Is There a Place for Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Previous Trials
3.2. Critical Appraisal of the OUTBACK Trial
3.2.1. Study Overview
3.2.2. External Validity
3.2.3. Internal Validity
3.3. Contribution of the OUTBACK Trial
4. Discussion
4.1. Predictive and Prognostic Factors That Could Have Caused the Inconsistencies
4.1.1. Nonadherence
4.1.2. Stage of the Disease
4.1.3. Regional Differences
4.1.4. Treatment
4.1.5. Duration of Enrollment and Follow-Up
4.2. Future Directions
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.-T.; Pötter, R.; Sturdza, A.; Fokdal, L.; Haie-Meder, C.; Schmid, M.; Gregory, D.; Petric, P.; Jürgenliemk-Schulz, I.; Gillham, C.; et al. Change in Patterns of Failure After Image-Guided Brachytherapy for Cervical Cancer: Analysis from the RetroEMBRACE Study. Int. J. Radiat. Oncol. 2019, 104, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, Radiation, and Adjuvant Hysterectomy Compared with Radiation and Adjuvant Hysterectomy for Bulky Stage IB Cervical Carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic Radiation with Concurrent Chemotherapy Compared with Pelvic and Para-Aortic Radiation for High-Risk Cervical Cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.-Y. Randomized Comparison of Fluorouracil Plus Cisplatin Versus Hydroxyurea as an Adjunct to Radiation Therapy in Stage IIB-IVA Carcinoma of the Cervix with Negative Para-Aortic Lymph Nodes: A Gynecologic Oncology Group and Southwest Oncology Group Study. J. Clin. Oncol. 1999, 17, 1339. [Google Scholar] [CrossRef] [Green Version]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared with Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Han, Y.; Guo, F.; Chen, C.; Chen, X.; Zou, W. Platinum single-agent vs. platinum-based doublet agent concurrent chemoradiotherapy for locally advanced cervical cancer: A meta-analysis of randomized controlled trials. Gynecol. Oncol. 2019, 154, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zou, H.; Li, H.; Lin, R.; Su, M.; Zhang, W.; Zhou, Y.; Zhang, P.; Hou, M.; Deng, X.; et al. Weekly Versus Triweekly Cisplatin-Based Chemotherapy Concurrent with Radiotherapy in the Treatment of Cervical Cancer: A Meta-Analysis. Int. J. Gynecol. Cancer 2017, 27, 344–349. [Google Scholar] [CrossRef]
- Vale, C. Reducing Uncertainties About the Effects of Chemoradiotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data From 18 Randomized Trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar]
- de Azevedo, C.R.A.S.; Thuler, L.C.S.; de Mello, M.J.G.; Ferreira, C.G. Neoadjuvant Chemotherapy Followed by Chemoradiation in Cervical Carcinoma: A Review. Int. J. Gynecol. Cancer 2016, 26, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Watari, H. Concurrent chemoradiotherapy for cervical cancer: Background including evidence-based data, pitfalls of the data, limitation of treatment in certain groups. Chin. J. Cancer Res. 2016, 28, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Dueñas-González, A.; Zarbá, J.J.; Patel, F.; Alcedo, J.C.; Beslija, S.; Casanova, L.; Pattaranutaporn, P.; Hameed, S.; Blair, J.M.; Barraclough, H.; et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J. Clin. Oncol. 2011, 29, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Lorvidhaya, V.; Chitapanarux, I.; Sangruchi, S.; Lertsanguansinchai, P.; Kongthanarat, Y.; Tangkaratt, S.; Visetsiri, E. Concurrent mitomycin C, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: A randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1226–1232. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, S.S.; Nam, J.-H.; Kim, Y.-T.; Kim, Y.-M.; Kim, J.H.; Choi, E.K. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol. Oncol. 2008, 108, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tangjitgamol, S.; Tharavichitkul, E.; Tovanabutra, C.; Rongsriyam, K.; Asakij, T.; Paengchit, K.; Sukhaboon, J.; Penpattanagul, S.; Kridakara, A.; Hanprasertpong, J.; et al. A randomized controlled trial comparing concurrent chemoradiation versus concurrent chemoradiation followed by adjuvant chemotherapy in locally advanced cervical cancer patients: ACTLACC trial. J. Gynecol. Oncol. 2019, 30, e82. [Google Scholar] [CrossRef]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.; Gebski, V.; Narayan, K.; Bradshaw, N.; Monk, B.J. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: The randomized phase III OUTBACK Trial (ANZGOG 0902, RTOG 1174, NRG 0274). J. Clin. Oncol. 2021, 39 (Suppl. S18), LBA3. [Google Scholar] [CrossRef]
