Magnetic Resonance Imaging of Peritoneal Carcinomatosis: Evaluation of High b-Value Computed Diffusion-Weighted Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
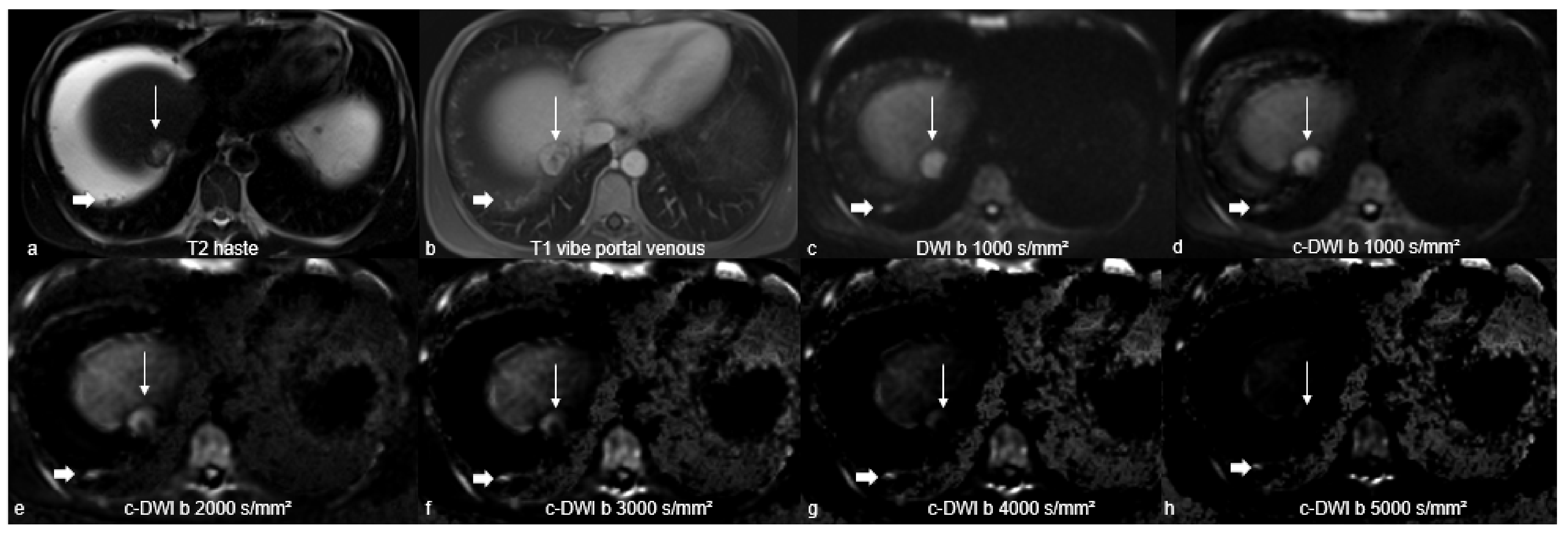
2.2. Magnetic Resonance Imaging Studies
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Quantitative Analysis
3.2. Qualitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfannenberg, C.; Schwenzer, N.F.; Bruecher, B.L. State-of-the-Art-Bildgebung bei Peritonealkarzinose [State-of-the-art imaging of peritoneal carcinomatosis]. Rofo 2012, 184, 205–213. [Google Scholar] [CrossRef]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- Iafrate, F.; Ciolina, M.; Sammartino, P.; Baldassari, P.; Rengo, M.; Lucchesi, P.; Sibio, S.; Accarpio, F.; Di Giorgio, A.; Laghi, A. Peritoneal carcinomatosis: Imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging. Abdom. Imaging 2012, 37, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. (Eds.) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Springer: Boston, MA, USA, 1996; Volume 82, pp. 359–374. [Google Scholar] [CrossRef]
- Dohan, A.; Hoeffel, C.; Soyer, P.; Jannot, A.-S.; Valette, P.; Thivolet, A.; Passot, G.; Glehen, O.; Rousset, P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 2017, 104, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Furtado, F.S.; Wu, M.Z.; Esfahani, S.A.; Ferrone, C.R.; Blaszkowsky, L.S.; Clark, J.W.; Ryan, D.P.; Goyal, L.; Franses, J.W.; Wo, J.Y.; et al. Positron Emission Tomography/Magnetic Resonance Imaging Versus the Standard of Care Imaging in the Diagnosis of Peritoneal Carcinomatosis. Ann. Surg. 2022, 17. [Google Scholar] [CrossRef]
- Lampe, B.; Kroll, N.; Piso, P.; Forner, D.M.; Mallmann, P. Prognostic Significance of Sugarbaker’s Peritoneal Cancer Index for the Operability of Ovarian Carcinoma. Int. J. Gynecol. Cancer 2015, 25, 135–144. [Google Scholar] [CrossRef]
- Kyriazi, S.; Collins, D.J.; Morgan, V.A.; Giles, S.L.; DeSouza, N.M. Diffusion-weighted Imaging of Peritoneal Disease for Noninvasive Staging of Advanced Ovarian Cancer 1. RadioGraphics 2010, 30, 1269–1285. [Google Scholar] [CrossRef]
- Satoh, Y.; Ichikawa, T.; Motosugi, U.; Kimura, K.; Sou, H.; Sano, K.; Araki, T. Diagnosis of Peritoneal Dissemination: Comparison of 18F-FDG PET/CT, Diffusion-Weighted MRI, and Contrast-Enhanced MDCT. Am. J. Roentgenol. 2011, 196, 447–453. [Google Scholar] [CrossRef]
- Woodhams, R.; Kakita, S.; Hata, H.; Iwabuchi, K.; Umeoka, S.; Mountford, C.E.; Hatabu, H. Diffusion-Weighted Imaging of Mucinous Carcinoma of the Breast: Evaluation of Apparent Diffusion Coefficient and Signal Intensity in Correlation With Histologic Findings. Am. J. Roentgenol. 2009, 193, 260–266. [Google Scholar] [CrossRef]
