The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Screening Participation
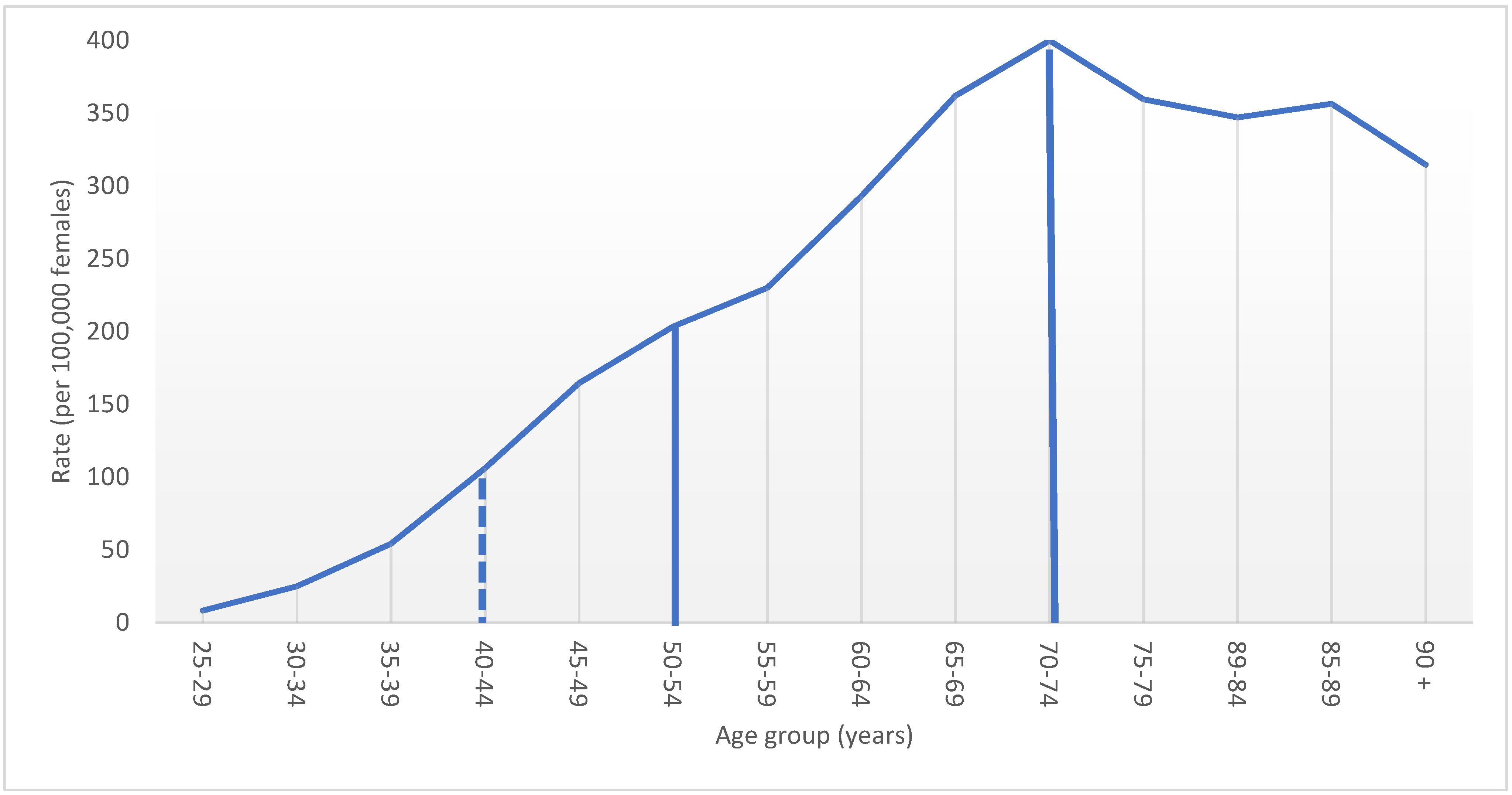
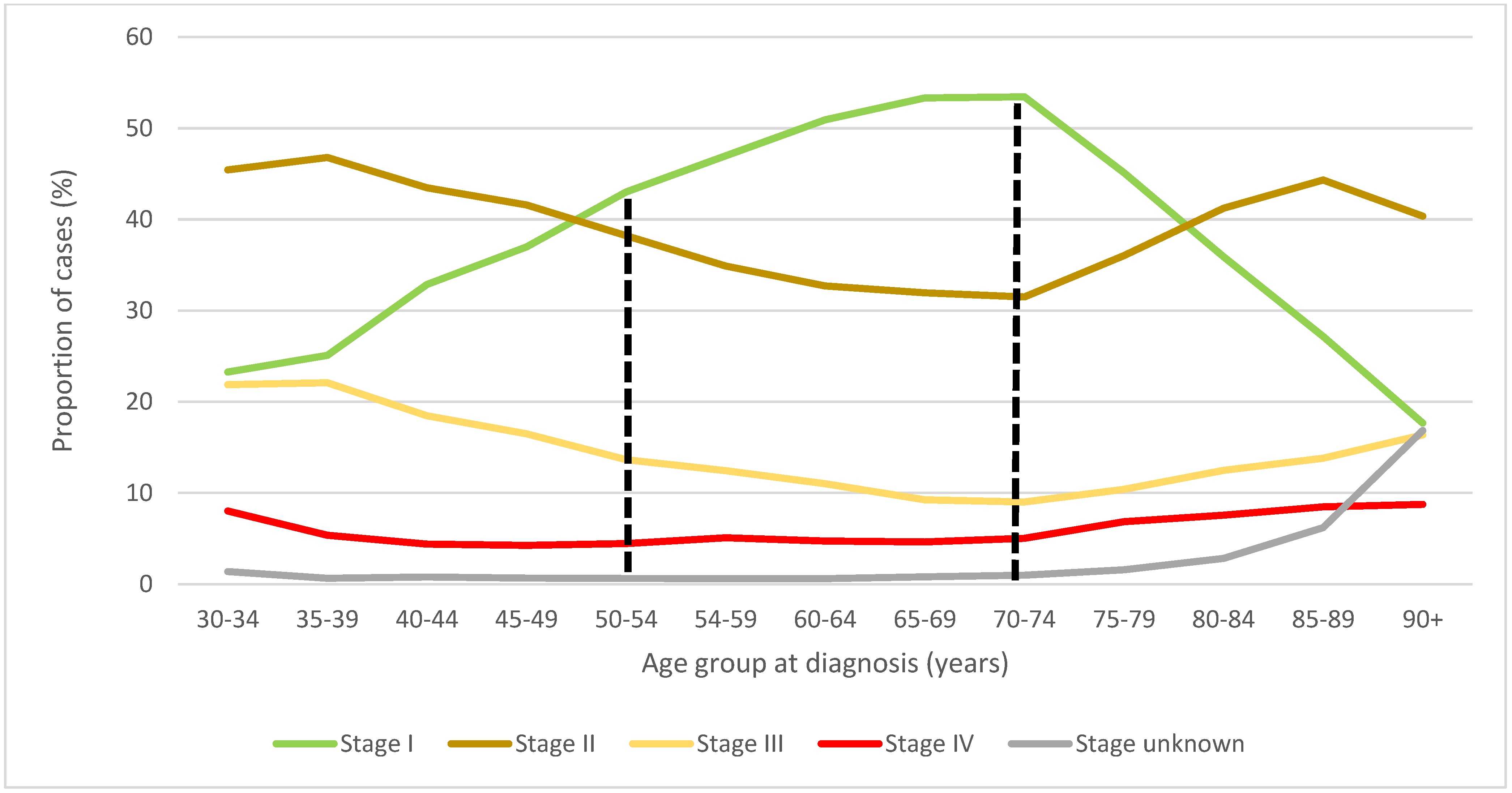
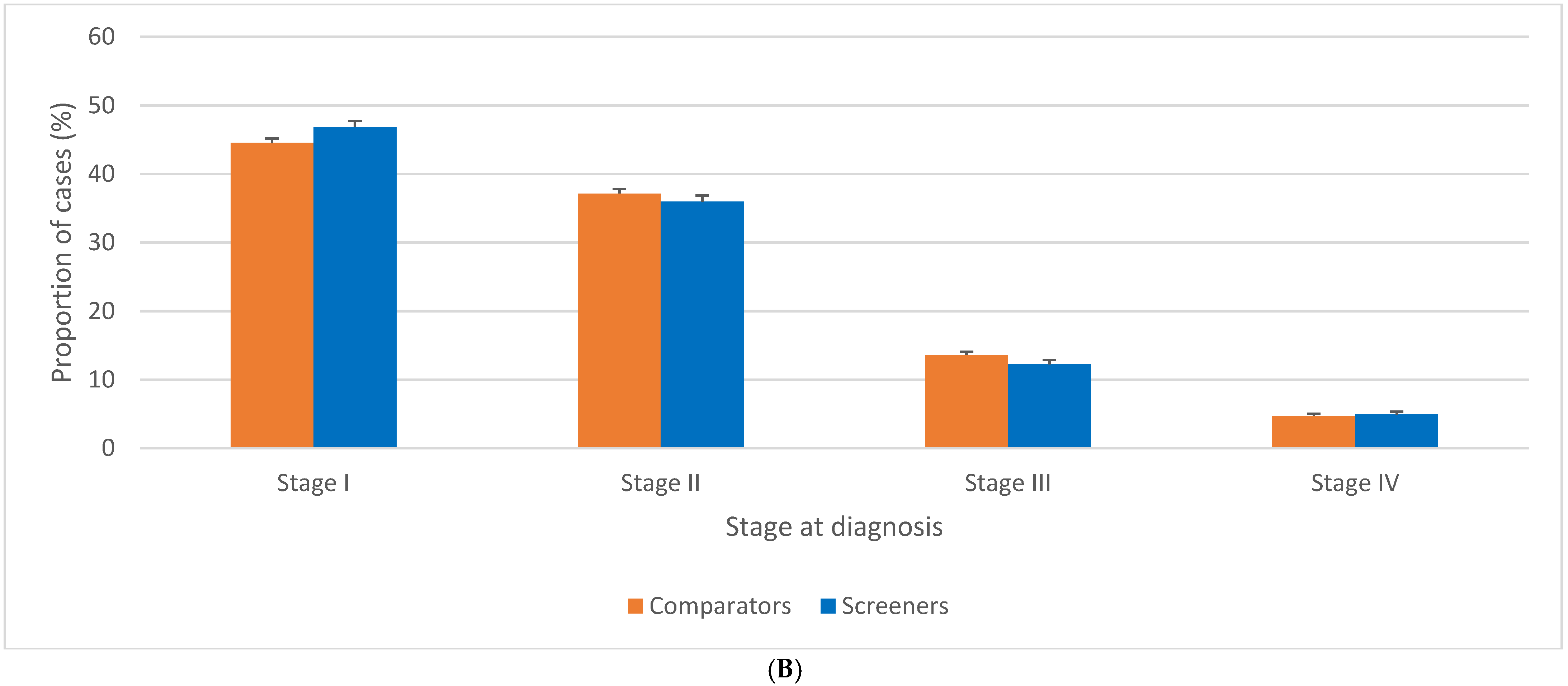
3.2. Stage Distribution of BC Related to Screening Guidelines for Women 40–49 Years Old
3.3. Stage Distribution of BC in Women 50–59 Related to Screening Guidelines for Women 40–49 Years Old
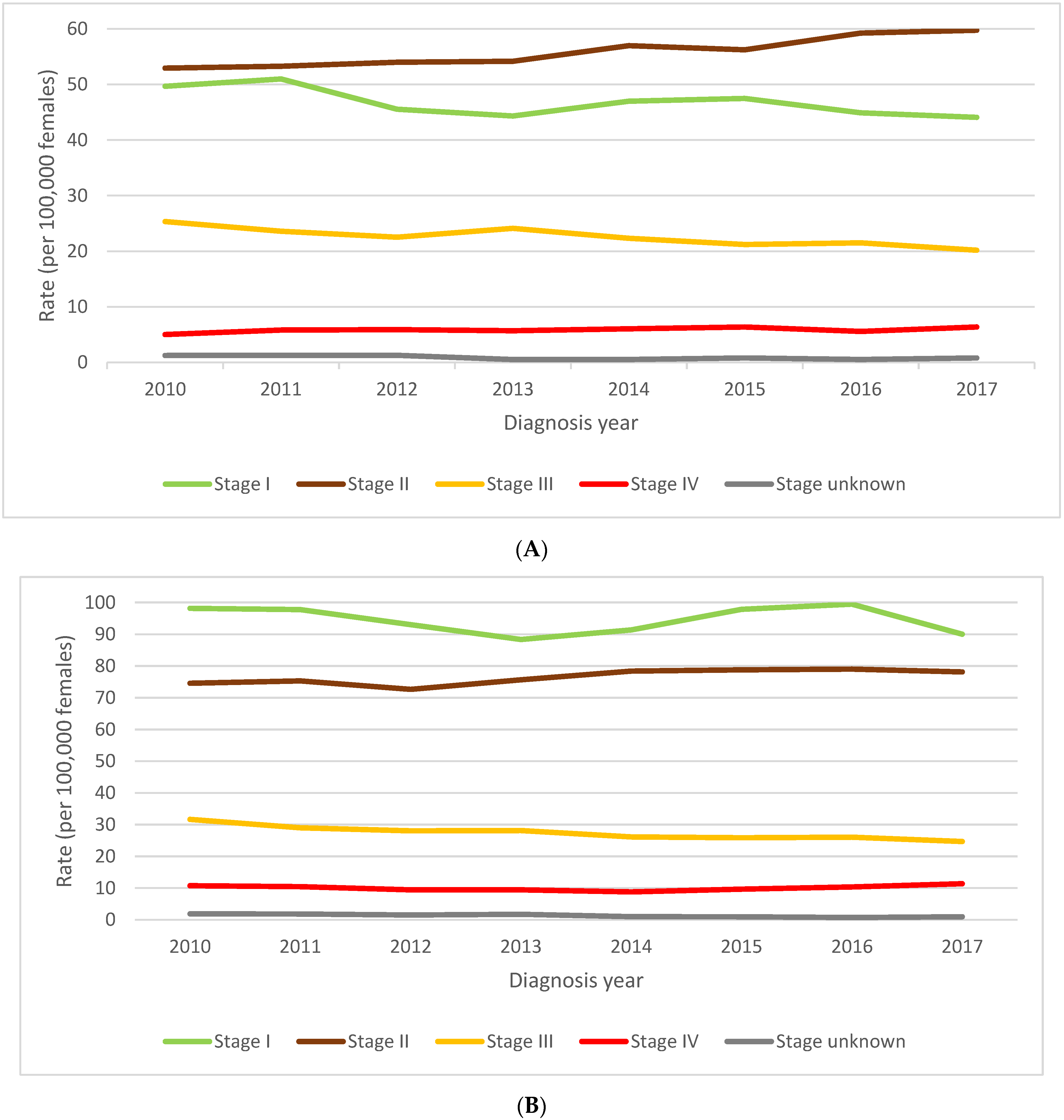
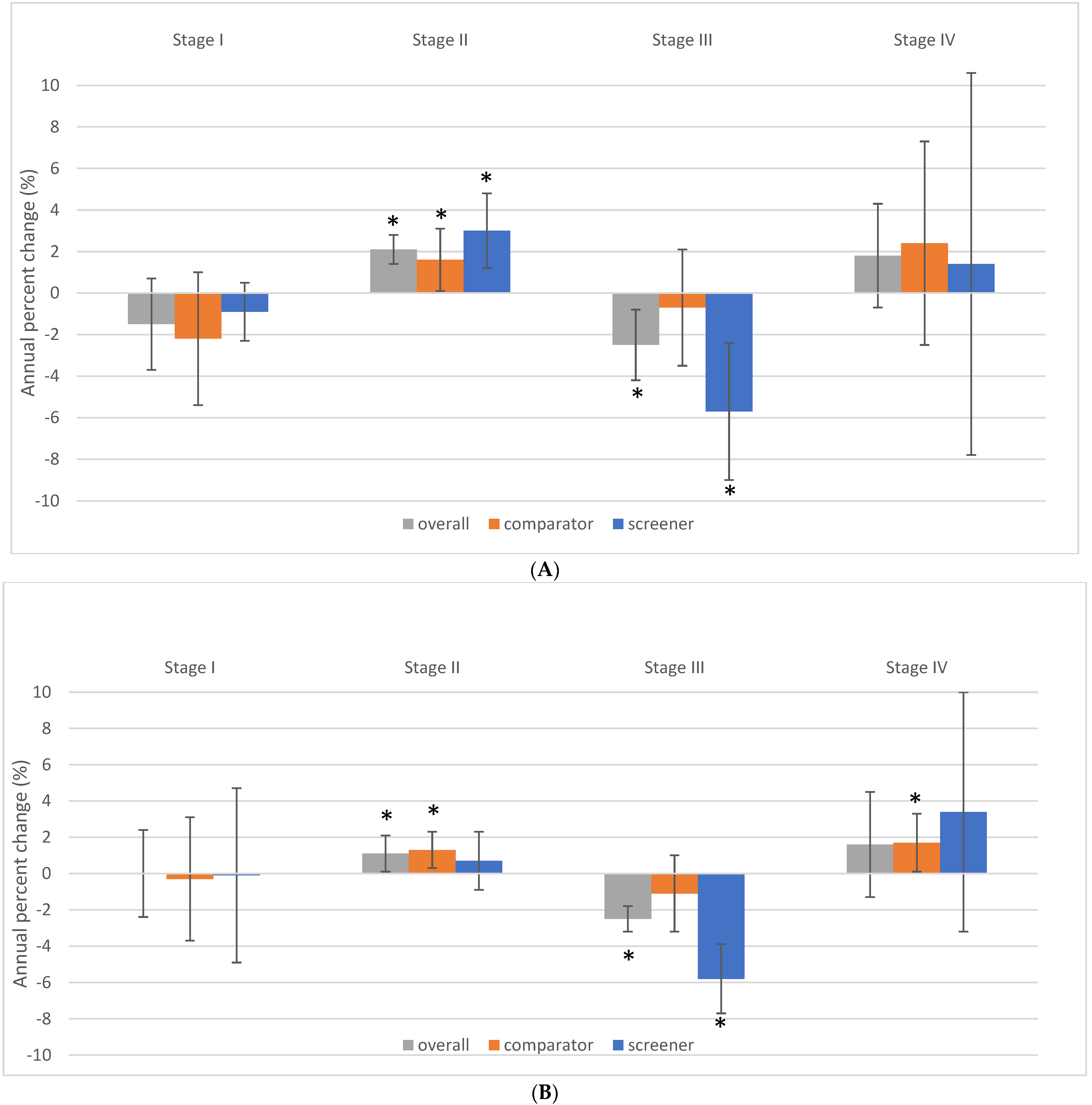
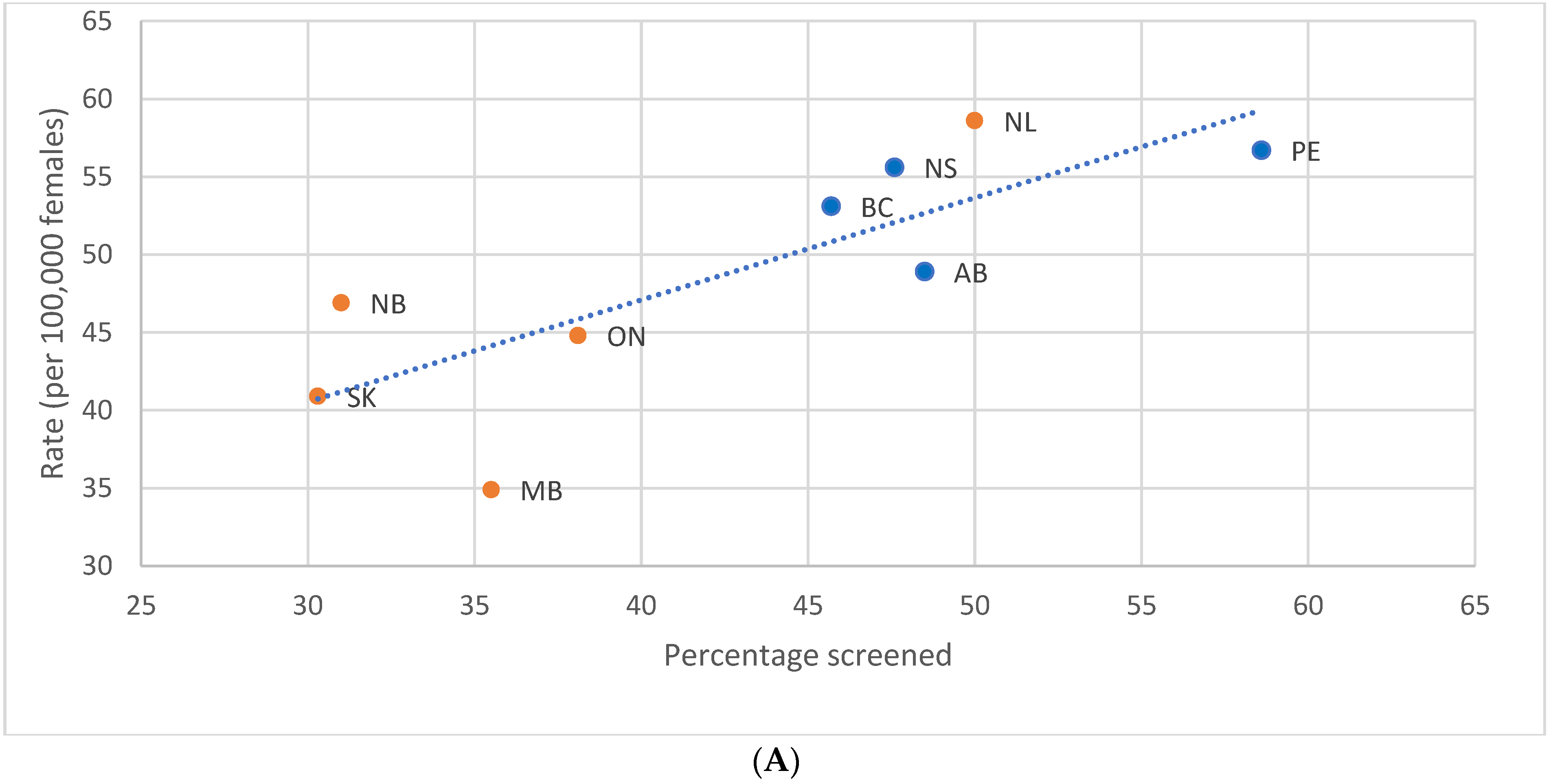
3.4. Impact of Provincial/Territorial Screening Status on BC Stage at Diagnosis in Women Aged 40–49
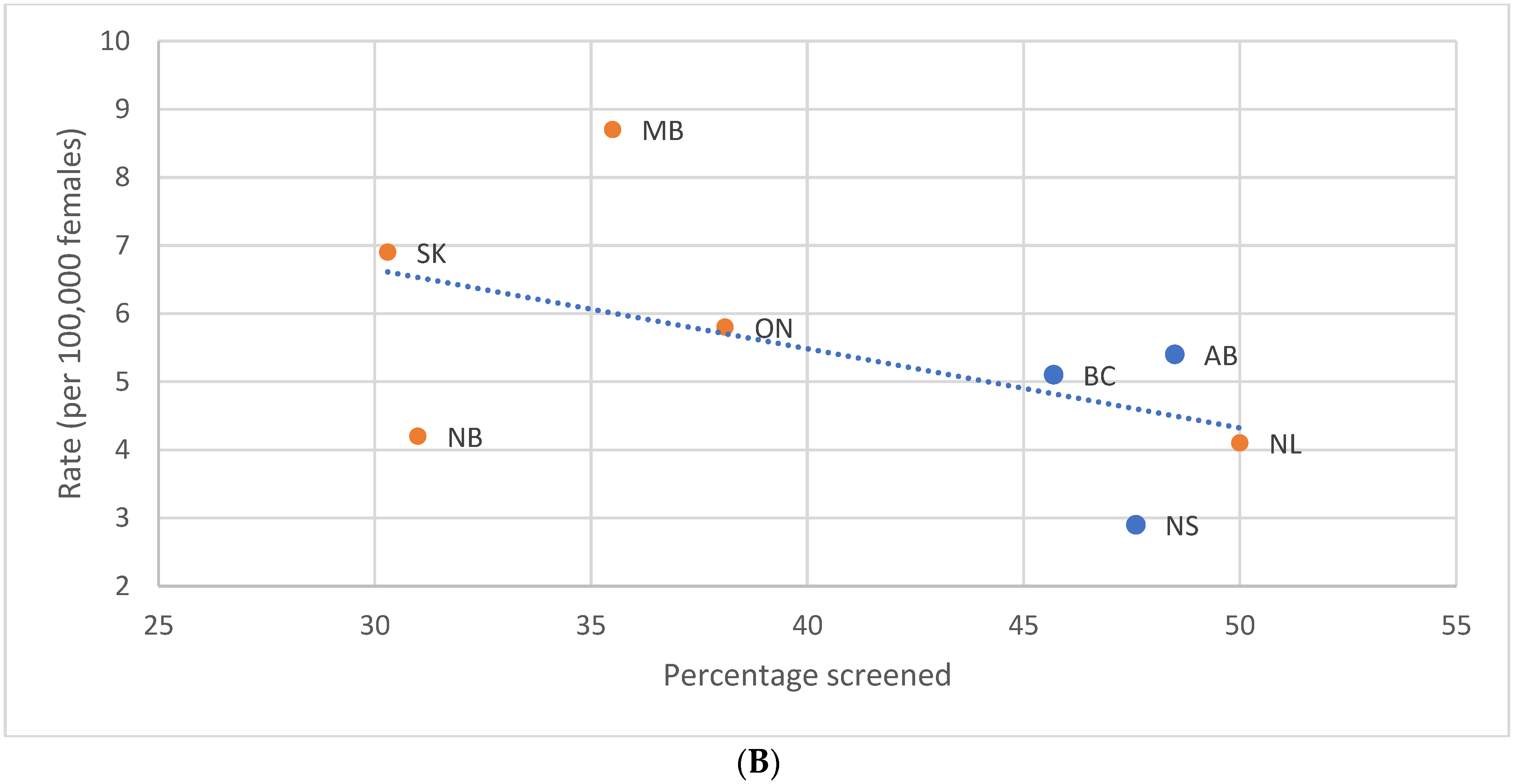
3.5. Impact of Provincial/Territorial Screening Status for Women Aged 40–49 on BC Stage at Diagnosis in Women Aged 50–59
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellison, L.F. The cancer survival index: Measuring progress in cancer survival to help evaluate cancer control efforts in Canada. Health Rep. 2021, 32, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada 2022. The Daily—Cancer Incidence and Mortality Trends, 1984 to 2020. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/220204/dq220204b-eng.htm (accessed on 22 July 2022).
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. CMAJ 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Avery, C.S.; Hendrick, E.; Baker, J.A. Benefits and Risks of Mammography Screening in Women Ages 40 to 49 Years. J. Prim. Care Community Health 2022, 13, 21501327211058322. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health Care. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ 2011, 183, 1991–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarenbach, S.; Sims-Jones, N.; Lewin, G.; Singh, H.; Thériault, G.; Tonelli, M.; Doull, M.; Courage, S.; Garcia, A.J.; Thombs, B.D. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. CMAJ 2018, 190, E1441–E1451. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.; Strax, P.; Venet, L. Periodic breast cancer screening in reducing mortality from breast cancer. JAMA 1971, 215, 1777–1785. [Google Scholar] [CrossRef]
- Andersson, I.; Aspegren, K.; Janzon, L.; Landberg, T.; Lindholm, K.; Linell, F.; Ljungberg, O.; Ranstam, J.; Sigfusson, B. Mammographic screening and mortality from breast cancer: The Malmö mammographic screening trial. Br. Med. J. 1988, 297, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Tabár, L.; Gad, A.; Holmberg, L.; Ljungquist, U.; Group, K.C.P.; Fagerberg, C.; Baldetorp, L.; Gröntoft, O.; Lundström, B.; Månson, J.; et al. Reduction in mortality from breast cancer after mass screening with mammography: Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985, 325, 829–832. [Google Scholar] [CrossRef]
- Huggins, A.; Muir, B.; Donnan, P.; Hepburn, W.; Prescott, R.; Anderson, T.; Lamb, J.; Alexander, F.; Chetty, U.; Forrest, P.; et al. Edinburgh trial of screening for breast cancer: Mortality at seven years. Lancet 1990, 335, 241–246. [Google Scholar] [CrossRef]
- Frisell, J.; Lidbrink, E.; Hellström, L.; Rutqvist, L.E. Followup after 11 years–update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res. Treat. 1997, 45, 263–270. [Google Scholar] [CrossRef]
- Miller, A.B.; Baines, C.J.; To, T.; Wall, C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ 1992, 147, 1459–1476, Erratum in Can. Med. Assoc. J. 1993, 148, 718. [Google Scholar]
- Miller, A.B.; Baines, C.J.; To, T.; Wall, C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ 1992, 147, 1477–1488, Erratum in Can. Med. Assoc. J. 1993, 148, 718. [Google Scholar]
