Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Recruitment and Consent
2.4. Baseline Data
2.5. Treatment
2.6. PRO Data Collection
2.7. Patient Body Type Characterization
2.8. Sample Size
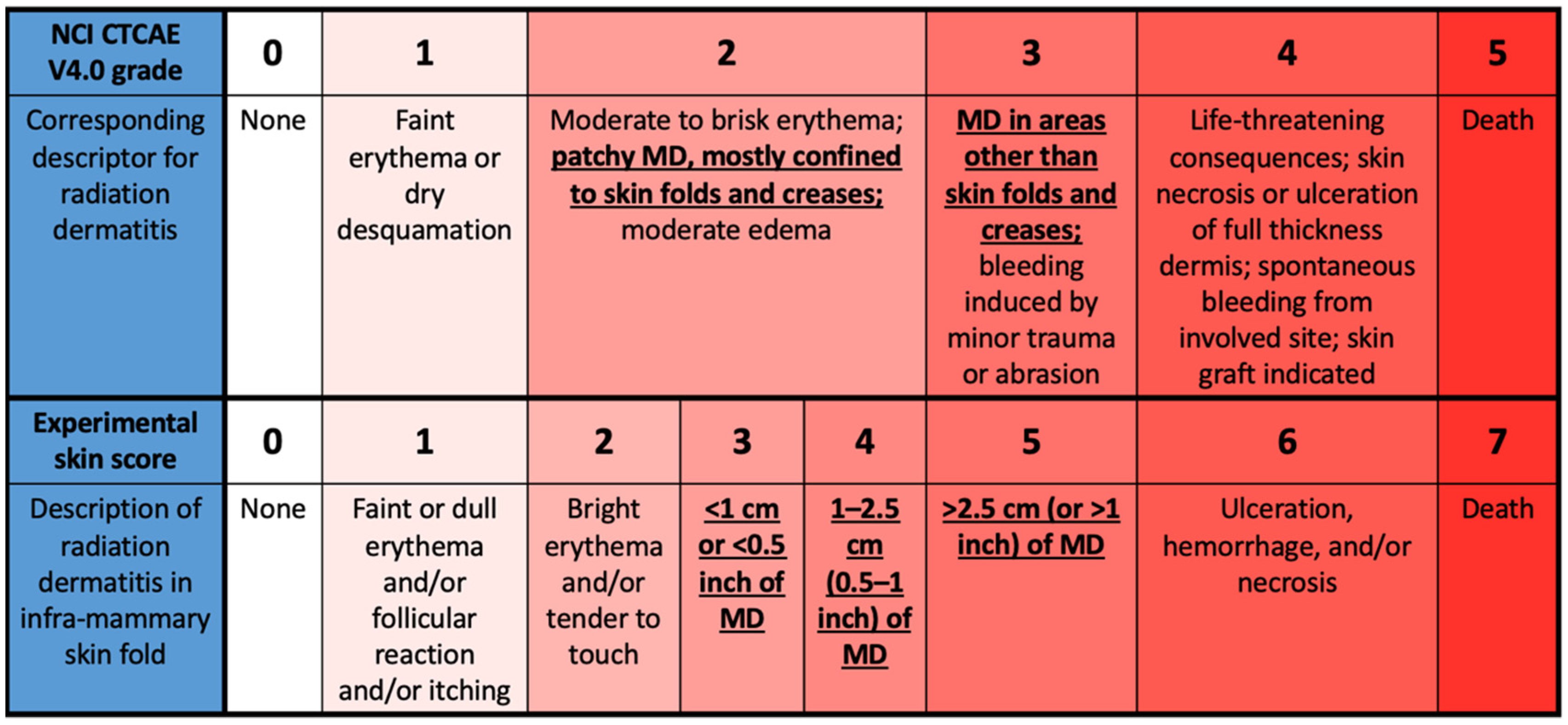
2.9. Skin Assessments
2.10. Skin Care
2.11. Data Analysis
3. Results
3.1. Patients
3.2. POSI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, R.A.; Howard, F.; Lapointe, V.; Schellenberg, D.; Nichol, A.; Bowering, G.; Curtis, S.; Walter, A.; Brown, S.; Thompson, C.; et al. Provincial development of a patient-reported outcome initiative to guide patient care, quality improvement, and research. Healthc. Manag. Forum 2018, 31, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caissie, A.; Olson, R.; Barbera, L.; O’Donnell, J.; Davis, C.A.; Croke, J.; Bird, L.; Kildea, J.; Brown, E.; Brundage, M.; et al. Striving to fill in gaps between clinical practice and standards: The evolution of a pan-Canadian approach to patient-reported outcomes use. Curr. Oncol. 2022, 29, 3698–3707. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Amylou, C.D.; Atkinson, T.M.; et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.S.; Haviland, J.S.; Hopwood, P.; Coles, C.E.; Yarnold, J.R.; Bliss, J.M.; Kirby, A.M.; IMPORT Trialists. Can patient-reported outcomes be used instead of clinician-reported outcomes and photographs as primary endpoints of late normal tissue effects in breast radiotherapy trials? Results from the IMPORT LOW trial. Radiother. Oncol. 2019, 134, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regional Radiotherapy in Biomarker Low-Risk Node Positive and T3N0 Breast Cancer (TAILOR RT). Identification Number NCT03488693. Available online: https://clinicaltrials.gov/ct2/show/NCT03488693 (accessed on 10 June 2022).
- Hypofractionated Radiation Therapy after Mastectomy in Preventing Recurrence in Patients with Stage IIa-IIIa Breast Cancer. Identification Number NCT03414970. Available online: https://clinicaltrials.gov/ct2/show/NCT03414970 (accessed on 10 June 2022).
- Lam, E.; Yee, C.; Wong, G.; Popovic, M.; Drost, L.; Pon, K.; Vesprini, D.; Lam, H.; Aljabri, S.; Soliman, H.; et al. A systematic review and meta-analysis of clinician-reported versus patient-reported outcomes of radiation dermatitis. Breast 2019, 50, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesprini, D.; Davidson, M.; Bosnic, S.; Truong, P.; Vallieres, I.; Fenkell, L.; Comsa, D.; El-Mallah, M.; Garcia, L.; Stevens, C.; et al. Effect of supine vs prone breast radiotherapy on acute toxic effects of the skin among women with large breast size: A randomized clinical trial. JAMA Oncol. 2022. [Google Scholar] [CrossRef]