- Horeweg, N.; Mittal, P.; Gradowska, P.L.; Boere, I.; Chopra, S.; Nout, R.A. Adjuvant Systemic Therapy after Chemoradiation and Brachytherapy for Locally Advanced Cervical Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1880. [Google Scholar] [CrossRef]
- Horeweg, N.; Mittal, P.; Gradowska, P.L.; Boere, I.; Nout, R.A.; Chopra, S. A systematic review and meta-analysis of adjuvant chemotherapy after chemoradiation for locally advanced cervical cancer. Crit. Rev. Oncol./Hematol. 2022, 172, 103638. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management, [E] Evidence Reviews for Adjuvant Chemotherapy. NICE Guideline NG 101. Evidence Reviews. 2018. Available online: https://www.nice.org.uk/guidance/ng101/evidence/evidence-review-e-adjuvant-chemotherapy-pdf-4904666610 (accessed on 11 November 2021).
- National Institute for Health and Care Excellence (NICE). Colorecatl Cancer (Update). [C8] Optimal Duration of Adjuvant Chemotherapy for Colorectal Cancer. NICE Guideline NG151. Evidence Reviews. 2020. Available online: https://www.nice.org.uk/guidance/ng151/evidence/c8-optimal-duration-of-adjuvant-chemotherapy-for-colorectal-cancer-pdf-253058083669 (accessed on 15 November 2021).
- Hellyer, J.A.; Wakelee, H.A. Adjuvant Chemotherapy. Thorac. Surg. Clin. 2020, 30, 179–185. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, R.; Zhang, Z.; Li, T.; Li, F.; Liu, H.; Li, G. Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: A systematic review and meta-analysis of randomized controlled trials. Colorectal Dis. 2016, 18, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.Y.; Jung, J.H.; Hah, Y.S.; Cho, K.S. Role of adjuvant cisplatin-based chemotherapy following radical cystectomy in locally advanced muscle-invasive bladder cancer: Systematic review and meta-analysis of randomized trials. Investig. Clin. Urol. 2019, 60, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, W.; Fu, H.; Lin, Y.; Chen, K.-N. Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: A meta-analysis. Thorac. Cancer 2018, 9, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Cervical Cancer. NCCN Guidelines Version 1.2022. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical_basic.pdf (accessed on 15 November 2021).
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Willson, M.L.; Burke, L.; Ferguson, T.; Ghersi, D.; Nowak, A.K.; Wilcken, N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Egger, S.J.; Willson, M.L.; Morgan, J.; Walker, H.S.; Carrick, S.; Ghersi, D.; Wilcken, N. Platinum-containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.; Gois, A.F.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, D.; Petrelli, F.; Coinu, A.; Raspagliesi, F.; Barni, S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014, 133, 117–123. [Google Scholar] [CrossRef]
- Liontos, M.; Kyriazoglou, A.; Dimitriadis, I.; Dimopoulos, M.-A.; Bamias, A. Systemic therapy in cervical cancer: 30 years in review. Crit. Rev. Oncol. Hematol. 2019, 137, 9–17. [Google Scholar]
- Osman, M. The role of neoadjuvant chemotherapy in the management of locally advanced cervix cancer: A systematic review. Oncol. Rev. 2014, 8, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Gomez, S.; Bodis, S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int. J. Radiat. Oncol. 2019, 103, 411–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangjitgamol, S.; Katanyoo, K.; Laopaiboon, M.; Lumbiganon, P.; Manusirivithaya, S.; Supawattanabodee, B. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst. Rev. 2014, 2014, CD010401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, M.K.B.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Čerina, D.; Matković, V.; Katić, K.; Belac Lovasić, I.; Šeparović, R.; Canjko, I.; Jakšić, B.; Petrić-Miše, B.; Bajić, Ž.; Boban, M.; et al. Real-World Efficacy and Safety of Bevacizumab in the First-Line Treatment of Metastatic Cervical Cancer: A Cohort Study in the Total Population of Croatian Patients. J. Oncol. 2021, 2021, 2815623. [Google Scholar] [CrossRef]
