Clinical Utility of Genomic Assay in Node-Positive Early-Stage Breast Cancer
Abstract
:1. Introduction
2. Oncotype DX
3. MammaPrint
- Both high clinical and genomic risk women (27%);
- Both low clinical and genomic risk women (41%);
- Low clinical risk and high genomic risk women (8.8%);
- High clinical risk and low genomic risk women (23.2%).
4. Prosigna®
5. Endopredict
6. Breast Cancer Index (BCI)
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markopoulos, C.; van de Velde, C.; Zarca, D.; Ozmen, V.; Masetti, R. Clinical evidence supporting genomic tests in early breast cancer: Do all genomic tests provide the same information? Eur. J. Surg. Oncol. (EJSO) 2017, 43, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sinn, P.; Seidman, A.D. Summary of head-to-head comparisons of patient risk classifications by the 21-gene Recurrence Score® (RS) assay and other genomic assays for early breast cancer. Int. J. Cancer 2019, 145, 882–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, S.; Kim, C.; Baehner, F.L.; Park, T.; Wickerham, D.L.; Wolmark, N. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Albain, K.S.; E Barlow, W.; Shak, S.; Hortobagyi, G.N.; Livingston, R.B.; Yeh, I.-T.; Ravdin, P.; Bugarini, R.; Baehner, F.L.; E Davidson, N.; et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Meric-Bernstam, F.; Gralow, J.R.; Albain, K.S.; Hayes, D.; Lin, L.; Perez, E.A.; Goldstein, L.J.; Chia, S.; et al. Abstract GS3-00: First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) in patients (pts) with 1–3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS) <25: SWOG S1007 (RxPonder). Cancer Res. 2021, 81 (Suppl. 4), GS3-00. [Google Scholar]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef]
- Van De Vijver, M.J.; He, Y.D.; Van’t Veer, L.J.; Dai, H.; Hart, A.A.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A Gene-Expression Signature as a Predictor of Survival in Breast Cancer. N. Engl. J. Med. 2002, 347, 1999–2009. [Google Scholar] [CrossRef] [Green Version]
- Van’t Veer, L.J.; Dai, H.; Van De Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Mook, S.; Schmidt, M.K.; Weigelt, B.; Kreike, B.; Eekhout, I.; van de Vijver, M.J.; Glas, A.M.; Floore, A.; Rutgers, E.J.T.; Veer, L.J.V. The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann. Oncol. 2010, 21, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Mook, S.; Schmidt, M.K.; Viale, G.; Pruneri, G.; Eekhout, I.; Floore, A.; Glas, A.M.; Bogaerts, J.; Cardoso, F.; Piccart-Gebhart, M.J.; et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res. Treat. 2009, 116, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyse, M.; Loi, S.; Veer, L.V.; Viale, G.; Delorenzi, M.; Glas, A.; D’Assignies, M.S.; Bergh, J.; Lidereau, R.; Ellis, P.; et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J. Natl. Cancer Inst. 2006, 98, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.Y.; Brain, E.; Causeret, S.; Delorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Veer, L.V.; Rutgers, E.; Loi, S.; Mook, S.; Piccart-Gebhart, M.J. Clinical Application of the 70-Gene Profile: The MINDACT Trial. J. Clin. Oncol. 2008, 26, 729–735. [Google Scholar] [CrossRef]
- Piccart, M.; van’t Veer, L.J.; Poncet, C.; Cardozo, J.M.L.; Delaloge, S.; Pierga, J.Y.; Vuylsteke, P.; Brain, P.E.; Vrijaldenhoven, S.; Neijenhuis, P.A.; et al. 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021, 22, 476–488. [Google Scholar] [CrossRef]
- Wallden, B.; Storhoff, J.; Nielsen, T.; Dowidar, N.; Schaper, C.; Ferree, S.; Liu, S.; Leung, S.; Geiss, G.; Snider, J.; et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med. Genom. 2015, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- A-Review-of-Gene-Expression. Available online: https://emj.emg-health.com/wp-content/uploads/sites/2/2019/05/A-Review-of-Gene-Expression....pdf (accessed on 7 May 2022).