- Pignol, J.P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.L.; Takita, C.; Reis, I.M.; Zhao, W.; Lee, E.; Nelson, O.L.; Hu, J.J. Prospective evaluation of radiation-induced skin toxicity in a race/ethnically diverse breast cancer population. Cancer Med. 2016, 5, 454–464. [Google Scholar] [CrossRef]
- Aquino-Parsons, C.; Lomas, S.; Smith, K.; Hayes, J.; Lew, S.; Bates, A.T.; Macdonald, A.G. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J. Med. Imaging Radiat. Sci. 2010, 41, 215–221. [Google Scholar] [CrossRef]
- Bhattacharya, I.S.; Haviland, J.S.; Kirby, A.M.; Kirwan, C.C.; Hopwood, P.; Yarnold, J.R.; Bliss, J.M.; Coles, C.E.; IMPORT Trialists. Patient-reported outcomes over 5 years after whole- or partial-breast radiotherapy: Longitudinal analysis of the IMPORT LOW (CRUK/06/003) phase III randomized controlled trial. J. Clin. Oncol. 2019, 37, 305–317. [Google Scholar] [CrossRef]
- Jagsi, R.; Griffith, K.A.; Boike, T.P.; Walker, E.; Nurushev, T.; Grills, I.S.; Moran, J.M.; Feng, M.; Hayman, J.; Pierce, L.J. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: Comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015, 1, 918–930. [Google Scholar] [CrossRef]
- Duzenli, C.; Koulis, T.; Menna, T.; Carpentier, E.; Arora, T.; Coope, R.; Gill, B.; Lim, P.; Aquino-Parsons, C.; Nichol, A.; et al. Reduction in doses to organs at risk and normal tissue during breast radiation therapy with a carbon-fiber adjustable reusable accessory. Pract. Radiat. Oncol. 2021, 11, 470–479. [Google Scholar] [CrossRef]
- Lapen, K.; Sabol, C.; Tin, A.L.; Lynch, K.; Kassa, A.; Mabli, X.; Ford, J.; Cha, E.; Bernstein, M.B.; Braunstein, L.Z.; et al. Development and pilot implementation of a remote monitoring system for acute toxicity using electronic patient-reported outcomes for patients undergoing radiation therapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 979–991. [Google Scholar] [CrossRef]
- Montpetit, C.; Singh-Carlson, S. Engaging patients with radiation related skin discomfort in self-care. Can. Oncol. Nurs. J. 2018, 28, 191–200. [Google Scholar] [CrossRef]
- Behroozian, T.; Milton, L.; Zhang, L.; Lou, J.; Karam, I.; Lam, E.; Wong, G.; Szumacher, E.; Chow, E. How do patient-reported outcomes compare with clinician assessments? A prospective study of radiation dermatitis in breast cancer. Radiother. Oncol. 2021, 159, 98–105. [Google Scholar] [CrossRef]
- Behroozian, T.; Milton, L.T.; Shear, N.H.; McKenzie, E.; Razvi, Y.; Karam, I.; Pon, K.; Lam, H.; Lam, E.; Chow, E. Radiation dermatitis assessment tools used in breast cancer: A systematic review of measurement properties. Support. Care Cancer 2021, 29, 2265–2278. [Google Scholar] [CrossRef]