- Bjurstam, N.; Björneld, L.; Duffy, S.W.; Smith, T.C.; Cahlin, E.; Eriksson, O.; Hafström, L.O.; Lingaas, H.; Mattsson, J.; Persson, S.; et al. The Gothenburg breast screening trial: First results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997, 80, 2091–2099. [Google Scholar] [CrossRef]
- Hendrick, R.E.; Smith, R.A.; Rutledge, J.H., III; Smart, C.R. Benefit of screening mammography in women aged 40–49: A new meta-analysis of randomized controlled trials. JNCI Monogr. 1997, 1997, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.B.; Wall, C.; Baines, C.J.; Sun, P.; To, T.; Narod, S.A. Twenty five year follow-up for BC incidence and mortality of the Canadian National Breast Screening Study: Randomised screening trial. Bmj 2014, 348, g366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaffe, M.J.; Seely, J.M.; Gordon, P.B.; Appavoo, S.; Kopans, D.B. The randomized trial of mammography screening that was not-A cautionary tale. J. Med. Screen 2022, 29, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Seely, J.M.; Eby, P.R.; Gordon, P.B.; Appavoo, S.; Yaffe, M.J. Errors in conduct of the CNBSS trials of breast cancer screening observed by research personnel. J. Breast Imaging 2022, 4, 135–143. [Google Scholar] [CrossRef]
- Smart, C.R.; Hendrick, R.E.; Rutledge, J.H., III; Smith, R.A. Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer 1995, 75, 1619–1626. [Google Scholar] [CrossRef]
- Coldman, A.; Phillips, N.; Wilson, C.; Decker, K.; Chiarelli, A.M.; Brisson, J.; Zhang, B.; Payne, J.; Doyle, G.; Ahmad, R. Pan-Canadian study of mammography screening and mortality from breast cancer. JNCI J. Natl. Cancer Inst. 2014, 106, dju261. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.W.; Tabár, L.; Yen, A.M.F.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.S.; Chiu, S.Y.H.; Fann, J.C.Y.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef]