- Cianci, R.; Pizzi, A.D.; Patriarca, G.; Massari, R.; Basilico, R.; Gabrielli, D.; Filippone, A. Magnetic Resonance Assessment of Peritoneal Carcinomatosis: Is There a True Benefit From Diffusion-Weighted Imaging? Curr. Probl. Diagn. Radiol. 2020, 49, 392–397. [Google Scholar] [CrossRef]
- Ablefoni, M.; Surup, H.; Ehrengut, C.; Schindler, A.; Seehofer, D.; Denecke, T.; Meyer, H.-J. Diagnostic Benefit of High b-Value Computed Diffusion-Weighted Imaging in Patients with Hepatic Metastasis. J. Clin. Med. 2021, 10, 5289. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, E.; Xu, H.; Ye, Q.; Li, J.; Ye, S.; Cheng, Q.; Zhao, L.; Su, M.; Wang, M. Feasibility and Diagnostic Performance of Voxelwise Computed Diffusion-Weighted Imaging in Breast Cancer. J. Magn. Reson. Imaging 2018, 49, 1610–1616. [Google Scholar] [CrossRef]
- Harder, F.N.; Jung, E.; McTavish, S.; Van, A.T.; Weiss, K.; Ziegelmayer, S.; Gawlitza, J.; Gouder, P.; Kamal, O.; Makowski, M.R.; et al. High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 470. [Google Scholar] [CrossRef]
- Higaki, T.; Nakamura, Y.; Tatsugami, F.; Kaichi, Y.; Akagi, M.; Akiyama, Y.; Baba, Y.; Iida, M.; Awai, K. Introduction to the Technical Aspects of Computed Diffusion-weighted Imaging for Radiologists. RadioGraphics 2018, 38, 1131–1144. [Google Scholar] [CrossRef]
- Ablefoni, M.; Ullrich, S.; Surov, A.; Hoffmann, K.-T.; Meyer, H.-J. Diagnostic benefit of high b-value computed diffusion-weighted imaging in acute brainstem infarction. J. Neuroradiol. 2020, 49, 47–52. [Google Scholar] [CrossRef]
- Ueno, Y.R.; Tamada, T.; Takahashi, S.; Tanaka, U.; Sofue, K.; Kanda, T.; Nogami, M.; Ohno, Y.; Hinata, N.; Fujisawa, M.; et al. Computed Diffusion-Weighted Imaging in Prostate Cancer: Basics, Advantages, Cautions, and Future Prospects. Korean J. Radiol. 2018, 19, 832–837. [Google Scholar] [CrossRef]
- Bozkurt, M.; Doganay, S.; Kantarci, M.; Yalcin, A.; Eren, S.; Atamanalp, S.S.; Yuce, I.; Yildirgan, M.I. Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values. Eur. J. Radiol. 2011, 80, 224–228. [Google Scholar] [CrossRef]
- Dong, L.; Li, K.; Peng, T. Diagnostic value of diffusion-weighted imaging/magnetic resonance imaging for peritoneal metastasis from malignant tumor. Medicine 2021, 100, e24251. [Google Scholar] [CrossRef]