- Berthelet, E.; Truong, P.T.; Musso, K.; Grant, V.; Kwan, W.; Moravan, V.; Patterson, K.; Olivotto, I.A. Preliminary reliability and validity testing of a new Skin Toxicity Assessment Tool (STAT) in breast cancer patients undergoing radiotherapy. Am. J. Clin. Oncol. 2004, 27, 626–631. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Lancellotta, V.; Zinicola, T.; Gentileschi, S.; Sollena, P.; Garganese, G.; Guinot, J.L.; Rembielak, A.; Soror, T.; Autorino, R.; et al. Cosmetic assessment in brachytherapy (interventional radiotherapy) for breast cancer: A multidisciplinary review. Brachytherapy 2019, 18, 635–644. [Google Scholar] [CrossRef]
- Shumway, D.A.; Kapadia, N.; Walker, E.M.; Griffith, K.A.; Do, T.T.; Feng, M.; Boike, T.; Helfrich, Y.; DePalma, B.; Gillespie, E.F.; et al. Development of an illustrated scale for acute radiation dermatitis in breast cancer patients. Pract. Radiat. Oncol. 2021, 11, 168–176. [Google Scholar] [CrossRef]
- Böhner, A.M.C.; Koch, D.; Schmeel, F.C.; Röhner, F.; Schoroth, F.; Sarria, G.R.; Abramian, A.V.; Baumert, B.G.; Giordano, F.A.; Schmeel, L.C. Objective evaluation of risk factors for radiation dermatitis in whole-breast irradiation using the spectrophotometric L*a*b color-space. Cancers 2020, 12, 2444. [Google Scholar] [CrossRef]
- King, M.T.; Link, E.K.; Whelan, T.J.; Olivotto, I.A.; Kunkler, I.; Westenberg, A.H.; Gruber, G.; Schofield, P.; Chua, B.H. Quality of life after breast-conserving therapy and adjuvant radiotherapy for non-low-risk ductal carcinoma in situ (BIG 3-07/TROG 07.01): 2-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 685–698. [Google Scholar] [CrossRef]
- Sun, L.M.; Huang, E.Y.; Liang, J.A.; Meng, F.Y.; Chang, G.H.; Tsao, M.J. Evaluation the consistency of location of moist desquamation and skin high dose area for breast cancer patients receiving adjuvant radiotherapy after breast conservative surgery. Radiat. Oncol. 2013, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.M.M.; Cohen, R.; Sopka, D.M.; Li, T.; Li, L.; Anderson, P.R.; Fowble, B.L.; Freedman, G.M. Effect of bra use during radiotherapy for large-breasted women: Acute toxicity and treated heart and lung volumes. Pract. Radiat. Oncol. 2013, 3, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagsi, R.; Griffith, K.A.; Vicini, F.; Boike, T.; Burmeister, J.; Dominello, M.M.; Grills, I.; Hayman, J.A.; Moran, J.M.; Paximadis, P.; et al. Toward improving patients’ experiences of acute toxicity from breast radiotherapy: Insights from the analysis of patient-reported outcomes in a large multicenter cohort. J. Clin. Oncol. 2020, 38, 4019–4029. [Google Scholar] [CrossRef]