- Tabár, L.; Dean, P.B.; Chen, T.H.H.; Yen, A.M.F.; Chen, S.L.S.; Fann, J.C.Y.; Lin, A.T.-Y.; Smith, R.A.; Duffy, S.W. The incidence of fatal BC measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019, 125, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.R.; Chiu, A.S.; Farrell, K.; Killelea, B.K.; Lannin, D.R. Why Has Breast Cancer Screening Failed to Decrease the Incidence of de Novo Stage IV Disease? Cancers 2019, 11, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleyer, A.; Welch, H.G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autier, P.; Boniol, M.; Middleton, R.; Doré, J.F.; Héry, C.; Zheng, T.; Gavin, A. Advanced breast cancer incidence following population-based mammographic screening. Ann. Oncol. 2011, 22, 1726–1735. [Google Scholar] [CrossRef]
- Kopans, D.B. Point: The New England Journal of Medicine article suggesting overdiagnosis from mammography screening is scientifically incorrect and should be withdrawn. J. Am. Coll. Radiol. 2013, 10, 317–319. [Google Scholar] [CrossRef]
- Garfinkel, L.; Boring, C.C.; Heath, C.W., Jr. Changing trends. An overview of breast cancer incidence and mortality. Cancer 1994, 74, 222–227. [Google Scholar] [CrossRef]
- Gaudette, L.A.; Silberberger, C.; Altmayer, C.A.; Gao, R.N. Trends in breast cancer incidence and mortality. Health Rep. 1996, 8, 29–37. [Google Scholar]
- Taplin, S.H.; Ichikawa, L.; Buist, D.S.; Seger, D.; White, E. Evaluating organized BC screening implementation: The prevention of late-stage disease? Cancer Epidemiol. Prev. Biomark. 2004, 13, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am. Health Drug Benefits 2016, 9, 23–32. [Google Scholar]