- Dueňas-González, A.; Orlando, M.; Zhou, Y.; Quinlivan, M.; Barraclough, H. Efficacy in high burden locally advanced cervical cancer with concurrent gemcitabine and cisplatin chemoradiotherapy plus adjuvant gemcitabine and cisplatin: Prognostic and predictive factors and the impact of disease stage on outcomes from a prospective. Gynecol. Oncol. 2012, 126, 334–340. [Google Scholar] [CrossRef]
- Mileshkin, L.; Barnes, E.; Moore, K.N.; Gebski, V.; King, M.; Narayan, K.; Kolodziej, I.K.; Sjoquist, K.; Fyles, A.; Small, W.; et al. Disparities starting adjuvant chemotherapy for locally advanced cervix cancer in the international, academic, randomised, phase III OUTBACK trial (ANZGOG 0902, RTOG 1174, NRG 0274). Ann. Oncol. 2019, 30, v428–v429. [Google Scholar] [CrossRef]
- Fabri, V.A.; Queiroz, A.C.M.; Mantoan, H.; Sanches, S.M.; Guimarães, A.P.G.; Ribeiro, A.R.G.; Souza, R.P.; Maya, J.M.L.; Santos, E.S.; Castro, F.S.; et al. The Impact of Addition of Consolidation Chemotherapy to Standard Cisplatin-Based Chemoradiotherapy in Uterine Cervical Cancer: Matter of Distant Relapse. J. Oncol. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, S.; Isohashi, F.; Okazawa, M.; Kitada, F.; Maruoka, S.; Ogawa, K.; Kimura, T. Chemoradiotherapy followed by consolidation chemotherapy involving paclitaxel and carboplatin and in FIGO stage IIIB/IVA cervical cancer patients. J. Gynecol. Oncol. 2017, 28, e15. [Google Scholar] [CrossRef] [Green Version]
- Yavas, G.; Yavas, C.; Sen, E.; Oner, I.; Celik, C.; Ata, O. Adjuvant carboplatin and paclitaxel after concurrent cisplatin and radiotherapy in patients with locally advanced cervical cancer. Int. J. Gynecol. Cancer 2019, 29, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, E.; Prskalo, T.; Omrcen, T.; Situm, K.; Boraska, T.; Frleta Ilić, N.; Janković, S.; Hamm, W. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: Results of a phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Jelavić, T.B.; Miše, B.P.; Strikic, A.; Ban, M.; Vrdoljak, E. Adjuvant Chemotherapy in Locally Advanced Cervical Cancer After Treatment with Concomitant Chemoradiotherapy--Room for Improvement? Anticancer Res. 2015, 35, 4161–4165. [Google Scholar] [PubMed]
- Petrić Miše, B.; Boraska Jelavić, T.; Strikic, A.; Hrepić, D.; Tomić, K.; Hamm, W.; Tomić, S.; Prskalo, T.; Vrdoljak, E. Long follow-up of patients with locally advanced cervical cancer treated with concomitant chemobrachyradiotherapy with cisplatin and ifosfamide followed by consolidation chemotherapy. Int. J. Gynecol. Cancer 2015, 25, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tang, Y.; Yang, J.; Huang, S. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol. Oncol. 2012, 125, 297–302. [Google Scholar] [CrossRef]
- Zhou, Z.; Maquilan, G.M.; Thomas, K.; Wachsmann, J.; Wang, J.; Folkert, M.R.; Albuquerque, K. Quantitative PET Imaging and Clinical Parameters as Predictive Factors for Patients with Cervical Carcinoma: Implications of a Prediction Model Generated Using Multi-Objective Support Vector Machine Learning. Technol. Cancer Res. Treat. 2020, 19, 153303382098380. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared with Cisplatin and Paclitaxel in Patients with Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- Kitagawa, R.; Katsumata, N.; Shibata, T.; Kamura, T.; Kasamatsu, T.; Nakanishi, T.; Nishimura, S.; Ushijima, K.; Takano, M.; Satoh, T.; et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. 2015, 33, 2129–2135. [Google Scholar] [CrossRef]
- NSCLC Meta-Analyses Collaborative Group; Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.; Pignon, J.P.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar]
- Watson, J.M.; Torgerson, D.J. Increasing recruitment to randomised trials: A review of randomised controlled trials. BMC Med. Res. Methodol. 2006, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Estimated Number of New Cases and Deaths in 2020, Worldwide, Females, All Ages. Cancer Today. 2020. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&i (accessed on 5 November 2021).
