |
| 1 February 2001 | Blood | Positive: CD20, CD19, CD22 and CD79b. Negative: CD5, CD10, CD23, CD11c, CD25, CD103 | ||
| 1 June 2002 | BM Bx | Hypercellular, nodular aggregates of small lymphocytes with interspersed large lymphocytes | Positive: CD20, kappa, BCL2, and BCL6 (mostly on few larger cells). Negative: Lambda, CD5, CD10 and MUM1 | |
| 1 April 2006 | Spleen | Composed predominantly of small lymphocytes, some with plasmacytic differentiation and some large lymphs mainly in white pulp | Positive: CD20, CD19, CD22 and CD79b | Ki67: 15–20%; focal P53 expression (few large cells) |
| 1 October 2007 | BM Bx | Unchanged | Karyotype 46XY, del (2) (q13q33); t (1; 17), (q25; q21) add (4) (p14), del (2) (5) (q13q15) | |
| 1 September 2016 | LAN Bx | Increased % of large cells | Unchanged | Ki67: up to 30% |
| 1 May 2018 | Liver Bx | Same as LN | Unchanged | Ki67: up to 30% |
| 1 June 2019 | BM Bx | Unchanged | Unchanged | Ki67: up to 30% |
| 1 January 2020 | LAN bx | Unchanged | Unchanged | Ki67: up to 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roche, K.C.; DeRosa, P.A.; Liu, M.-L.; Nava, V.E.; Aggarwal, A. Refractory Splenic Marginal Zone Lymphoma Responsive to Combination Venetoclax and Bortezomib (Velcade) (V2) Therapy. Curr. Oncol. 2022, 29, 4117-4124. https://doi.org/10.3390/curroncol29060328
Roche KC, DeRosa PA, Liu M-L, Nava VE, Aggarwal A. Refractory Splenic Marginal Zone Lymphoma Responsive to Combination Venetoclax and Bortezomib (Velcade) (V2) Therapy. Current Oncology. 2022; 29(6):4117-4124. https://doi.org/10.3390/curroncol29060328
Chicago/Turabian StyleRoche, Kyle C., Peter A. DeRosa, Min-Ling Liu, Victor E. Nava, and Anita Aggarwal. 2022. "Refractory Splenic Marginal Zone Lymphoma Responsive to Combination Venetoclax and Bortezomib (Velcade) (V2) Therapy" Current Oncology 29, no. 6: 4117-4124. https://doi.org/10.3390/curroncol29060328
APA StyleRoche, K. C., DeRosa, P. A., Liu, M. -L., Nava, V. E., & Aggarwal, A. (2022). Refractory Splenic Marginal Zone Lymphoma Responsive to Combination Venetoclax and Bortezomib (Velcade) (V2) Therapy. Current Oncology, 29(6), 4117-4124. https://doi.org/10.3390/curroncol29060328