- Shen, Y.; Yang, Y.; Inoue, L.Y.; Munsell, M.F.; Miller, A.B.; Berry, D.A. Role of detection method in predicting BC survival: Analysis of randomized screening trials. J. Natl. Cancer Inst. 2005, 97, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Ugnat, A.M.; Xie, L.; Morriss, J.; Semenciw, R.; Mao, Y. Survival of women with breast cancer in Ottawa, Canada: Variation with age, stage, histology, grade and treatment. Br. J. Cancer 2004, 90, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, S.R.; Autier, P.; Støvring, H. Change in effectiveness of mammography screening with decreasing breast cancer mortality: A population-based study. Eur. J. Public Health 2022, 32, 630–635. [Google Scholar] [CrossRef]
- Yaffe, M.J.; Mainprize, J.G. Overdetection of Breast Cancer. Curr. Oncol. 2022, 29, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Poelhekken, K.; Greuter, M.J.; Truyen, I.; De Schutter, H.; Goossens, M.; Houssami, N.; Van Hal, G.; de Bock, G.H. Overdiagnosis of invasive breast cancer in population-based breast cancer screening: A short-and long-term perspective. Eur. J. Cancer 2022, 173, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Stefanini, V.; Senore, C.; Frigerio, A.; Castagno, R.; Marra, V.; Dalmasso, M.; del Turco, M.R.; Paci, E.; Segnan, N. The impact of different communication and organizational strategies on mammography screening uptake in women aged 40–45 years. Eur. J. Public Health 2012, 22, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Loy, E.Y.; Molinar, D.; Chow, K.Y.; Fock, C. National Breast Cancer Screening Programme, Singapore: Evaluation of participation and performance indicators. J. Med. Screen. 2015, 22, 194–200. [Google Scholar] [CrossRef]