- Dowsett, M.; Sestak, I.; Lopez-Knowles, E.; Sidhu, K.; Dunbier, A.K.; Cowens, J.W.; Ferree, S.; Storhoff, J.; Schaper, C.; Cuzick, J. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J. Clin. Oncol. 2013, 31, 2783–2790. [Google Scholar] [CrossRef]
- Gnant, M.; Filipits, M.; Greil, R.; Stoeger, H.; Rudas, M.; Bago-Horvath, Z.; Mlineritsch, B.; Kwasny, W.; Knauer, M.; Singer, C.; et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: Using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014, 25, 339–345. [Google Scholar] [CrossRef]
- Lænkholm, A.-V.; Jensen, M.-B.; Eriksen, J.O.; Rasmussen, B.B.; Knoop, A.; Buckingham, W.; Ferree, S.; Schaper, C.; Nielsen, T.O.; Haffner, T.; et al. PAM50 Risk of Recurrence Score Predicts 10-Year Distant Recurrence in a Comprehensive Danish Cohort of Postmenopausal Women Allocated to 5 Years of Endocrine Therapy for Hormone Receptor-Positive Early Breast Cancer. J. Clin. Oncol. 2018, 36, 735–740. [Google Scholar] [CrossRef]
- Dombernowsky, P.; Andersen, J.A.; Andersen, K.W.; Axelsson, C.K.; Blichert-Toft, M.; Hansen, M.; Krag, C.; Mouridsen, H.T.; Overgaard, M.; Rasmussen, B.B. Adjuvant chemotherapy in premenopausal and menopausal high-risk patients with breast cancer. 4. Results of the DBCG (Danish Breast Cancer Cooperative Group) 77B study. Ugeskr. Laeger 1991, 153, 2280–2283. [Google Scholar] [PubMed]
- Fitzal, F.; Filipits, M.; Fesl, C.; Rudas, M.; Greil, R.; Balic, M.; Moinfar, F.; Herz, W.; Dubsky, P.; Bartsch, R.; et al. PAM-50 predicts local recurrence after breast cancer surgery in postmenopausal patients with ER+/HER2- disease: Results from 1204 patients in the randomized ABCSG-8 trial. Br. J. Surg. 2021, 108, 308–314. [Google Scholar] [CrossRef]
- Sestak, I.; Cuzick, J.; Dowsett, M.; Knowles, E.L.; Filipits, M.; Dubsky, P.; Cowens, J.W.; Ferree, S.; Schaper, C.; Fesl, C.; et al. Prediction of late distant recurrence after 5 years of endocrine treatment: A combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J. Clin. Oncol. 2015, 33, 916–922. [Google Scholar] [PubMed]
- Ejlertsen, B.; Jensen, M.B.; Eriksen, J.O.; Kibøll, T.; Bruun Rasmussen, B.; Knoop, A.S.; Ferree, S.; Haffner, T.; Schaper, C.; Lankholm, A.-V. Validation of prediction of distant recurrence (DR) by Prosigna (PAM50) in subgroups of a Danish Breast Cancer Cooperative Group (DBCG) cohort of node-positive (N1), hormone receptor positive (HR+), postmenopausal early breast cancer (EBC) patients allocated 5yr of endocrine therapy (ET). J. Clin. Oncol. 2015, 33 (Suppl. 15), 513. [Google Scholar]
- Filipits, M.; Rudas, M.; Jakesz, R.; Dubsky, P.; Fitzal, F.; Singer, C.F.; Dietze, O.; Greil, R.; Jelen, A.; Sevelda, P.; et al. A New Molecular Predictor of Distant Recurrence in ER-Positive, HER2-Negative Breast Cancer Adds Independent Information to Conventional Clinical Risk Factors. Clin. Cancer Res. 2011, 17, 6012–6020. [Google Scholar] [CrossRef] [Green Version]