- Lapen, K.; King, C.; Braunstein, L.Z.; Khan, A.J.; Kamrava, M.R.; Gillespie, E.F.; Cook, K.A. A comparison of patient- and clinician-reported acute toxicity during radiotherapy for primary breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022. [Google Scholar] [CrossRef]
- Kishan, A.U.; Wang, P.C.; Sharif, J.; Kupelian, P.A.; Steinberg, M.L.; McCloskey, S.A. Clinical indicators of psychosocial distress predict for acute radiation-induced fatigue in patients receiving adjuvant radiation therapy for breast cancer: An analysis of patient-reported outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 946–955. [Google Scholar] [CrossRef]
- McNeely, M.L.; Campbell, K.L.; Rowe, B.H.; Klassen, T.P.; Mackey, J.R.; Courneya, K.S. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ 2006, 175, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Perrucci, E.; Lancellotta, V.; Bini, V.; Falcinelli, L.; Farneti, A.; Margaritelli, M.; Capezzali, G.; Palumbo, I.; Aristei, C. Quality of life and cosmesis after breast cancer: Whole breast radiotherapy vs partial breast high-dose-rate brachytherapy. Tumori 2015, 101, 161–167. [Google Scholar] [CrossRef]
- Wadasadawala, T.; Maitre, P.; Sinha, S.; Parmar, V.; Pathak, R.; Gaikar, M.; Verma, S.; Sarin, R. Patient-reported quality of life with interstitial partial breast brachytherapy and external beam whole breast radiotherapy: A comparison using propensity-score matching. J. Contemp. Brachytherapy 2021, 13, 387–394. [Google Scholar] [CrossRef]




| Characteristic | Number of Patients | Percentage of Patients |
|---|---|---|
| Smoking status | ||
| Never | 86 | 64.2% |
| Current | 6 | 4.5% |
| Previous | 42 | 31.3% |
| Patient body type characterization | ||
| Group 1: BMI < 25, cup size A–C, IMF < 1cm | 38 | 28.4% |
| Group 2: BMI ≥ 25, cup size ≥ D, IMF ≥ 1cm | 27 | 20.1% |
| Group 3: BMI < 25, cup size ≥ D, IMF ≥ 1cm | 4 | 3.0% |
| Group 4: BMI ≥ 25, any cup size, IMF < 1cm | 41 | 30.6% |
| Group 5: post-mastectomy | 24 | 17.9% |
| Radiotherapy dose (without boost) | ||
| ≤4500 cGy | 97 | 72.4% |
| ≥4800 cGy | 37 | 27.6% |
| Chemotherapy | ||
| No | 79 | 59.0% |
| Yes | 55 | 41.0% |
| Hormone therapy | ||
| No | 103 | 76.9% |
| Yes | 31 | 23.1% |
| Total Dose (Gy)/Fractions | Patient Body Group | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| 42.5/16 | 37 (97.4%) | 16 (59.3.%) | 3 (75.0%) | 32 (78.0%) | 8 (33.3%) |
| 45 | 0 | 0 | 0 | 1 (2.4%) | 0 |
| 48.0/24 | 0 | 0 | 0 | 0 | 1 (4.2%) |