- Pocock, S.J.; Simon, R. Sequential Treatment Assignment with Balancing for Prognostic Factors in the Controlled Clinical Trial. Biometrics 1975, 31, 103. [Google Scholar] [CrossRef] [PubMed]
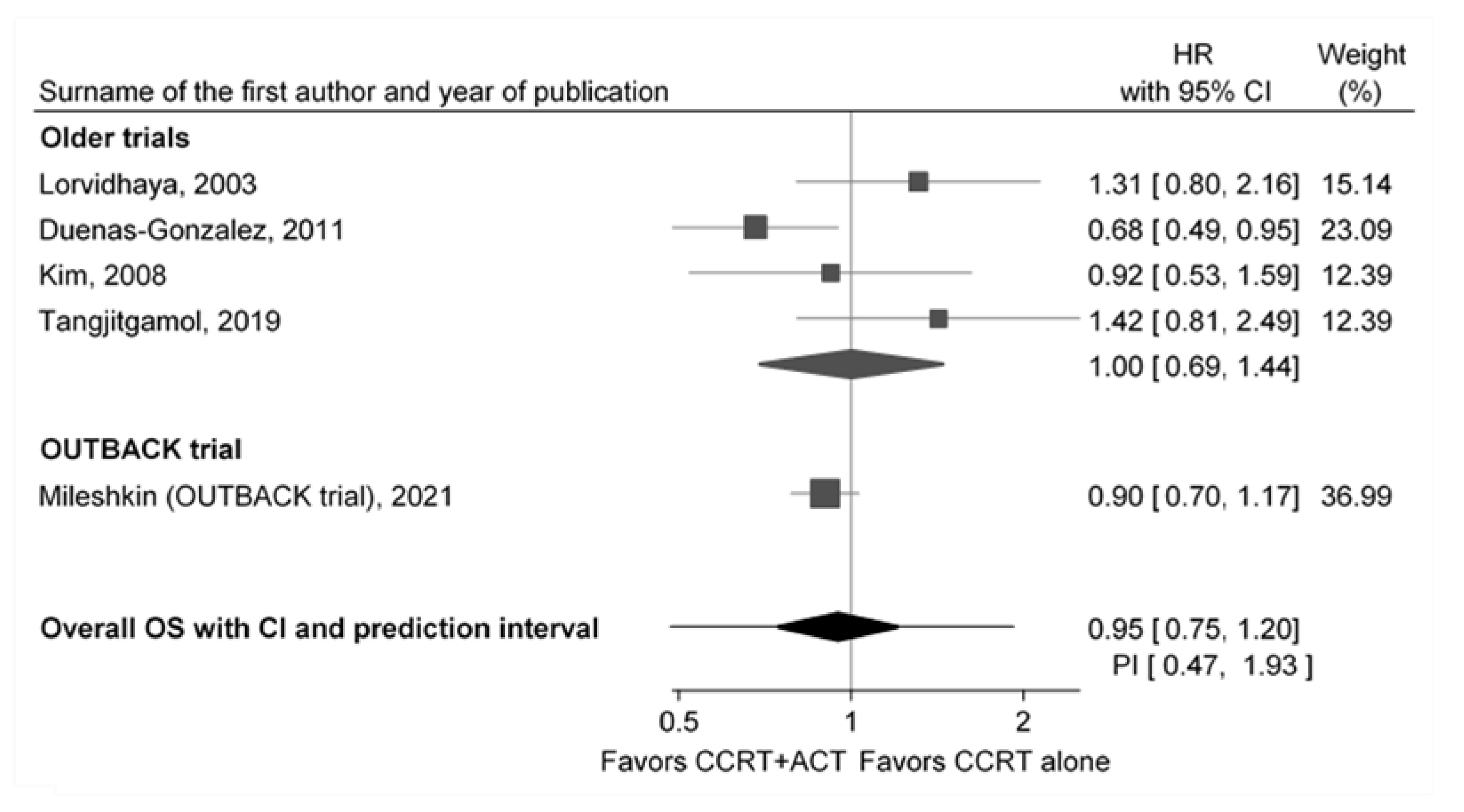
| Lorvidhaya [14] | Dueňas- González [13] | Kim [15] | Tangjitgamol [16] | Mileshkin OUTBACK [17] | |
|---|---|---|---|---|---|
| Year of publication | 2003 | 2012 | 2008 | 2019 | 2021 |
| Country | Thailand | Multiple a | Korea | Thailand | Multiple b |
| Outcomes | |||||
| The main result favors ACT | no | yes | no | no | no |
| Overall survival (HR) | 1.41 c | 0.68 | 0.92 c | 1.42 | 0.90 |
| (95% CI) | (0.79; 2.16) | (0.49; 0.95) | (0.53; 1.59) | (0.81; 2.49) | (0.70; 1.17) |
| Randomization | Stratified e | Minimization d | Stratified f | Stratified g | Stratified h |
| Concealed allocation | no | yes | no | not clear/yes | no |
| Masking/blinding | no | no | no | outcome ass | no |
| Patients | |||||
| Number of patients randomized | 230/233 | 259/256 | 78/77 | 130/129 | 461/465 |
| Enrollment (start-end year) | 1988–1994 | 2002–2004 | 1998–2005 | 2015–2017 | 2011–2017 |
| Duration of enrollment (years) | 6 | 2 | 7 | 2 | 6 |
| Patient median age (years) | 50/48 i | 45/46 | 58/57 | 49/50 | 46/45 |
| Range of patient age (years) | <65 | 22–68/18–70 | 36–75/34–73 | 23–68/26–68 j | 21–99/22–88 |
| Disease | |||||
| Stage (%) | |||||
| IB1 (all node+), IB2, IIA | 0/0 | 0/0 | 0/0 | 0/0 | 33/33 |
| IIB | 43/50 | 62/61 | 67/75 | 65/62 | 43/43 |
| IIIA | 1/1 | <1/<1 | 6/3 | 1/3 | 0/0 |
| IIIB | 55/49 | 36/37 | 22/17 | 31/35 | 24/24 k |
| IVA | 0/0 | 2/2 | 5/5 | 3/0 | - |
| Median tumor diameter (cm) | n.a. | 6/6 | 5/5 l | 5/5 | 5/5 |