- Filipits, M.; Dubsky, P.; Rudas, M.; Greil, R.; Balic, M.; Bago-Horvath, Z.; Singer, C.F.; Hlauschek, D.; Brown, K.; Bernhisel, R.; et al. Prediction of Distant Recurrence Using EndoPredict Among Women with ER+, HER2− Node-Positive and Node-Negative Breast Cancer Treated with Endocrine Therapy Only. Clin. Cancer Res. 2019, 25, 3865–3872. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Brase, J.C.; Calvo, L.; Krappmann, K.; Ruiz-Borrego, M.; Fisch, K.; Ruiz, A.; Weber, K.E.; Munarriz, B.; Petry, C.; et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− breast cancer patients: Results from the GEICAM 9906 trial. Breast Cancer Res. 2014, 16, R38. [Google Scholar] [CrossRef] [Green Version]
- Sestak, I.; Martín, M.; Dubsky, P.; Kronenwett, R.; Rojo, F.; Cuzick, J.; Filipits, M.; Ruiz, A.; Gradishar, W.; Soliman, H.; et al. Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast Cancer Res. Treat. 2019, 176, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Jerevall, P.-L.; Ma, X.-J.; Li, H.; Salunga, R.; Kesty, N.C.; Erlander, M.G.; Sgroi, D.C.; Holmlund, B.; Skoog, L.; Fornander, T.; et al. Prognostic utility of HOXB13: IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br. J. Cancer 2011, 104, 1762–1769. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnabel, C.A.; Schroeder, B.E.; Jerevall, P.L.; Jankowitz, R.C.; Fornander, T.; Stal, O.; Brufsky, A.M.; Sgroi, D.; Erlander, M.G.; et al. Breast Cancer Index Identifies Early-Stage Estrogen Receptor–Positive Breast Cancer Patients at Risk for Early- and Late-Distant Recurrence. Clin. Cancer Res. 2013, 19, 4196–4205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schroeder, B.E.; Jerevall, P.L.; Ly, A.; Nolan, H.; Schnabel, C.A.; Sgroi, D.C. A Novel Breast Cancer Index for Prediction of Distant Recurrence in HR + Early-Stage Breast Cancer with One to Three Positive Nodes. Clin. Cancer Res. 2017, 23, 7217–7224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgroi, D.C.; Carney, E.; Zarrella, E.; Steffel, L.; Binns, S.N.; Finkelstein, D.M.; Szynonifka, J.; Bhan, A.K.; Shepherd, L.E. Prediction of Late Disease Recurrence and Extended Adjuvant Letrozole Benefit by the HOXB13/IL17BR Biomarker. JNCI J. Natl. Cancer Inst. 2013, 105, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.; Sgroi, D.; Treuner, K.; Zhang, Y.; Ahmed, I.; Piper, T.; Salunga, R.; Brachtel, E.; Pirrie, S.; Schnabel, C.; et al. Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial. Ann. Oncol. 2019, 30, 1776–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
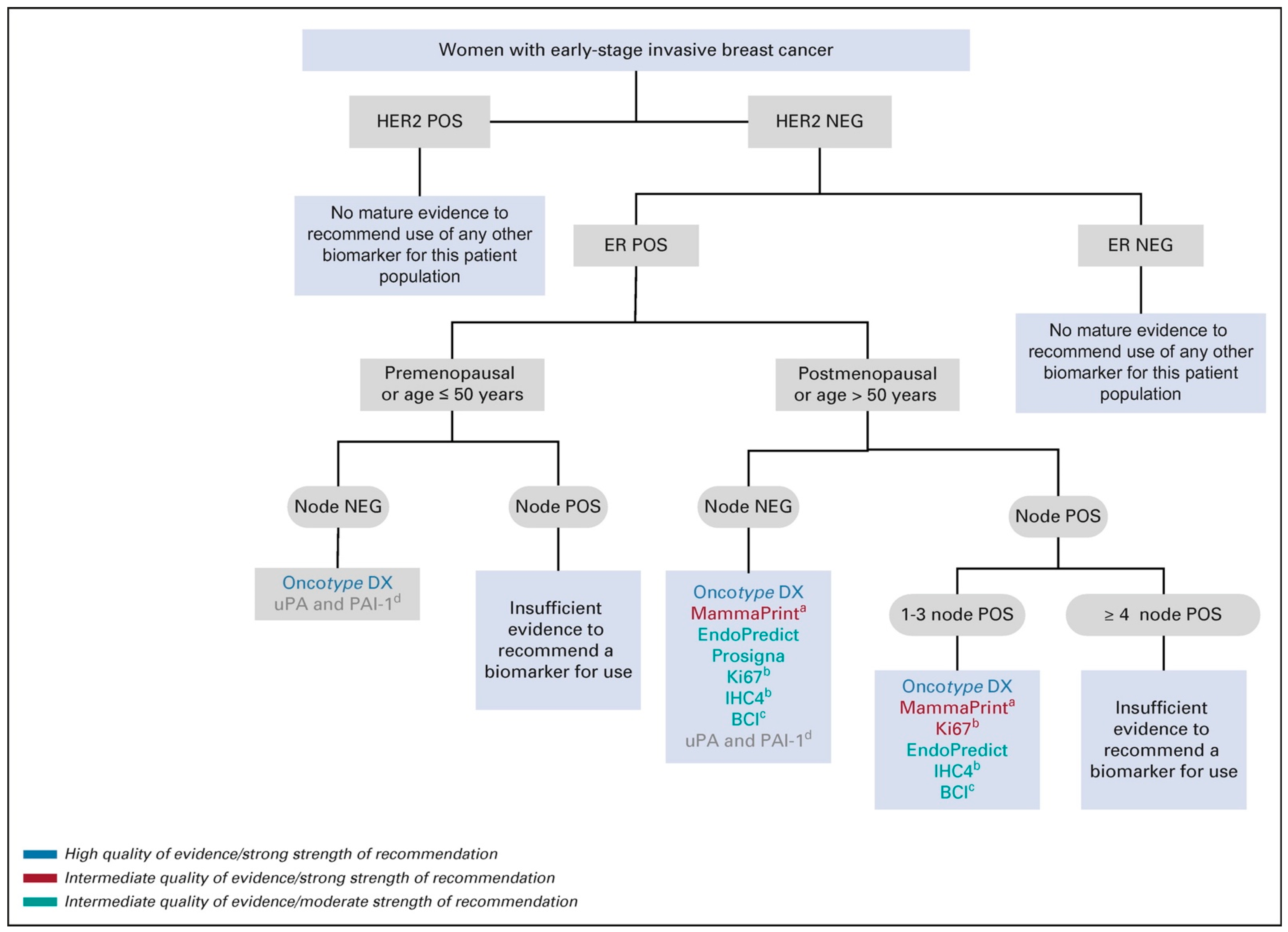
| Assay | Platform | Number of Gene Selected | Pivotal Studies Associated with the Assay for 1–3 Node-Positive BC | Recommendation by ASCO Clinical Practice Guideline for Postmenopausal Women with 1–3 Node-Positive ER+/HER-2 BC | Recommendation by ASCO Clinical Practice Guideline for Premenopausal Women with 1–3 Node-Positive ER+/HER-2 Negative BC |
|---|---|---|---|---|---|
| Oncotype DX | RT–PCR | 21 genes | SWOG-8814 (retrospective trial) RxPONDER trial (prospective trial) | Oncotype DX should be offered or used to guide systemic endocrine and chemotherapy decisions Evidence quality: high: Strength of recommendation: strong | Oncotype Dx should NOT be offered or used to guide systemic endocrine and chemotherapy decisions Evidence quality: high; Strength of recommendation: moderate |