- Arita, Y.; Yoshida, S.; Waseda, Y.; Takahara, T.; Ishii, C.; Ueda, R.; Kwee, T.C.; Miyahira, K.; Ishii, R.; Okuda, S.; et al. Diagnostic value of computed high b-value whole-body diffusion-weighted imaging for primary prostate cancer. Eur. J. Radiol. 2021, 137, 109581. [Google Scholar] [CrossRef]
- Jendoubi, S.; Wagner, M.; Montagne, S.; Ezziane, M.; Mespoulet, J.; Comperat, E.; Estellat, C.; Baptiste, A.; Renard-Penna, R. MRI for prostate cancer: Can computed high b-value DWI replace native acquisitions? Eur. Radiol. 2019, 29, 5197–5204. [Google Scholar] [CrossRef]
- DelPriore, M.R.; Biswas, D.; Hippe, D.S.; Zecevic, M.; Parsian, S.; Scheel, J.R.; Rahbar, H.; Partridge, S.C. Breast Cancer Conspicuity on Computed Versus Acquired High b-Value Diffusion-Weighted MRI. Acad. Radiol. 2020, 28, 1108–1117. [Google Scholar] [CrossRef]
- Bickel, H.; Polanec, S.H.; Wengert, G.; Pinker, K.; Bogner, W.; Helbich, T.H.; Baltzer, P.A. Diffusion-Weighted MRI of Breast Cancer: Improved Lesion Visibility and Image Quality Using Synthetic b-Values. J. Magn. Reson. Imaging 2019, 50, 1754–1761. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, S.; Kromrey, M.-L.; Motosugi, U.; Onishi, H. Optimal target b-value on computed diffusion-weighted magnetic resonance imaging for visualization of pancreatic ductal adenocarcinoma and focal autoimmune pancreatitis. Abdom. Radiol. 2020, 46, 636–646. [Google Scholar] [CrossRef]
- Kawahara, S.; Isoda, H.; Fujimoto, K.; Shimizu, H.; Furuta, A.; Arizono, S.; Ohno, T.; Yamashita, R.; Ono, A.; Togashi, K. Additional benefit of computed diffusion-weighted imaging for detection of hepatic metastases at 1.5T. Clin. Imaging 2016, 40, 481–485. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, X. Combining Diffusion-Weighted Imaging and T2-Weighted Imaging to Delineate Tumorous Tissue in Peritoneal Carcinomatosis: A Comparative Study with 18F-Fluoro-Deoxyglucose Positron Emission Tomography with Computed Tomography (FDG PET/CT). Med. Sci. Monit. 2022, 28, 664. [Google Scholar] [CrossRef]


| 1.5 T MRI Scanner | |||
|---|---|---|---|
| Parameters | DWI | T2-Haste | T1-Fat-Saturated |
| FOV [mm × mm] | 295 × 449 | 312 × 400 | 300 × 400 |
| Matrix | 134 × 88 | 320 × 200 | 320 × 180 |
| ST [mm] | 5 | 5 | 3 |
| number of slices | 114 | 40 | 72 |
| TR [ms] | 7750 | 1100 | 3.56 |
| TE [ms] | 50.5 | 119 | 1.36 |
| Flip angle [°] | 90 | 160 | 10 |
| b-values [s/mm²] | (n = 21; 52.5%) 50, 400, and 800 | ||
| (n = 19; 47.5%) 50, 400, and 1000 | |||
| Characteristics | All Patients (n = 40) | |
|---|---|---|
| n | % | |
| female | 21 | 52.5 |
| male | 19 | 47.5 |
| age (years) | mean 63.2 | range 55–70.5 |
| primary tumor | ||
