Abstract
The lack of new drugs and resistance to existing drugs are serious problems in gastric cancer(GC) treatment. The research found polyphenols possess anti-Helicobacter pylori(Hp) and antitumor activities and may be used in the research and development of drugs for cancer prevention and treatment. However, polyphenols are affected by their chemical structures and physical properties, which leads to relatively low bioavailability and bioactivity in vivo. The intestinal flora can improve the absorption, utilization, and biological activity of polyphenols, whereas polyphenol compounds can increase the richness of the intestinal flora, reduce the activity of carcinogenic bacteria, stabilize the proportion of core flora, and maintain homeostasis of the intestinal microenvironment. Our review summarizes the gastrointestinal flora-mediated mechanisms of polyphenol against GC.
1. Introduction
According to the Global Cancer Epidemiology Statistics (GLOBOCAN) in 2020 worldwide [1], gastric cancer (GC) remains important cancer worldwide and is responsible for over one million new cases and an estimated 769,000 deaths (equating to 1 in every 13 deaths globally), ranking fifth for incidence and fourth for mortality globally. Considering the new cases of and deaths due to GC, China ranks third worldwide. According to data from China [2], the five-year survival rate for most early GCs after radical endoscopic therapy is more than 90, but the 5-year survival rate is still less than 30% even after comprehensive treatment-based surgery [3]. Achieving early diagnosis and treatment is a key part of the global GC prevention and control work. However, most areas with a high incidence of GC in the world still lack a mature prevention and control system for gastric cancer. More than 50% of the patients were at the advanced stage of GC at the time of initial diagnosis, so they lost the chance of radical surgery, and thus, they could only adopt a comprehensive treatment scheme based on anti-tumor drugs [4]. A recent meta-analysis showed that third-line therapy (TLT) is effective and safe in the treatment of advanced or metastatic GC, such as overall survival (OS), progression-free survival (PFS) and disease control rate (DCR) [5]. Chemotherapy, in the dominant position, has a relatively wide application scope. Fluorouracil plus platinum is the first line regimen, but the benefit of chemotherapy alone is limited, and the Median Survival Time (MS) is only 8 months, so chemotherapy is recommended combined with targeted therapy [6]. Targeted therapy is in targeting human epidermal growth factor receptor-2 (HER-2), vascular endothelial growth factor receptor (VEGF), tyrosine kinase inhibitor (TKI), and so on [7]. As early as 2010, the ToGA trial established the first-line treatment for patients with advanced GC who were HER-2 positive with trastuzumab combined with chemotherapy [8]. With the development of research on HER-2-targeted therapy for advanced GC, in January 2021, the Food and Drug Administration (FDA) of the United States approved trastuzumab deruxtecan (T-Dxd) for the treatment of unresectable, locally advanced, or metastatic GC, which previously received a trastuzumab regimen, thus further perfecting the targeted treatment of GC. However, the population suitable for targeted therapy is relatively limited, and the therapeutic effect is also different among individuals [9]. With the in-depth study of the tumor immune microenvironment (TME), the efficacy of immunotherapy represented by immune checkpoint inhibitors (ICIs) has been clear, especially in GC, with high expression of programmed death protein-1 (PD-1), programmed death ligand-1 (PD-L1), EB virus infection (EBV) and microsatellite instability (MSI), where the curative effect of ICIs is the most significant. The therapeutic effects of genomic stable type (GS) and chromosome unstable type need to be further studied [10]. However, ICIs work only against specific immune checkpoints (cell-surface molecules) and are almost ineffective in patients with low immune checkpoint expression levels [11]. However, with the continuous improvement in antineoplastic drug treatment for GC, the problem of tumor drug resistance has obviously not been improved [12]. Therefore, development of new drugs and complimentary medicine is essential, and plant polyphenols have been reported to have a good anti-cancer effect, which has attracted the wide attention of researchers.
Polyphenols are secondary metabolites from plants, widely present in foods and beverages with plant origins (e.g., fruits, vegetables, grains, soy, tea, and wine) [13]. Results of epidemiological research and meta-analyses implied that a polyphenol-rich diet has a protective effect against tumor, cardiovascular disease, diabetes mellitus, osteoporosis, and neurodegenerative diseases [14,15]. Polyphenols and polyphenol subclasses intake may reduce GC risk [16]. In addition, several literature findings have suggested that dietary polyphenols inhibit proliferation, induce apoptosis and reduce drug resistance in GC cells [17]. However, polyphenols’ function is affected by many factors, both intrinsic and extrinsic. For example, the gastrointestinal flora plays significant roles in the process of polyphenol absorption and metabolism. Most natural polyphenolic compounds must be absorbed and utilized under the action of specific intestinal flora, and phenolic metabolites can have activities that are not found in the original compounds [18]. On the contrary, polyphenols have a regulatory effect on the intestinal flora. They function as prebiotics by providing substrates required for microbial metabolism and interacting with microbial-related enzymes, enhancing beneficial flora growth, inhibiting carcinogenic flora proliferation, and maintaining the homeostasis of the intestinal microenvironment [19]. Therefore, our review concentrates on the anti-GC mechanisms of polyphenols mediated by gastrointestinal flora.
2. Polyphenol Anti-GC Mechanism
Previous studies have indicated polyphenols’ chemopreventive effect as antioxidant, antiproliferative, antibacterial, apoptosis-promoting compounds, and their role in regulating signaling pathways that prevent or reverse tumor differentiation. This includes two main aspects: polyphenols directly inhibit the occurrence of GC, and polyphenols eliminate GC risk factors, such as the infection by Helicobacter pylori (Hp).
2.1. Direct Protective Effect of Polyphenols
2.1.1. Polyphenols Protect against DNA Damage
Polyphenols have the same average reduction potential as vitamin E and are considered to be the richest antioxidants in the daily diet [20,21]. The biological activity depends on chemical structure, including the hydroxylation position of a single compound and the substitution of specific hydroxyl groups. The presence of hydroxyl groups makes polyphenols excellent hydrogen-bond donors [22]. Polyphenols have a high affinity for proteins and DNA, which promotes antioxidant properties and anti-free radical-mediated anti-DNA damage effects [23]. For example, curcumin inhibits GC growth by generating many reactive oxygen species, leading to the depletion of mitochondrial DNA content and DNA polymerase, altering the bioenergetics of the cells [24]. The mechanism is mainly regulated by the p53-p21/Gadd45a cyclin/CDK Rb/E2f-dnmt1 axis in damaged DNA repair [24]. Furthermore, studies have shown that curcumin analogs target topoisomerase II in human cancer cells, thus directly blocking the activity of topoisomerase II, and the chain in the DNA chain cannot be reconnected, leading to cancer cell apoptosis [25]. However, a Peng et al. study showed that polyphenols seemed to only have an antioxidant effect but did not repair the oxidized cells. This conclusion needs to be confirmed by more studies [26].