| Histology (%) | |||||
| Squamous cell carcinoma | 90/88 | 93/94 m | 96/95 | 77/76 | 83/79 |
| Adenocarcinoma | 6/9 | 7/6 | 3/3 | 20/22 | 15/17 |
| Adenosquamous carcinoma | 1/0 | - | 1/3 | 2/2 | 3/4 |
| Small-cell carcinoma | 3/3 | - | 0/0 | 0/0 | 0/0 |
| Positive pelvic lymph nodes | yes | n.a. | yes | yes | yes |
| Para-aortic lymph nodes >1 cm | yes | no n | no | no | no |
| Previous chemotherapy or RT | no | no | no | yes o | yes p |
| Intervention (%) | |||||
| Completed CCRT | 95 | n.a. | 73 | 80 | 83 |
| Received at least one ACT dose | n.a. | 86 | n.a. | 77 | 78 |
| Completed CCRT + ACT | 92 | 77 | 65 | 65 | 62 |
| CCRT in control arm (cycle × DRUG mg/m2 or AUC) | 2 × MIT 10 2 × FU 300 mg/day | 6 × CIS 40 | 6 × CIS 30 | 6 × CIS 40 | 5 × CIS 40 |
| CCRT in ACT arm | 2 × MIT 10 2 × FU 300 mg/day | 6 × CIS 40 6 × GEM 125 | 2 × CIS 20 2 × FU 1000 | 6 × CIS 40 | 5 × CIS 40 |
| ACT protocol | 3 × FU 200 mg/day | 2 × CIS 50 2 × GEM 1000 | 1 × CIS 20 1 × FU 1000 | 3 × PAC 175 3 × CAR 5 | 4 × PAC 155 4 × CAR 5 |
| Follow-up | |||||
| Median follow-up (months) q | 89 | 46 | 39 | 27 | 60 |
| Worldwide | Income Levels | Continent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Low Middle | Upper Middle | High | Asia | Europe | Northern America | Latin America | Africa | Oceania | ||
| Incidence | |||||||||||
| Breast | 79.7 | 56.2 | 51.6 | 73.3 | 135.0 | 61.3 | 123.8 | 149.0 | 86.5 | 67.8 | 146.3 |
| Cervix | 22.1 | 39.7 | 28.2 | 21.2 | 13.9 | 21.1 | 17.7 | 10.2 | 24.7 | 42.7 | 16.8 |
| Ratio cervix to breast | 0.28 | 0.71 | 0.55 | 0.29 | 0.10 | 0.34 | 0.14 | 0.07 | 0.29 | 0.63 | 0.11 |
| Mortality | |||||||||||