- Ray, K.M.; Joe, B.N.; Freimanis, R.I.; Sickles, E.A.; Hendrick, R.E. Screening mammography in women 40–49 years old: Current evidence. Am. J. Roentgenol. 2018, 210, 264–270. [Google Scholar] [CrossRef]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. JNCI J. Natl. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Seely, J.M.; Peddle, S.E.; Yang, H.; Chiarelli, A.M.; McCallum, M.; Narasimhan, G.; Zakaria, D.; Earle, C.C.; Fung, S.; Bryant, H.; et al. Breast Density and Risk of Interval Cancers: The Effect of Annual Versus Biennial Screening Mammography Policies in Canada. Can. Assoc. Radiol. J. 2022, 73, 90–100. [Google Scholar] [CrossRef]
- Statistics Canada. Canadian Cancer Registry. Available online: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207 (accessed on 6 June 2022).
- Public Health Agency of Canada. Organized Breast Cancer Screening Programs in Canada. Report on Program Performance in 2003 and 2004. 2008. Available online: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/publicat/2008/obcsp-podcs-03-04/pdf/obcsp-podcs-03-04-eng.pdf (accessed on 22 June 2022).
- Government of Canada SC. Canadian Community Health Survey: Public Use Microdata File. 2020. Available online: http://www150.statcan.gc.ca/n1/en/catalogue/82M0013X (accessed on 6 June 2022).
- Cronin, K.A.; Miglioretti, D.L.; Krapcho, M.; Yu, B.; Geller, B.M.; Carney, P.A.; Onega, T.; Feuer, E.J.; Breen, N.; Ballard-Barbash, R. Bias associated with self-report of prior screening mammography. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics Canada. 2021. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/210519/dq210519b-eng.htm (accessed on 6 June 2022).
- International Agency for Research on Cancer; World Health Organization; International Association of Cancer Registries; European Network of Cancer Registries. International Rules for Multiple Primary Cancers (ICD-O Third Edition); IARC: Lyon, France, 2004; Available online: http://www.iacr.com.fr/images/doc/MPrules_july2004.pdf (accessed on 22 June 2022).