2.1.2. Apoptosis of Tumor Cells Induced by Polyphenols
Polyphenols have great potential for cancer prevention through the induction of apoptosis [27]. Natural polyphenols promote GC cell apoptosis by regulating target kinases. Researchers found that terminal ascorbic acids can activate the p38 MAPK-c-Jun-terminal kinase (JNK) pathway and promote apoptosis of GC cells [28]. Otherwise, phenolic compounds in the Begonia fruit extract inhibit the tumor cells’ growth, mainly by increasing the expression of Bcl-2 and Bcl-xL, and inhibiting Bax and Bak expression [29]. Similarly, curcumin significantly downregulate the expression level of Bcl-2, CDK4, and cyclin D1 in cells and tissues, thereby inhibiting SGC-7901 GC cell proliferation and inducing cell apoptosis [30]. It also regulates the proliferation, autophagy, and apoptosis of GC cells by affecting the PI3K and p53 signaling pathways [31]. Kaempferol activates IRE1-JNK-CHOP signaling pathway from cytoplasm to nucleus and inhibits epigenetic changes mediated by G9a (HDAC/G9a axis), thus activating autophagic death of GC cells [32]. Pectolina rigenin may lead to cell cycle arrest, autophagy and apoptosis in G2/M phase of GC cells by downregulating PI3K/AKT/mTOR pathway [33]. Resveratrol promotes cell apoptosis and against proliferation by combining with PIM-1 and inhibiting its catalytic activity [34]. The anti-apoptosis effect of polyphenols may be related to the inhibition of the activation of NF-κB involved in Notch and Wnt pathways [35]. Ho et al. indicated that gallic acid’s inhibitory effect on GC cells might connect with the NF-κB activity [36]. Curcumin inhibits the growth and promotes apoptosis of GC cells by the Wnt/-catenin pathway [37].
2.1.3. Tumor Metastasis Inhibition and Invasion
Epithelial-mesenchymal transition (EMT) is vital for tumor cells to achieve metastatic ability and invasiveness. After EMT, patients with GC are more likely to develop resistance to various therapeutic drugs, which worsens their clinical outcomes. For example, resveratrol regulates EMT by interfering with the hedgehog pathway inhibiting the GC invasion and metastasis [38]. In addition, resveratrol can regulate the PTEN/Akt pathway to inhibit EMT of GC cells [39]. Lignin-like sauchinone downregulate the PI3K/Akt and Smad2/3 pathways to prevent TGF-β1-relevant EMT [40]. Luteolin reverses EMT and inhibits GC progression by restraining the Notch pathway [41]. Plant polyphenols reduce tumor metastasis and invasion by regulating the EMT pathway. Recent studies have shown that resveratrol may also prevent IL-6-induced GC metastasis through downregulating the activation of the Raf/MAPK pathway [42]. Pagliara et al. reported that the lemon peel polyphenol extract inhibits the invasiveness of GC cells by decreasing the MM9/2 expression level [43]. Polyphenolic compounds inhibit tumor metastasis and invasion through other mechanisms. For example, curcumin may inhibit liver metastasis in primary GC by inhibiting the CD1/CXCR4-related pathway [44] and HMGB1/VEGF-D pathway GC [45].
2.1.4. Tumor Metastasis Inhibition and Invasion
Chemotherapeutic drug resistance has become a problem in GC. Studies have reported that compared with simple chemotherapy drug treatment group, the polyphenol-containing drug combined with chemotherapy increased the effect of GC chemotherapy [46]. The combination of oxaliplatin and rutin reduces the toxicity effects of chemotherapeutics and improves chemotherapy effect; the combination of luteolin and oxaliplatin can change the cell cycle ratio of SGC-7901 cells [47]. Baicalein promotes apoptosis and autophagy of GC cells through the Akt/mTOR and Nrf2/keap 1 pathway to improve sensitivity to cisplatin, and its effect is more intense than that of cisplatin or baicalein alone [48]. Similarly, cisplatin combined with avicularin significantly induces tumor cell apoptosis and reduces proliferation [17]. Cisplatin and resveratrol synergize the antitumor effect through endoplasmic reticulum stress-induced apoptosis and G2/M phase arrest [49]. The concentration and expression of angiogenesis-related factors are significantly downregulated after the combined treatment of quercetin and irinotecan, which may enhance the curative effect of irinotecan on the human GC cells [50]. Troxerutin inhibits STAT3/NF- B and Bcl-2 pathways to enhance the therapeutic function of 5- fluorouracil (5-FU) on GC [51]. The combination of 5-FU and catechin shows a better cytotoxic effect on tumor cells, and promotes the apoptosis of GC cells through reactive oxygen species [52]. Flavonoids can promote autophagy, inhibit EMT, block cell cycle and target ERK1/2/MAP pathway, showing selective anti-proliferation activity of adriamycin-resistant GC cells [53]. Rosmarinic acid combined with targeted therapy for GC has an excellent anticancer effect [54]. In addition, some polyphenol compounds, such as flavonoid polyphenols, have shown a more substantial anticancer effect than chemotherapeutic drugs [48]. Studies have shown that silibinin has significant cytotoxic activity on gastric adenocarcinoma cells (CI50: 60.17 ± 0.95 μg/mL) with a higher selectivity index compared with cisplatin. After metabolization silibinin showed an increase of cytotoxicity with a CI50 six-fold decrease (10.46 ± 0.25) [55].
3. Polyphenols Protect Indirectly from GC by Inhibiting Hp
Hp is considered the most critical member of the gastric microbiota, and its infection is a risk bacterium factor for GC [56]. Therefore, eradication of the infection is important for GC prevention and treatment. Because Hp can invade and colonize the gastric mucosa, its eradication has become a problem worldwide, but most antibiotics are not active in the acidic gastric environment. Therefore, new antibacterial compounds are actively being explored. Natural polyphenols and their secondary metabolites inhibit Hp activity. Based on Hp pathogenic factors, the mechanism of action of polyphenols against the bacterium mainly includes the following.
