Treatment Strategies for Residual Disease following Neoadjuvant Chemotherapy in Patients with Early-Stage Breast Cancer
Abstract
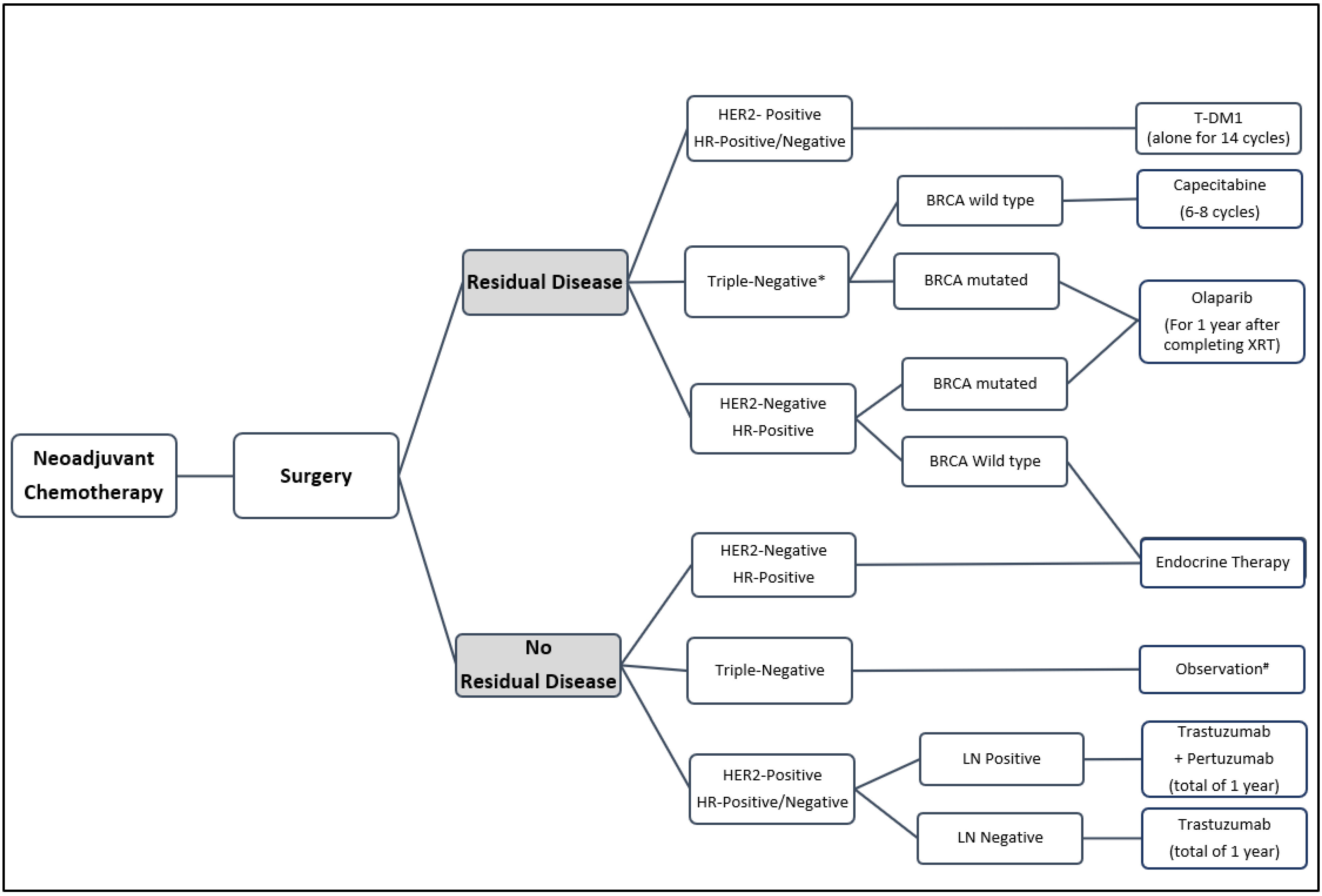
:1. Introduction
2. Pathological Complete Response (pCR)
3. Management of Residual HER2-Positive Disease
3.1. Current Neoadjuvant Standards
3.2. Post Neoadjuvant Treatment
3.3. Ongoing Trials Investigating Other Anti-HER2 Agents in the Post Neoadjuvant Settings
4. Management of Residual HER2-Negative Disease
4.1. Current Neoadjuvant Standards
4.2. Residual Disease in HER2-Negative Breast Cancer
5. Immunotherapy
5.1. Immunotherapy in Neoadjuvant Settings
5.2. Immunotherapy for Residual Disease
6. PARP Inhibitors in BRCA-Positive Patients
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global Patterns of Breast Cancer Incidence and Mortality: A Population-Based Cancer Registry Data Analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Mieog, S.; van der Hage, J.; van de Velde, C. Preoperative Chemotherapy for Women with Operable Breast Cancer. Cochrane Database Syst Rev. 2007, 18, CD005002. [Google Scholar] [CrossRef]
- Caparica, R.; Lambertini, M.; Pondé, N.; Fumagalli, D.; de Azambuja, E.; Piccart, M. Post-Neoadjuvant Treatment and the Management of Residual Disease in Breast Cancer: State of the Art and Perspectives. Ther. Adv. Med. Oncol. 2019, 11, 175883591982771. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Kuroi, K.; Toi, M.; Ohno, S.; Nakamura, S.; Iwata, H.; Masuda, N.; Sato, N.; Tsuda, H.; Kurosumi, M.; Akiyama, F. Prognostic Significance of Subtype and Pathologic Response in Operable Breast Cancer; a Pooled Analysis of Prospective Neoadjuvant Studies of JBCRG. Breast Cancer 2013, 22, 486–495. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; la Haba-Rodriguez, J.D.; Im, S.-A.; Pedrini, J.L.; et al. 5-Year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients with Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef]
- Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval (2020). Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use (accessed on 1 May 2022).