- Byrd, D.R.; Carducci, M.A.; Compton, C.C.; Fritz, A.G.; Greene, F. AJCC Cancer Staging Manual; Edge, S.B., Ed.; Springer: New York, NY, USA, 2010; Volume 7, pp. 97–100. [Google Scholar]
- Organized Breast Cancer Screening Programs in Canada. Available online: https://s22457.pcdn.co/wp-content/uploads/2019/01/Breast-Cancer-Screen-Perform-2008-EN.pdf (accessed on 22 June 2022).
- Breast Cancer Screening in Canada; Monitoring & Evaluation of Quality Indicators–Results Report, January 2011 to December 2012. Available online: https://s22457.pcdn.co/wp-content/uploads/2019/01/Breast-Cancer-Screen-Quality-Indicators-Report-2012-EN.pdf (accessed on 22 June 2022).
- Statistics Canada. Canadian Community Health Survey (CCHS)—Annual Component—2020 Microdata File. User Guide. 2021. Available online: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3226 (accessed on 6 June 2022).
- JoinPoint Regression Program, Version 4.9.0.0; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute: Bethesda, MD, USA, 2020.
- Agresti, A.; Coull, B.A. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar]
- Ellison, L.F.; Saint-Jacques, N. Five-Year Cancer Survival by Stage at Diagnosis in Canada. [Manuscript Submitted for Publication]; Centre for Population Health Data, Statistics Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- Wilkinson, A.N.; Boutet, C.E. Breast Cancer Survivorship Tool: Facilitating breast cancer survivorship care for family physicians and patients. Can. Fam. Physician 2020, 66, 321–326. [Google Scholar] [PubMed]
- Mittmann, N.; Stout, N.K.; Tosteson, A.N.; Trentham-Dietz, A.; Alagoz, O.; Yaffe, M.J. Cost-effectiveness of mammography from a publicly funded health care system perspective. Can. Med. Assoc. Open Access J. 2018, 6, E77–E86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittmann, N.; Porter, J.; Rangrej, J.; Seung, S.; Liu, N.; Saskin, R.; Cheung, M.; Leighl, N.; Hoch, J.S.; Trudeau, M.; et al. Health system costs for stage-specific breast cancer: A population-based approach. Curr. Oncol. 2014, 21, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Brezden-Masley, C.; Fathers, K.E.; Coombes, M.E.; Pourmirza, B.; Xue, C.; Jerzak, K.J. A population-based comparison of treatment patterns, resource utilization, and costs by cancer stage for Ontario patients with hormone receptor-positive/HER2-negative breast cancer. Breast Cancer Res. Treat. 2021, 185, 507–515. [Google Scholar] [CrossRef]
- pCODR Expert Review Committee (pERC) Final Recommendation. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10195RibociclibFulvestrantMBC_fnRec_pERC%20Chair%20Approved_22April2020_final.pdf (accessed on 22 June 2022).
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Bianchi, G.V.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Gagnon, J.; Lévesque, E.; Borduas, F.; Chiquette, J.; Diorio, C.; Duchesne, N.; Dumais, M.; Eloy, L.; Foulkes, W.; Gervais, N.; et al. Recommendations on breast cancer screening and prevention in the context of implementing risk stratification: Impending changes to current policies. Curr. Oncol. 2016, 23, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Puliti, D.; Duffy, S.W.; Miccinesi, G.; De Koning, H.; Lynge, E.; Zappa, M.; Paci, E. Overdiagnosis in mammographic screening for breast cancer in Europe: A literature review. J. Med. Screen. 2012, 19 (Suppl. S1), 42–56. [Google Scholar] [CrossRef]