3.1. Restriction of Hp Colonization through Urease Inhibition
Urease is considered as one of the virulence factors of Hp and a necessary condition for infection. The apple polyphenol improves the chronic gastrointestinal effects caused by Hp through inhibiting urease effect [57]. Paulo et al. reported that resveratrol and red wine inhibit the growth of Hp through downregulating urease activity [58]. Procyanidins also have inhibitory effect against Hp urease, which is significantly related to the molecular size of procyanidins [59].
3.2. Inhibitory Effect of Bacterial Sialic Acid-Specific Adhesin and Downregulation on Expression of Inducible Cytidine Deaminase
Hp is parasitic on the human gastric mucosa and causes inflammation, atrophic gastritis, and GC. Adhesion is an essential component of the pathogen invasion and is a key event in the establishment of infection. Studies have indicated that polyphenols decrease the adhesion between Hp and the gastric mucosa, reduce the Hp-related inflammatory response, and reduce the incidence of Hp-associated GC. Additionally, 3.9 g/mL flavonoids inhibit approximately 90% of Hp growth by inhibiting adhesion [60]. In addition, in Hp-infected gastric epithelial cells, the activation of NF-κB can promote the abnormal expression of inducible cytidine deaminase, which is considered as one of the key mechanisms of Hp -related GC. Therefore, the inhibitory effect of NF-κB downregulates AID expression and plays a protective role. Curcumin may downregulate AID induced by inhibiting the NF-κB pathway and combat Hp-related gastric carcinogenesis [61].
3.3. Inhibition of the Release of Inflammatory Cytokines
IL-8 is the key cytokines involved in Hp-related inflammatory response. Torres et al. synthesized epicatechin semisynthetic derivatives from avocado peel and observed their adhesion to human GC cells and the induction of the proinflammatory release of IL-8 [62]. The study found that at 700 g/mL, the Hp adhesion rate to human stomach adenocarcinoma cells was less than 20% The production rate of IL-8 was less than 10%, indicating that epicatechin has anti-inflammatory functions on Hp-infected GC. The resveratrol pretreatment significantly inhibits Hp-induced IL-8 secretion and reactive oxygen species production [61]. The inhibitory function of resveratrol and epicatechin on IL-8 is probably related to their inhibitory activity on the NF-κB pathway, which downregulates the expression level of IL-8. Research has found that Walnut polyphenol extracts prevent Hp-related tumor growth by inhibiting STAT3 phosphorylation and nuclear translocation in gastric mucosal cells [63], and reversing precancerous atrophic gastritis [64]. Nobiletin has been confirmed to inhibit Hp infection and prevent Hp-mediated GC [65]. Notably, silymarin has 100% inhibitory effect on cytokines and NO related to Hp infection [55].
3.4. Inhibition of the Cytotoxic Activities of Hp Vacuolar Protein A (Vac A) and Cytotoxic Associated Protein A (Cag A)
Vac A and Cag A have important impacts in Hp pathogenesis. Infection with Vac A+ strain leads to vacuolization and apoptosis, whereas Cag A+ strain infection leads to severe gastritis and GC. Kaempferol plays anti-inflammatory and anti-cancer roles by decreasing the translocation of Cag A and Vac A [66]. In addition, the degree of gastric damage is quantitatively determined in mice, which tips that high-molecular-weight catechin-polymerized hop bud leaf extract (HBT) inhibits the activity of Vac A in vivo. Additionally, HBT can inhibit the activity and absorption of Vac A, while inhibiting Vac A-induced vacuolation of sensitive cells to inhibit the occurrence of gastric ulcer and inflammation [67]. Black rice extract, with anthocyanin as the main component, can also impede mRNA and protein levels of Cag A and Vac A [68]. Mahady et al. proved that resveratrol inhibits the Cag A+ Hp growth [69]. Animal studies have further confirmed that polyphenols limit damage to the gastric epithelium in mice model infected with Hp or treated by Vac A toxin [70]. Similarly, curcumin has an obvious inhibitory function on the activity of Cag A+ Hp [71]. All in all, polyphenols exert a variety of biological activities inhibiting the appearance of GC directly and indirectly, and have a protective impact by regulating gastrointestinal flora, such as Hp. Indeed, there is an extremely close relationship between polyphenols and intestinal microbes, which is closely related to the occurrence of GC. Importantly, gastrointestinal flora involves in the complete metabolic process of polyphenols, which significantly improves the absorption, utilization, and biological activity of polyphenols. In addition, polyphenols have a strong regulatory effect on the intestinal flora, thereby triggering an increase in the body beneficial bacteria to prevent GC occurrence.
4. Intestinal Flora Promotes the Transformation and Absorption of Polyphenols and Regulates Their Biological Activity
4.1. Absorption and Metabolism of Polyphenols in Gastrointestinal Tract
Studies have verified it is not natural polyphenols that ultimately make effects on cells and tissues, which is due to the transformation of their structure and activity in the process of absorption and utilization. Most dietary polyphenols exist in food as esters, glycosides or polymers, which cannot be used directly and must be absorbed after the action of gastrointestinal flora and enzymes. In the past, the biological community generally believed that polyphenols’ digestion and metabolism mainly occurred in the small intestine. However, recent research on the morphology of polyphenols and gastrointestinal organisms have led to a new understanding of their absorption and metabolism. Figure 1 shows the absorption and metabolism of the ingested polyphenols in the body. Dietary polyphenols are divided into free and conjugated polyphenols [72]. It is estimated that only 5–10% of free phenols with a simple structure, such as aglycons, flavonol monomers or dimers, and some polyphenol sugars, are absorbed by small intestine cells [73]. Some free phenols are transformed into metabolites available for resident microorganisms to produce biomass. These metabolites may even be more bioactive than their precursors. These simple phenols undergo phase I and II reactions in intestinal cells and hepatocytes, such as methylation, glucuronidation, and sulfated derivatives, to produce many water-soluble metabolites released into blood and various organs, and finally discharged from the urine. Almost 90–95% of dietary phenols cannot be absorbed by small intestine cells [74]. Conjugated phenols, such as oligomeric and polymerized phenols with a molecular weight of nearly 40 kDa, enter the colon. Only a few are absorbed by colon cells. Most participate in the catabolism of the intestinal flora, enter the enterohepatic circulation, and are finally absorbed and utilized by the human body, thus promoting health [75].
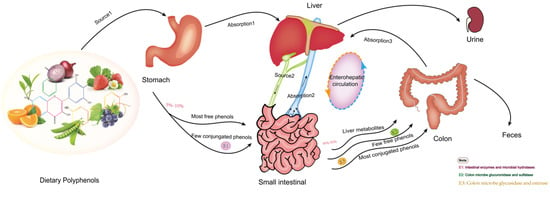
Figure 1.