| 50.0/25 | 1 (2.6%) | 11 (40.7%) | 1 (25.0%) | 8 (19.6%) | 12 (50.0%) |
| 50.4/28 | 0 | 0 | 0 | 0 | 3 (12.5%) |
| Score | “Not at All” | “A Little Bit” | “Quite a Bit” | “Very Much” |
|---|---|---|---|---|
| Peak patient fatigue | 7 (5.2%) | 45 (33.6%) | 61 (45.5%) | 21 (15.7%) |
| Peak patient pain | 5 (3.7%) | 60 (44.8%) | 45 (33.6%) | 24 (17.9%) |
| Peak patient activity | 53 (39.6%) | 42 (31.3%) | 30 (22.4%) | 9 (6.7%) |
| Peak patient sleep | 33 (24.6%) | 61 (45.5%) | 31 (23.1%) | 9 (6.7%) |
| Peak pain medication | 67 (50.0%) | 50 (37.3%) | 12 (9.0%) | 5 (3.7%) |
| Peak POSI Score | Peak Staff Skin Score on Modified MD Scale | |
|---|---|---|
| 0–2 (N = 97) | 3–5 (N = 37) | |
| 0 | 79% (N = 77) | 14% (N = 5) |
| 1–3 | 21% (N = 20) | 86% (N = 32) |
| Variable | Dose ≥ 48 Gy | Body Group 2 | ||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Staff skin score | 3.2 (1.4–7.3) * | 4.4 (1.5–13.1) * | 14.6 (3.8–56.2) * | 9.6 (2.2–42.1) * |
| POSI open skin score | 2.8 (1.3–6.0) * | 3.0 (1.2–7.9) * | 3.9 (1.4–11.1) * | 2.6 (0.9–8.0) |
| POSI pain score (“quite a bit” to “very much”) | 2.9 (1.3–6.5) * | 2.9 (1.3–6.7) * | 1.6 (0.6–4.3) | N/A |
| POSI fatigue score (“quite a bit” to “very much”) | 3.5 (1.4–8.7) * | 3.5 (1.4–8.7) * | 2.0 (0.7–5.8) | N/A |
| POSI sleep interruption score (“quite a bit” to “very much”) | 3.2 (1.4–7.1) * | 2.8 (1.1–7.1) * | 1.8 (0.6–5.6) | 1.2 (0.4–4.0) |
| Antibiotic use | 3.3 (1.5–7.2) * | 3.0 (1.2–7.8) * | 5.3 (1.8–15.4) * | 3.6 (1.2–11.2) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duzenli, C.; Chan, E.K.; Koulis, T.; Grahame, S.; Singer, J.; Morris, D.; Spence, J.; Lee, T.; Burns, L.; Olson, R.A. Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients. Curr. Oncol. 2022, 29, 4734-4747. https://doi.org/10.3390/curroncol29070376
Duzenli C, Chan EK, Koulis T, Grahame S, Singer J, Morris D, Spence J, Lee T, Burns L, Olson RA. Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients. Current Oncology. 2022; 29(7):4734-4747. https://doi.org/10.3390/curroncol29070376
Chicago/Turabian StyleDuzenli, Cheryl, Elisa K. Chan, Theodora Koulis, Sheri Grahame, Joel Singer, David Morris, Josslynn Spence, Terry Lee, Levi Burns, and Robert A. Olson. 2022. "Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients" Current Oncology 29, no. 7: 4734-4747. https://doi.org/10.3390/curroncol29070376
APA StyleDuzenli, C., Chan, E. K., Koulis, T., Grahame, S., Singer, J., Morris, D., Spence, J., Lee, T., Burns, L., & Olson, R. A. (2022). Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients. Current Oncology, 29(7), 4734-4747. https://doi.org/10.3390/curroncol29070376