| hepatocellular carcinoma | 14 | 33.3 |
| colorectal cancer | 8 | 19 |
| cholangiocarcinoma | 6 | 14.3 |
| ovarian cancer | 6 | 14.3 |
| pancreatic cancer | 2 | 4.8 |
| gastric cancer | 1 | 2.4 |
| breast cancer | 1 | 2.4 |
| appendiceal adenocarcinoma | 1 | 2.4 |
| Peritoneal Carcinomatosis | ||
|---|---|---|
| Imaging characteristics | n | % |
| 40 | 100 | |
| DWI characteristics | ||
| hyperintensity on high b-value DWI | 39 | 97.5 |
| high | 37 | 92.5 |
| intermediate | 2 | 5 |
| none | 1 | 2.5 |
| ADC characteristics | ||
| decreased ADC | 36 | 90 |
| low | 26 | 65 |
| intermediate | 10 | 25 |
| none | 4 | 10 |
| ADC value [×10−3 mm²/s] | mean 0.93 | range 0.69–1.14 |
| Imaging findings | ||
| peritoneal enhancement | 8 | 20 |
| presence of discrete nodules | 32 | 80 |
| omental cake | 7 | 17.5 |
| omental cake [mm] | mean 23.5 | range 17.3–30.3 |
| ascites | 12 | 27 |
| large volume | 10 | 25 |
| low volume | 2 | 5 |
| no ascites | 28 | 70 |
| splenomegaly | 16 | 40 |
| Imaging Characteristics | DWI | c-DWI b 1000 | c-DWI b 2000 | c-DWI b3000 | c-DWI b 4000 | c-DWI b 5000 |
|---|---|---|---|---|---|---|
| c-DWI derived from DWI b 800-images | ||||||
| Volume cm³ [IQR] | 1 [1–7.5] | 1 [1–6.5] | 1 [0–6] | 1 [0–6] | not measurable | not measurable |
| p-value (comparison with DWI b 800-images) | 0.766 | 0.062 | 0.125 | |||
| c-DWI derived from DWI b 1000-images | ||||||
| volume cm³ [IQR] | 7 [1–26] | 6 [1–26] | 6 [1–83] | 7 [1–70] | not measurable | not measurable |
| p-value (comparison with DWI b 1000-images) | 0.102 | 0.021 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ablefoni, M.; Leonhardi, J.; Ehrengut, C.; Mehdorn, M.; Sucher, R.; Gockel, I.; Denecke, T.; Meyer, H.-J. Magnetic Resonance Imaging of Peritoneal Carcinomatosis: Evaluation of High b-Value Computed Diffusion-Weighted Imaging. Curr. Oncol. 2022, 29, 4593-4603. https://doi.org/10.3390/curroncol29070364
Ablefoni M, Leonhardi J, Ehrengut C, Mehdorn M, Sucher R, Gockel I, Denecke T, Meyer H-J. Magnetic Resonance Imaging of Peritoneal Carcinomatosis: Evaluation of High b-Value Computed Diffusion-Weighted Imaging. Current Oncology. 2022; 29(7):4593-4603. https://doi.org/10.3390/curroncol29070364
Chicago/Turabian StyleAblefoni, Maxime, Jakob Leonhardi, Constantin Ehrengut, Matthias Mehdorn, Robert Sucher, Ines Gockel, Timm Denecke, and Hans-Jonas Meyer. 2022. "Magnetic Resonance Imaging of Peritoneal Carcinomatosis: Evaluation of High b-Value Computed Diffusion-Weighted Imaging" Current Oncology 29, no. 7: 4593-4603. https://doi.org/10.3390/curroncol29070364