The transformation and absorption of polyphenols in the human body.
4.2. Intestinal Flora Regulates Biotransformation and Activity of Polyphenols
Intestinal flora has irreplaceable impacts on the complete metabolism of polyphenol glycosides. Polyphenols decompose into small-molecule metabolites absorbed and distributed in various tissues by intestinal microorganisms. Studies have reported that differences in intestinal flora affect biological functions of polyphenols [76]. The regulatory function of polyphenols is mainly reflected in the following aspects: (1) intestinal microorganisms secrete enzymes promote the transformation of polyphenols from conjugated to unconjugated [77]; (2) intestinal flora directly promotes the decomposition of free polyphenols into more active and easily absorbed molecules, which are absorbed into the intestinal liver circulation through intestinal mucosal cells; (3) through the depolymerization of intestinal microbial enzymes, phenol metabolites are excreted through the bile duct and some are reabsorbed; (4) through microbial metabolism, small phenolic metabolites with higher absorbability, utilization, and biological activity than precursor compounds, and some even have broader natural characteristics than original polyphenols [78]; (5) intestinal flora has specificity for the metabolic degradation of polyphenols. Different types of polyphenols can be absorbed by different flora. If there is no particular flora in the intestine, even if some polyphenols are ingested, they are not biologically active. Table 1 lists representative studies on the impact of the intestinal flora on polyphenol conversion and absorption.

Table 1.
The effect of intestinal flora on transformation and absorption of polyphenol.
4.3. Regulation of Polyphenols on the Intestinal Flora
The intestinal flora and the human body constitute the intestinal microecosystem and are closely related to health. The proportion of beneficial bacteria in the intestines of healthy people is 70%, whereas that of patients with cancer is only 10%. As shown in Table 2, studies including vitro fermentation models, animal models and clinical trials, have revealed polyphenols and metabolites’ regulatory functions on intestinal flora. Polyphenols selectively promote the proliferation of beneficial intestinal flora, inhibit pathogenic bacteria, reduce their virulence through prebiotic effects, regulate the composition of intestinal flora, enrich the diversity of intestinal flora, and promote intestinal microenvironment homeostasis [86]. The regulatory effect of polyphenols on gut microbiota might be affected to their structure, concentration, and microbial species. The reported mechanisms of polyphenols and intestinal flora are as follows: (1) polyphenol metabolites provide metabolic substrates for microbial growth and (2) polyphenols affect the activity of enzymes related to microbial growth. The specific impact may be as follows: (1) They affect the type and quantity of enzymes in the intestine by changing the type and content of gut microbiota and (2) chelating metal ions in the body. Some microbial enzyme systems with metal ions as coenzymes lose their activity due to the lack of auxiliary groups. Additionally, polyphenols and iron binding inhibit the heme group production in some aerobic microorganisms, which affects the microorganism and its enzymatic system. (3) They directly inhibit the activity of some intestinal microbial enzymes. For example, studies have shown that condensed tannins can inhibit the activity of bacterial extracellular enzymes, such as endoglucanase, which is mainly achieved by the combination of polyphenols and enzyme protein molecules. (4) There may be action on microbial cell membranes: the hydroxyl structure of polyphenols can combine with the bacterial cell membrane to inhibit bacteria and (5) influence microbial adhesion. For example, procyanidins B1 and B2 significantly increase the adhesion ability of Lactobacillus spp. In a word, polyphenols regulate intestinal flora, promote the growth of intestinal probiotics, inhibit pathogenic bacteria, inhibit carcinogenic enzymes activity in the microbiota, and reduce the probability of carcinogenesis.

Table 2.
The regulation of polyphenols and polyphenol-rich extracts on intestinal flora.
5. Gut Microbiome and GC Treatment
The intestinal microbiome is closely related to GC, which influences the curative effect of different treatment strategies for GC, including surgery, chemotherapy, radiotherapy, and immunotherapy. Intestinal probiotics regulate the homeostasis of intestinal microbiota and maintain the intestinal barrier and immune state, which is beneficial to the recovery and improvement of post-operative prognosis [102]. In chemotherapy, intestinal flora can enhance efficacy, reduce drug resistance and reduce adverse events. Intestinal flora interaction promotes inflammation and provides inflammatory mediators for the treatment of oxaliplatin, cisplatin and CpG oligonucleotides. Research in mice has shown that antibiotic-treated mice (which killed the gut microbiome) did not respond as well to platinum chemotherapy or CpG-oligonucleotide immunotherapy as mice with intact gut microbes [103]. Other studies have reported that regulating the microbiome through nutrition or probiotic supplements can reduce chemotherapy toxicity and subsequent adverse events in mice and humans [104]. Additionally, research demonstrated that intestinal flora improves chemoresistance. For instance, Fusobacterium nucleatum regulating the molecular network of Toll-like receptors, microRNAs, and autophagy control the chemotherapy resistance of colorectal cancer clinically, biologically and mechanically [105]. Similarly, Fusobacterium nucleatum promotes chemoresistance of oxaliplatin by activating autophagy in tumor cells [106]. In radiotherapy, fecal flora transplantation (FMT) improves the survival rate of irradiated animals, gastrointestinal function, and intestinal epithelial integrity, and prevents radiation-induced toxicity. Moreover, intestinal microbiological disorders may become a potential biomarker for the prediction and prevention of radiation-induced bowel disease or other complications in the future [107]. In immunotherapy, the effect of intestinal microorganisms on the therapeutic efficacy and toxicity of immune checkpoint inhibitors (ICIs) has also been explored to a great extent [108]. Although the exact mechanism is unclear, Gopalakrishnan et al. showed that intestinal microflora remotely controls the central role of lymphocyte and myeloid cell regulation [109]. The release of lipopolysaccharide (LPS) from intestinal microorganisms stimulates innate immunity through TLR4 pathway, thus promoting anti-tumor CD8+ T cell immune response [110]. Certain bacteria, such as Bacteroidetes thetaiotaomicron and Faecali bacterium prausnitzii, have been reported to enhance the effectiveness of checkpoint inhibitors [111]. Hp is recognized as a pathogenic factor in gastric cancer, but recently, researchers have found that Hp influences gastric cancer immunotherapy. Liu et al. demonstrated that 59.3% of Hp+ GC patients expressed PD-L1, suggesting that Hp might imply anti-PD-1/PD-L1 therapy efficacy [112]. Wu et al. proved that PD-L1 expression in primary human gastric epithelial cells is strongly enhanced by Hp, and significantly induces T cell apoptosis to enhance the efficacy of immune checkpoint inhibitors [113]. Finally, in a recent study (DELIVER test: UMIN000030850), Bacterial genome analysis of 501 patients with advanced GC treated with nivolumab showed that Odoribacter and Veillonella were associated with tumor response to nivolumab, and GC-specific intestinal microflora may predict the response to ICIs [114]. In all, the role of gastrointestinal microflora in GC treatment needs to be further clarified in multicenter prospective studies to identify specific bacterial species and pathways, as well as changes in microbiota associated with the progression of GC.