| Breast | 22.6 | 30.5 | 24.5 | 20.2 | 21.5 | 19.9 | 24.7 | 20.9 | 22.5 | 32.3 | 24.5 |
| Cervix | 12.1 | 29.0 | 17.7 | 10.8 | 4.2 | 11.7 | 6.3 | 3.5 | 12.6 | 29.4 | 7.7 |
| Ratio cervix to breast | 0.54 | 0.95 | 0.72 | 0.53 | 0.20 | 0.59 | 0.26 | 0.17 | 0.56 | 0.91 | 0.31 |
| Mortality-to-incidence ratio | |||||||||||
| Breast | 0.28 | 0.54 | 0.47 | 0.28 | 0.16 | 0.32 | 0.20 | 0.14 | 0.26 | 0.48 | 0.17 |
| Cervix | 0.55 | 0.73 | 0.63 | 0.51 | 0.30 | 0.55 | 0.36 | 0.34 | 0.51 | 0.69 | 0.46 |
| Ratio cervix to breast | 1.93 | 1.35 | 1.32 | 1.85 | 1.90 | 1.71 | 1.78 | 2.45 | 1.96 | 1.45 | 2.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čerina, D.; Boraska Jelavić, T.; Buljubašić Franić, M.; Tomić, K.; Bajić, Ž.; Vrdoljak, E. Is There a Place for Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer? Curr. Oncol. 2022, 29, 5223-5237. https://doi.org/10.3390/curroncol29080415
Čerina D, Boraska Jelavić T, Buljubašić Franić M, Tomić K, Bajić Ž, Vrdoljak E. Is There a Place for Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer? Current Oncology. 2022; 29(8):5223-5237. https://doi.org/10.3390/curroncol29080415
Chicago/Turabian StyleČerina, Dora, Tihana Boraska Jelavić, Matea Buljubašić Franić, Krešimir Tomić, Žarko Bajić, and Eduard Vrdoljak. 2022. "Is There a Place for Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer?" Current Oncology 29, no. 8: 5223-5237. https://doi.org/10.3390/curroncol29080415
APA StyleČerina, D., Boraska Jelavić, T., Buljubašić Franić, M., Tomić, K., Bajić, Ž., & Vrdoljak, E. (2022). Is There a Place for Adjuvant Chemotherapy in the Treatment of Locally Advanced Cervical Cancer? Current Oncology, 29(8), 5223-5237. https://doi.org/10.3390/curroncol29080415