- Anders, C.K.; Johnson, R.; Litton, J.; Phillips, M.; Bleyer, A. Breast cancer before age 40 years. Semin. Oncol. 2009, 36, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Partridge, A.H.; Hughes, M.E.; Warner, E.T.; Ottesen, R.A.; Wong, Y.-N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 2016, 34, 3308–3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, S.M.; Oseni, T.O.; Bababekov, Y.J.; Hung, Y.C.; Chang, D.C. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018, 153, 594–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppong, B.A.; Obeng-Gyasi, S.; Relation, T.; Adams-Campbell, L. Call to action: Breast cancer screening recommendations for Black women. Breast Cancer Res. Treat. 2021, 187, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Rebner, M.; Pai, V.R. Breast cancer screening recommendations: African American women are at a disadvantage. J. Breast Imaging 2020, 2, 416–421. [Google Scholar] [CrossRef]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048, Erratum in J. Natl. Cancer Inst. 2015, 107, djv121. [Google Scholar] [CrossRef] [PubMed]
- Monticciolo, D.L. Current guidelines and gaps in breast cancer screening. J. Am. Coll. Radiol. 2020, 17, 1269–1275. [Google Scholar] [CrossRef]
- Chapman, C.H.; Schechter, C.B.; Cadham, C.J.; Trentham-Dietz, A.; Gangnon, R.E.; Jagsi, R.; Mandelblatt, J.S. Identifying Equitable Screening Mammography Strategies for Black Women in the United States Using Simulation Modeling. Ann. Intern. Med. 2021, 174, 1637–1646. [Google Scholar] [CrossRef]

| Province/Territory | Screening Programmatic Information (2007–2008) | Screening Participation Rates | ||||
|---|---|---|---|---|---|---|
| Referral 40–49 | Recall | 2003 | 2008 | 2012 | 2017 | |
| British Columbia * | Self | Annual | 44.2 | 47.4 | 45.7 | 39.0 |
| Alberta ** | Self | Annual | 43.2 | 52.9 | 48.5 | 44.1 |
| Saskatchewan | No | None | 26.6 | 27.9 | 30.3 | 32.6 |
| Manitoba | MD | Biennial | 24.5 | 21.8 | 35.5 | 18.1 |
| Ontario | MD-High Risk only | High Risk-Annual | 33.1 | 37.5 | 38.1 | 27.0 |
| Quebec | MD | None | 26.0 | 31.4 | 25.2 | 21.4 |
| New Brunswick | MD | None | 42.2 | 41.4 | 31.0 | 20.8 |
| Nova Scotia | Self | Annual | 50.2 | 50.7 | 47.6 | 45.5 |
| Prince Edward Island | Self | Annual | 31.2 | 35.2 | 58.6 | 45.4 |
| Newfoundland and Labrador | No | None | 41.9 | 51.8 | 50.0 | 51.3 |
| Northwest Territories | Self | Annual | 43.9 | 42.7 | 63.6 | n/a |
| Yukon | Self | None | 10.0 | 32.6 | 11.4 | n/a |
| Nunavut | n/a | n/a | 12.2 | 13.2 | 41.0 | n/a |
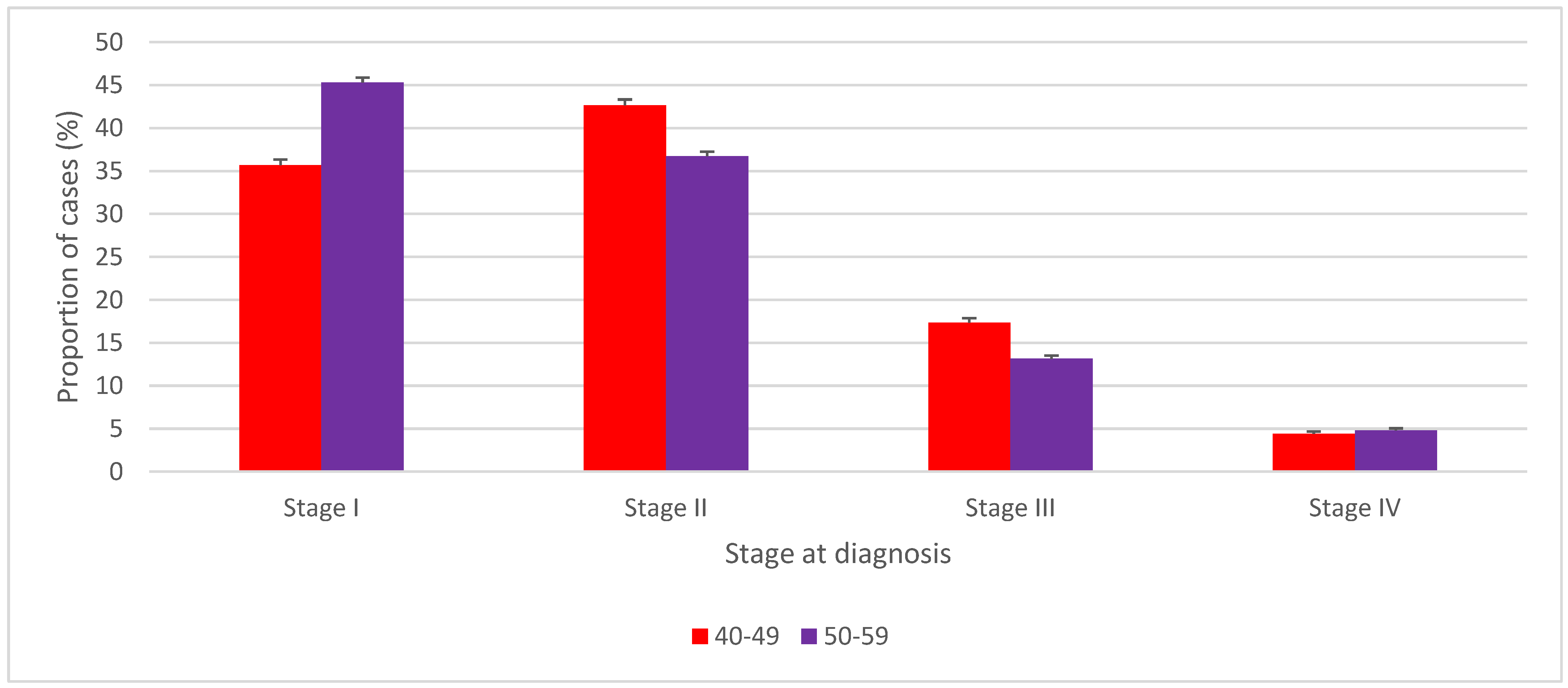
| Stage at Diagnosis | 40 to 49 Years | 50 to 59 Years | p-Value for Differences in Stage-Specific Proportions | ||||
|---|---|---|---|---|---|---|---|
| Number | Proportion of Cases Diagnosed at Stages I to IV (%) | 95% CI of the Proportion | Number | Proportion of Cases Diagnosed at Stages I to IV (%) | 95% CI of the Proportion | ||
| Stage I | 7200 | 35.7 | (35.0, 36.3) | 15,125 | 45.3 | (44.8, 45.8) | <0.001 |
| Stage II | 8600 | 42.6 | (41.9, 43.3) | 12,265 | 36.7 | (36.2, 37.3) | <0.001 |
| Stage III | 3500 | 17.3 | (16.8, 17.9) | 4385 | 13.1 | (12.8, 13.5) | <0.001 |
| Stage IV | 880 | 4.4 | (4.1, 4.7) | 1610 | 4.8 | (4.6, 5.1) | 0.005 |
| Unknown | 140 | NA | NA | 210 | NA | NA | NA |
| Unstaged | 645 | NA | NA | 930 | NA | NA | NA |
| Total | 20,965 | NA | NA | 34,525 | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, A.N.; Billette, J.-M.; Ellison, L.F.; Killip, M.A.; Islam, N.; Seely, J.M. The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59. Curr. Oncol. 2022, 29, 5627-5643. https://doi.org/10.3390/curroncol29080444
Wilkinson AN, Billette J-M, Ellison LF, Killip MA, Islam N, Seely JM. The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59. Current Oncology. 2022; 29(8):5627-5643. https://doi.org/10.3390/curroncol29080444
Chicago/Turabian StyleWilkinson, Anna N., Jean-Michel Billette, Larry F. Ellison, Michael A. Killip, Nayaar Islam, and Jean M. Seely. 2022. "The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59" Current Oncology 29, no. 8: 5627-5643. https://doi.org/10.3390/curroncol29080444
APA StyleWilkinson, A. N., Billette, J.-M., Ellison, L. F., Killip, M. A., Islam, N., & Seely, J. M. (2022). The Impact of Organised Screening Programs on Breast Cancer Stage at Diagnosis for Canadian Women Aged 40–49 and 50–59. Current Oncology, 29(8), 5627-5643. https://doi.org/10.3390/curroncol29080444