6. Summary and Challenge
As natural plant compounds, dietary polyphenols have great potential for chemical prevention and therapy of GC. Polyphenols are anti-inflammatory, antibacterial, antioxidant, and anti-proliferative compounds that induce apoptosis or autophagy, inhibit EMT, cause the hindering of angiogenesis and metastasis, enhance chemotherapy sensitivity, and regulate gastrointestinal flora to play a protective role against GC [41,115,116].
Currently, although much progress has been made in understanding the anti-GC mechanism related to polyphenols, the details are still unclear as to how dietary polyphenols affect these mechanisms. Polyphenols act as localized small-molecule inhibitors in signal transduction and block their protein–protein interactions or their interactions with DNA, in particular the disruption of multimeric forms of transcription factors such as c-jun/c-fos (Activator Protein-1; AP-1) [117], c-myc/max, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [118] and β-catenin/T cell factor (Tcf), thus having an anti-tumor effect. The development of polyphenol drugs, such as polyphenol transcription factor inhibitors, has significant clinical application value [119]. Additionally, as mentioned above, human topoisomerase may serve as a potential molecular target of polyphenol compounds, which can inhibit enzyme activity and ultimately prevent the growth of cancer cells [120]. In the future, we can develop polyphenol compounds as cancer cell topoisomerase inhibitors, providing more possibilities for anticancer drugs. However, polyphenols, as natural compounds, have low bioavailability in our bodies. More attention should be paid to how to deliver higher concentrations of polyphenols to target organs to improve their absorption and utilization. The existing polyphenol nano-drug delivery technology may have great potential in this regard [121]. In fact, studies have demonstrated that polyphenols can provide a powerful environment for tumor immunotherapy by regulating the tumor immune microenvironment (TME) [122]. Conversely, there are indications that polyphenols may also play harmful roles [123], which means we should choose carefully when immunotherapy is used [124]. Future studies focused on precise immunotherapeutic protocols and well-defined cell and animal models will probably help us explore new ways to fight cancer. Importantly, studies have reported that the polyphenol compound naringin cannot be hydrolyzed by rhamnosidase in probiotics but can be hydrolyzed by fungal rhamnosidases, which indicates that intestinal fungi have a specific effect on the catabolism of polyphenols [79]. Similarly, studies have reported the effect of polyphenols on fungi [125], but the interaction mechanism between polyphenols and fungi still needs to be further studied. What is the interaction between intestinal fungi and intestinal bacteria in the anti-GC activity of polyphenols? What are the potential connections between polyphenols, intestinal bacteria, and intestinal fungi? In the future, more basic and clinical research will be required to understand the interaction mechanisms among polyphenols, GC and other influencing factors.
Author Contributions
Y.Z. (Yongning Zhou) and M.L. (Matu Li) conceived and proposed the idea. M.L. (Matu Li), Y.Z. (Ya Zheng) and J.Z. designed and wrote the manuscript. M.L. (Meimei Liu), X.S. and Q.L. revised the manuscript. Y.W. contacted the company to polish the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Several foundations supported our work: the National Natural Science Foundation of China (the funder: Yongning Zhou No. 71964021), the National Key R&D Program of China (the funder: Yuping Wang No. 2016YFC1302201) and the Foundation of The First Hospital of Lanzhou University, China (the funder: Ya Zheng No. ldyyyn2021-59).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Sun, T.; Wu, H.; Yang, F.; Zou, W.B. Consensus on screening, Endoscopic diagnosis and treatment of early gastric Cancer in China. Gastroenterology 2014, 19, 408–427. [Google Scholar]
- Ajani, J.A.; Bentrem, D.J.; Besh, S.; D’Amico, T.A.; Das, P.; Denlinger, C.; Fakih, M.G.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; et al. Gastric cancer, version 2.2013: Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2013, 11, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Mollica, V.; Ricci, A.D.; Maggio, I.; Massucci, M.; Rojas Limpe, F.L.; Fabio, F.D.; Ardizzoni, A. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 2020, 16, 4409–4418. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Ekstrom, K.; Hoffman, K.; Graf, W.; Sjoden, P.O.; Haglund, U.; Svensson, C.; Enander, L.K.; Linne, T.; Sellstrom, H.; et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann. Oncol. 1997, 8, 163–168. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer, C.; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Haffner, I.; Schierle, K.; Raimundez, E.; Geier, B.; Maier, D.; Hasenauer, J.; Luber, B.; Walch, A.; Kolbe, K.; Riera Knorrenschild, J.; et al. HER2 Expression, Test Deviations, and Their Impact on Survival in Metastatic Gastric Cancer: Results From the Prospective Multicenter VARIANZ Study. J. Clin. Oncol. 2021, 39, 1468–1478. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Rojas Llimpe, F.L.; Di Fabio, F.; De Biase, D.; Rihawi, K. Novel HER2-Directed Treatments in Advanced Gastric Carcinoma: AnotHER Paradigm Shift? Cancers 2021, 13, 1664. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.-C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Ning, T.; Yang, H.; Liu, D.; Zhang, Q.; Lin, D.; Ge, S.; Bai, M.; et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 2020, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Koch, M.; Bang, C.; Franke, A.; Lieb, W.; Cassidy, A. Microbial Diversity and Abundance of Parabacteroides Mediate the Associations Between Higher Intake of Flavonoid-Rich Foods and Lower Blood Pressure. Hypertension 2021, 78, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Vitelli-Storelli, F.; Rossi, M.; Pelucchi, C.; Rota, M.; Palli, D.; Ferraroni, M.; Lunet, N.; Morais, S.; Lopez-Carrillo, L.; Zaridze, D.G.; et al. Polyphenol Intake and Gastric Cancer Risk: Findings from the Stomach Cancer Pooling Project (StoP). Cancers 2020, 12, 3064. [Google Scholar] [CrossRef]