| MammaPrint | Microarray analysis | 70-gene | MINDACT trial (prospective trial) | MammaPrint should be offered to guide adjuvant endocrine and chemotherapy decision among patients older than 50 with high clinical riskEvidence quality: intermediate; Strength of recommendation: strong | MammaPrint test is NOT recommended to guide adjuvant treatment among women age 50 or younger with high clinical risk Evidence quality: high -Strength of recommendation: strong. |
| Prosigna® | NanoString nCounter | 50 genes | ATAC trial, ABCSG A Danish cohort study DBCG 36 endocrine-treated women with early-stage BC DBCG 77B (all retrospective trials) OPTIMA (prospective trial)-awaiting results | Inconclusive to recommend using the Prosigna® test to guide decisions for adjuvant endocrine and chemotherapy Evidence quality: intermediate; Strength of recommendation: moderate | Prosigna® test is NOT recommended to guide decisions for adjuvant systemic endocrine and chemotherapy Evidence quality: insufficient; Strength of recommendation: moderate |
| Endopredict | RT–PCR | 8 genes | Predictive value of Endopredict is from pooled population from five large retrospective clinical trials (ABCSG-6, ABCSG-8, TransATAC trials, GEICAM 9906, GEICAM 2003/02) | EndoPredict can be used by clinicians to guide decisions for adjuvant endocrine and chemotherapy Evidence quality: intermediate; Strength of recommendation: moderate | EndoPredict test is NOT recommended to guide adjuvant endocrine and chemotherapy Evidence quality: insufficient; Strength of recommendation: moderate |
| Breast Cancer Index (BCI) | RT–PCR | 7 genes | MA.17 trial Trans-aTTom trial) | BCI test can be offered to guide decisions ONLY regarding extended endocrine therapy among patients with node-negative or 1–3 node-positive BC who have been treated with five years of primary endocrine therapy without evidence of recurrence Evidence quality: intermediate; Strength of recommendation: moderate | BCI test can be offered to guide decisions ONLY regarding extended endocrine therapy among patients with node-negative or 1–3 node-positive BC who have been treated with five years of primary endocrine therapy without evidence of recurrence Evidence quality: intermediate; Strength of recommendation: moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution-NonCommercial (CC-BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/).
Share and Cite
Pauls, M.; Chia, S. Clinical Utility of Genomic Assay in Node-Positive Early-Stage Breast Cancer. Curr. Oncol. 2022, 29, 5139-5149. https://doi.org/10.3390/curroncol29070407
Pauls M, Chia S. Clinical Utility of Genomic Assay in Node-Positive Early-Stage Breast Cancer. Current Oncology. 2022; 29(7):5139-5149. https://doi.org/10.3390/curroncol29070407
Chicago/Turabian StylePauls, Mehrnoosh, and Stephen Chia. 2022. "Clinical Utility of Genomic Assay in Node-Positive Early-Stage Breast Cancer" Current Oncology 29, no. 7: 5139-5149. https://doi.org/10.3390/curroncol29070407
APA StylePauls, M., & Chia, S. (2022). Clinical Utility of Genomic Assay in Node-Positive Early-Stage Breast Cancer. Current Oncology, 29(7), 5139-5149. https://doi.org/10.3390/curroncol29070407