- Guo, X.F.; Liu, J.P.; Ma, S.Q.; Zhang, P.; Sun, W.D. Avicularin reversed multidrug-resistance in human gastric cancer through enhancing Bax and BOK expressions. Biomed. Pharmacother. 2018, 103, 67–74. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Duncan, K.R.; Suzuki, Y.J. Vitamin E Nicotinate. Antioxidants 2017, 6, 20. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.; Gwon, H.; Lee, K.-Y.; Choi, S.-G.; Atiqual Islam, M.; Chun, J.; Hwang, J. Phytochemical profile and antioxidant activity of Dracocephalum moldavica L. seed extracts using different extraction methods. Food Chem. 2021, 350, 128531. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Pottier, L.; Dufrasne, F.; Nève, J.; Dubois, J.; Kornienko, A.; Kiss, R.; Ingrassia, L. Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr. Med. Chem. 2010, 17, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.Y.; Wu, X.; Liu, Y.; Liu, Y.; Zhou, J.G.; Jiang, X.Y.; Zhang, L.; He, X.Y.; Ma, L.B. Curcumin-Induced DNA Demethylation in Human Gastric Cancer Cells Is Mediated by the DNA-Damage Response Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2543504. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Duffield, K.M.; Ness, M.R.; Ravoori, S.; Andrews, G.; Bhullar, K.S.; Rupasinghe, H.P.; Balzarini, J. Curcumin-inspired cytotoxic 3,5-bis(arylmethylene)-1-(N-(ortho-substituted aryl)maleamoyl)-4-piperidones: A novel group of topoisomerase II alpha inhibitors. Bioorganic Med. Chem. 2015, 23, 6404–6417. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Yang, X.; Zhang, Y.S.; Qi-Feng, J.I.; Wang, W.; School, A.H. Experimental study on intracellular antioxidant activity of water-soluble polyphenols from tea and coffee. Chin. Heart J. 2019, 31, 139–143. [Google Scholar]
- Li, T.; Zhang, X.; Cheng, L.; Li, C.; Wu, Z.; Luo, Y.; Zhou, K.; Li, Y.; Zhao, Q.; Huang, Y. Modulation of lncRNA H19 enhances resveratrol-inhibited cancer cell proliferation and migration by regulating endoplasmic reticulum stress. J. Cell Mol. Med. 2022, 26, 2205–2217. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chiu, Y.M.; Ho, T.Y.; Hsieh, C.T.; Shieh, D.C.; Lee, Y.J.; Tsay, G.J.; Wu, Y.Y. Gallic Acid Induces Apoptosis in Human Gastric Adenocarcinoma Cells. Anticancer Res. 2018, 38, 2057–2067. [Google Scholar] [CrossRef]
- Han, M.L.; Li, A.; Shen, T.; Meng, J.X.; Lei, Y.Q.; Zhang, X.; Liu, P.Y.; Gan, L.X.; Ao, L.; Li, H.H. Phenolic compounds present in fruit extracts of Malus spp. show antioxidative and pro-apoptotic effects on human gastric cancer cell lines. J. Food Biochem. 2019, 43, e13028. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641. [Google Scholar] [CrossRef]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 2018, 233, 4634–4642. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Venkatarame Gowda Saralamma, V.; Kim, S.M.; Ha, S.E.; Raha, S.; Lee, W.S.; Kim, E.H.; Lee, S.J.; Heo, J.D.; Kim, G.S. Pectolinarigenin Induced Cell Cycle Arrest, Autophagy, and Apoptosis in Gastric Cancer Cell via PI3K/AKT/mTOR Signaling Pathway. Nutrients 2018, 10, 1043. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Kim, D.H.; Jang, J.H.; Kim, S.J.; Park, S.A.; Hahn, H.; Han, B.W.; Na, H.K.; Chun, K.S.; et al. Resveratrol suppresses gastric cancer cell proliferation and survival through inhibition of PIM-1 kinase activity. Arch. Biochem. Biophys. 2020, 689, 108413. [Google Scholar] [CrossRef] [PubMed]
- Kunze, B.; Wein, F.; Fang, H.-Y.; Anand, A.; Baumeister, T.; Strangmann, J.; Gerland, S.; Ingermann, J.; Münch, N.S.; Wiethaler, M.; et al. Notch Signaling Mediates Differentiation in Barrett’s Esophagus and Promotes Progression to Adenocarcinoma. Gastroenterology 2020, 159, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.H.; Chang, C.S.; Ho, W.C.; Liao, S.Y.; Wu, C.H.; Wang, C.J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/beta-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 23, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yuan, Y.; Gan, H.Z.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387. [Google Scholar] [CrossRef]
- Xu, J.H.; Liu, D.Y.; Niu, H.L.; Zhu, G.F.; Xu, Y.W.; Ye, D.L.; Li, J.; Zhang, Q.L. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef]
- He, Z.; Dong, W.; Li, Q.; Qin, C.; Li, Y. Sauchinone prevents TGF-beta-induced EMT and metastasis in gastric cancer cells. Biomed Pharm. 2018, 101, 355–361. [Google Scholar] [CrossRef]
- Zang, M.D.; Hu, L.; Fan, Z.Y.; Wang, H.X.; Zhu, Z.L.; Cao, S.; Wu, X.Y.; Li, J.F.; Su, L.P.; Li, C.; et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, J.; Zhou, J.; Zhu, M.; Wang, L.; Yan, L. Resveratrol inhibits Interleukin-6 induced invasion of human gastric cancer cells. Biomed Pharm. 2018, 99, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, V.; Nasso, R.; Di Donato, P.; Finore, I.; Poli, A.; Masullo, M.; Arcone, R. Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines. Biomolecules 2019, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.X.; Zhang, Q.Q.; Zhang, W.; Zhu, L. Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging 2019, 11, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Zhang, J.; Zhang, R.; Zhu, J. Curcumin inhibits the lymphangiogenesis of gastric cancer cells by inhibiton of HMGB1/VEGF-D signaling. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419861600. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Ren, L.-Q.; Li, Q.; Zhang, Y. Luteolin Suppresses the Proliferation of Gastric Cancer Cells and Acts in Synergy with Oxaliplatin. Biomed Res. Int. 2020, 2020, 9396512. [Google Scholar] [CrossRef]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef]
- Lei, C.S.; Hou, Y.C.; Pai, M.H.; Lin, M.T.; Yeh, S.L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Xu, G.-Y.; Tang, X.-J. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed. Pharmacother. 2017, 92, 95–107. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, M.; Kim, H.J.; Jang, S.B.; Bae, S.J.; Lee, I.K.; Ryu, D.; Ha, K.T. Targeting Lactate Dehydrogenase A with Catechin Resensitizes SNU620/5FU Gastric Cancer Cells to 5-Fluorouracil. Int. J. Mol. Sci. 2021, 22, 5406. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Xu, J.; Zhu, Z.; Hu, Z.; Cai, Q. Phloretin flavonoid exhibits selective antiproliferative activity in doxorubicin-resistant gastric cancer cells by inducing autophagy, inhibiting cell migration and invasion, cell cycle arrest and targeting ERK1/2 MAP pathway. J. BUON 2020, 25, 308–313. [Google Scholar] [PubMed]
- Radziejewska, I.; Supruniuk, K.; Bielawska, A. Anti-cancer effect of combined action of anti-MUC1 and rosmarinic acid in AGS gastric cancer cells. Eur. J. Pharmacol. 2021, 902, 174119. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.L.F.; Rodrigues, R.P.; Kitagawa, R.R.; Goncalves, R.C.R. The gastroprotective potential of silibinin against Helicobacter pylori infection and gastric tumor cells. Life Sci. 2020, 256, 117977. [Google Scholar] [CrossRef]
- Stewart, O.A.; Wu, F.; Chen, Y. The role of gastric microbiota in gastric cancer. Gut Microbes 2020, 11, 1220–1230. [Google Scholar] [CrossRef]
- Pastene, E.; Troncoso, M.; Figueroa, G.; Alarcon, J.; Speisky, H. Association between Polymerization Degree of Apple Peel Polyphenols and Inhibition of Helicobacter pylori Urease. J. Agric. Food Chem. 2009, 57, 416–424. [Google Scholar] [CrossRef]
- Paulo, L.O.M.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Anti-Helicobacter pylori and urease inhibitory activies of resveratrol and red wine. Food Res. Int. 2011, 44, 964–969. [Google Scholar] [CrossRef]
- Chavez, F.; Aranda, M.; Garcia, A.; Pastene, E. Antioxidant polyphenols extracted from Avocado epicarp (Persea americana var. Hass) inhibit Helicobacter pylori urease. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 265–280. [Google Scholar]
- Gomez-Chang, E.; Uribe-Estanislao, G.V.; Martinez-Martinez, M.; Galvez-Mariscal, A.; Romero, I. Anti-Helicobacter pylori Potential of Three Edible Plants Known as Quelites in Mexico. J. Med. Food 2018, 21, 1150–1157. [Google Scholar] [CrossRef]
- Zaidi, S.F.; Yamamoto, T.; Refaat, A.; Ahmed, K.; Sakurai, H.; Saiki, I.; Kondo, T.; Usmanghani, K.; Kadowaki, M.; Sugiyama, T. Modulation of activation-induced cytidine deaminase by curcumin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter 2009, 14, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.G.A.; Aranda, M. One-step purification of two procyanidins. semisynthetic epicatechin adducts from avocado skin and their anti-inflammatory effects on gastric cancer cells infected by Helicobacter pylori. J. Chil. Chem. Soc. 2018, 63, 4222–4228. [Google Scholar] [CrossRef]
- Park, J.M.; An, J.M.; Han, Y.M.; Surh, Y.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Walnut polyphenol extracts inhibit -induced STAT3 phosphorylation through activation of PPAR-γ and SOCS1 induction. J. Clin. Biochem. Nutr. 2020, 67, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Han, Y.M.; Lee, H.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Transcriptome profiling analysis of the response to walnut polyphenol extract in Helicobacter pylori-infected cells. J. Clin. Biochem. Nutr. 2021, 68, 201–214. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, L.; Ling, P. Nobiletin Inhibits Helicobacterium pylori Infection-Induced Gastric Carcinogenic Signaling by Blocking Inflammation, Apoptosis, and Mitogen-Activated Protein Kinase Events in Gastric Epithelial-1 Cells. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 77–88. [Google Scholar] [CrossRef]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.H.; Kim, J.B. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 166–173. [Google Scholar] [CrossRef]
- Yahiro, K.; Shirasaka, D.; Tagashira, M.; Wada, A.; Morinaga, N.; Kuroda, F.; Choi, O.; Inoue, M.; Aoyama, N.; Ikeda, M.; et al. Inhibitory effects of polyphenols on gastric injury by Helicobacter pylori VacA toxin. Helicobacter 2005, 10, 231–239. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.H.; Park, M.; Woo, H.J.; Kim, Y.S.; Tharmalingam, N.; Seo, W.D.; Kim, J.B. Regulatory Effects of Black Rice Extract on Helicobacter pylori Infection-Induced Apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700586. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Chadwick, L.R. Resveratrol and red wine extracts inhibit the growth of CagA+ strains of Helicobacter pylori in vitro. Am. J. Gastroenterol. 2003, 98, 1440–1441. [Google Scholar] [CrossRef]
- Ruggiero, P.; Tombola, F.; Rossi, G.; Pancotto, L.; Lauretti, L.; Del Giudice, G.; Zoratti, M. Polyphenols reduce gastritis induced by Helicobacter pylori infection or VacA toxin administration in mice. Antimicrob. Agents Chemother. 2006, 50, 2550–2552. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, D.; Roy, B.K. Structural Interactions of Curcumin Biotransformed Molecules with the N-Terminal Residues of Cytotoxic-Associated Gene A Protein Provide Insights into Suppression of Oncogenic Activities. Interdiscip. Sci. -Comput. Life Sci. 2017, 9, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Friedman, M.; Brown, E.F. Molecular binding of black tea theaflavins to biological membranes: Relationship to bioactivities. J. Agric. Food Chem. 2011, 59, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef] [PubMed]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1271527. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Gimenez-Bastida, J.A.; Nunez-Sanchez, M.A.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef]
- Mueller, M.; Zartl, B.; Schleritzko, A.; Stenzl, M.; Viernstein, H.; Unger, F.M. Rhamnosidase activity of selected probiotics and their ability to hydrolyse flavonoid rhamnoglucosides. Bioproc. Biosyst. Eng. 2018, 41, 221–228. [Google Scholar] [CrossRef]
- Xinhui, L.I.; Liang, X. Simulated gastrointestinal tract metabolism. of the isoflavones from chickpeas. Bull. Ferment. Sci. Technol. 2017, 46, 147–152. [Google Scholar]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Akaza, H. Identification of an enzyme system for daidzein-to-equol conversion in Slackia sp. strain NATTS. Appl. Environ. Microbiol. 2012, 78, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Nakagawa, H.; Hori, S.; Suzuki, T.; Hirayama, K. Plasma quercetin metabolites are affected by intestinal microbiota of human microbiota-associated mice fed with a quercetin-containing diet. J. Clin. Biochem. Nutr. 2019, 65, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Shpigelman, A.; Lorenzo, J.M.; Montesano, D.; Barba, F.J. Direct and indirect measurements of enhanced phenolic bioavailability from litchi pericarp procyanidins by Lactobacillus casei-01. Food Funct. 2017, 8, 2760–2770. [Google Scholar] [CrossRef]
- Quartieri, A.; Garcia-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomas-Barberan, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S44–S55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Y.L. Effect of betel nut seed polyphenols on in vitro. fermentation of intestinal microorganisms. Food Mach. 2019, 35, 41–46. [Google Scholar] [CrossRef]
- Guo, H.; Xiangyu, X.U.; Chen, Y.; Li, N.I.; Liu, Z. Effect of green tea. infusions on obesity-associated gut microbiota. J. Tea Sci. 2016, 36, 354–362. [Google Scholar]
- Massot-Cladera, M.; Perez-Berezo, T.; Franch, A.; Castell, M.; Perez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef]
- Larrosa, M.; Gonzalez-Sarrias, A.; Yanez-Gascon, M.J.; Selma, M.V.; Azorin-Ortuno, M.; Toti, S.; Tomas-Barberan, F.; Dolara, P.; Espin, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sembries, S.; Dongowski, G.; Mehrlander, K.; Will, F.; Dietrich, H. Physiological effects of extraction juices from apple, grape, and red beet pomaces in rats. J. Agric. Food Chem. 2006, 54, 10269–10280. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Guo, X.J.; Cheng, M.; Zhang, X.; Cao, J.X.; Wu, Z.F.; Weng, P.F. Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int. J. Food Sci. Technol. 2017, 52, 1723–1730. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Zeng, B.-H.; Wang, W.; Li, G.-H.; Wu, F.; Wang, L.; Zhong, Q.-P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal in High-Fat-Fed ApoE Mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Wei, W.; Buick, B.C.; Mehtisaidi, Z.; Remu, N.A.; You, Y.; Hu, X. Effect of Grape Seed Pol-yphenols on Gut Microbiota in Immunosuppressive Mice. Storage Process 2021, 21, 116–124. [Google Scholar] [CrossRef]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominiguez, R.; Zamora-Ros, R.; Peron, G.; Marino, M.; et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: The MaPLE randomised controlled trial. Clin. Nutr. 2021, 40, 3006–3018. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Lu, J.; Wei, K.; Tian, H.; Liu, W.; Xu, T.; Wang, X.; Wang, S.; Yang, R.; et al. Adjuvant treatment and molecular mechanism of probiotic compounds in patients with gastric cancer after gastrectomy. Food Funct. 2021, 12, 6294–6308. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Sun, L.; Chen, S.W.; Guo, S.H.; Yue, T.H.; Hou, Q.S.; Feng, M.; Xu, H.; Liu, Y.C.; Wang, P.Y.; et al. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan-induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sci. 2019, 231, 116529. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563. [Google Scholar] [CrossRef]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; Ruiz de Porras, V.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef]
- Reis Ferreira, M.; Andreyev, H.J.N.; Mohammed, K.; Truelove, L.; Gowan, S.M.; Li, J.; Gulliford, S.L.; Marchesi, J.R.; Dearnaley, D.P. Microbiota- and Radiotherapy-Induced Gastrointestinal Side-Effects (MARS) Study: A Large Pilot Study of the Microbiome in Acute and Late-Radiation Enteropathy. Clin. Cancer Res. 2019, 25, 6487–6500. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 2007, 117, 2197–2204. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.-M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol. Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Lin, C.W.; Cheng, K.S.; Lin, C.; Wang, Y.M.; Lin, I.T.; Chou, Y.H.; Hsu, P.N. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin. Exp. Immunol. 2010, 161, 551–559. [Google Scholar] [CrossRef]
- Sunakawa, Y.M.R.; Inoue, E.; Sakamoto, Y.; Kawabata, R.; Ishiguro, A.; Akamaru, Y.; Kito, Y.; Takahashi, M.; Matsuyama, J. Genomic pathway of gut microbiome to predict efficacy of nivolumab in advanced gastric cancer: DELIVER trial (JACCROGC-08). J. Clin. Oncol. 2021, 39 (Suppl. 3), 161. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Jin, J.; Xing, J.; Zhang, Y.; Jia, N.; Ren, X.; Lin, Z.; Jin, N.; Chen, L.; Piao, Y. Baicalein Inhibits Gastric Cancer Cell Proliferation and Migration through a FAK Interaction via AKT/mTOR Signaling. Am. J. Chin. Med. 2021, 49, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Cheon, G.; Lee, J.; Kim, B.; Park, C.; Yang, C.-H. New and known symmetrical curcumin derivatives inhibit the formation of Fos-Jun-DNA complex. Cancer Lett. 2002, 184, 89–96. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800. [Google Scholar] [CrossRef]
- Park, S. Polyphenol Compound as a Transcription Factor Inhibitor. Nutrients 2015, 7, 8987–9004. [Google Scholar] [CrossRef]
- Youssef, D.; Potter, E.; Jha, M.; De Clercq, E.; Balzarini, J.; Stables, J.P.; Jha, A. Design, synthesis and bioevaluation of novel maleamic amino acid ester conjugates of 3,5-bisarylmethylene-4-piperidones as cytostatic agents. Bioorg. Med. Chem. Lett. 2009, 19, 6364–6367. [Google Scholar] [CrossRef]
- Deng, G.; Wu, Y.; Song, Z.; Li, S.; Du, M.; Deng, J.; Xu, Q.; Deng, L.; Bahlol, H.S.; Han, H. Tea Polyphenol Liposomes Overcome Gastric Mucus to Treat Helicobacter Pylori Infection and Enhance the Intestinal Microenvironment. ACS Appl. Mater. Interfaces 2022, 14, 13001–13012. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Romero, P.; Barreto, A.; Fiorentino, S. An Immunomodulatory Gallotanin-Rich Fraction From Enhances the Therapeutic Effect of Anti-PD-L1 in Melanoma. Front. Immunol. 2020, 11, 584959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sang, W.; Xie, L.; Li, W.; Li, B.; Li, J.; Tian, H.; Yuan, Z.; Zhao, Q.; Dai, Y. Polyphenol-Based Nanomedicine Evokes Immune Activation for Combination Cancer Treatment. Angew. Chem. Int. Ed. 2021, 60, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Benohoud, M.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).