- Agency, E.M. The Role of the Pathological Complete Response as an Endpoint in Neoadjuvant Breast Cancer Studies. 2014. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guidelinerole-pathological-complete-response-endpoint-neoadjuvant-breast-cancer-studies_en.pdf (accessed on 1 May 2022).
- Broglio, K.R.; Quintana, M.; Foster, M.; Olinger, M.; McGlothlin, A.; Berry, S.M.; Boileau, J.-F.; Brezden-Masley, C.; Chia, S.; Dent, S.; et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2 Positive Breast Cancer with Long Term Outcomes. JAMA Oncol. 2016, 2, 751. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-Analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; Schiff, R.; Osborne, C.K. Targeting HER2 for the Treatment of Breast Cancer. Annu. Rev. Med. 2015, 66, 111–128. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Lambertini, M.; Pondé, N.F.; Solinas, C.; de Azambuja, E. Adjuvant Trastuzumab: A 10-Year Overview of Its Benefit. Expert Rev. Anticancer. Ther. 2016, 17, 61–74. [Google Scholar] [CrossRef]
- Brandão, M.; Pondé, N.F.; Poggio, F.; Kotecki, N.; Salis, M.; Lambertini, M.; de Azambuja, E. Combination Therapies for the Treatment of HER2-Positive Breast Cancer: Current and Future Prospects. Expert Rev. Anticancer. Ther. 2018, 18, 629–649. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Gianni, L.; Dafni, U.; Gelber, R.D.; Azambuja, E.; Muehlbauer, S.; Goldhirsch, A.; Untch, M.; Smith, I.; Baselga, J.; Jackisch, C.; et al. Treatment with Trastuzumab for 1 Year after Adjuvant Chemotherapy in Patients with HER2-Positive Early Breast Cancer: A 4-Year Follow-up of a Randomised Controlled Trial. Lancet Oncol. 2011, 12, 236–244. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Gelber, R.D.; Piccart-Gebhart, M.J.; de Azambuja, E.; Procter, M.; Suter, T.M.; Jackisch, C.; Cameron, D.; Weber, H.A.; Heinzmann, D.; et al. 2 Years versus 1 Year of Adjuvant Trastuzumab for HER2-Positive Breast Cancer (HERA): An Open-Label, Randomised Controlled Trial. Lancet 2013, 382, 1021–1028. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Davidson, N.E.; Geyer, C.E.; Martino, S.; Mamounas, E.P.; Kaufman, P.A.; Wolmark, N. Four-Year Follow-Up of Trastuzumab Plus Adjuvant Chemotherapy for Operable Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Joint Analysis of Data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. 2011, 29, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Spielmann, M.; Roché, H.; Delozier, T.; Canon, J.-L.; Romieu, G.; Bourgeois, H.; Extra, J.-M.; Serin, D.; Kerbrat, P.; Machiels, J.-P.; et al. Trastuzumab for Patients with Axillary-Node-Positive Breast Cancer: Results of the FNCLCC-PACS 04 Trial. J. Clin. Oncol. 2009, 27, 6129–6134. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harker, G.; Masuda, N.; Konstantinovic, Z.B.N.; Petrakova, K.; Zotano, A.L.G.; et al. Abstract S5-02: Neratinib after Trastuzumab-Based Adjuvant Therapy in Early-Stage HER2+ Breast Cancer: 3-Year Analysis from a Phase 3 Randomized, Placebo-Controlled, Double-Blind Trial (ExteNET). Cancer Res. 2016, 76, S5-02. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, N.; Chavez-MacGregor, M.; Telli, M.L.; Eisen, A.; Graff, S.L.; Hassett, M.J.; Holloway, J.N.; Hurria, A.; King, T.A.; Lyman, G.H.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2433–2443. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus Trastuzumab in Combination with Standard Neoadjuvant Anthracycline-Containing and Anthracycline-Free Chemotherapy Regimens in Patients with HER2-Positive Early Breast Cancer: A Randomized Phase II Cardiac Safety Study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (Breast Cancer), Version 4-2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast (accessed on 8 August 2022).
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Cosimo, S.D.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with Trastuzumab for HER2-Positive Early Breast Cancer (NeoALTTO): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; la Haba-Rodriguez, J.d.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Carey, L.A.; Berry, D.A.; Cirrincione, C.T.; Barry, W.T.; Pitcher, B.N.; Harris, L.N.; Ollila, D.W.; Krop, I.E.; Henry, N.L.; Weckstein, D.J.; et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab with or Without Lapatinib. J. Clin. Oncol. 2016, 34, 542–549. [Google Scholar] [CrossRef]
- Robidoux, A.; Tang, G.; Rastogi, P.; Geyer, C.E.; Azar, C.A.; Atkins, J.N.; Fehrenbacher, L.; Bear, H.D.; Baez-Diaz, L.; Sarwar, S.; et al. Lapatinib as a Component of Neoadjuvant Therapy for HER2-Positive Operable Breast Cancer (NSABP Protocol B-41): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 1183–1192. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant Chemotherapy Induces Breast Cancer Metastasis through a TMEM-Mediated Mechanism. Sci. Transl. Med. 2017, 9, eaan0026, Erratum in Sci. Transl. Med. 2017, 9, aao3817. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Kummel, S.; Vogel, P.; Hanusch, C.; Eidtmann, H.; Hilfrich, J.; Gerber, B.; Huober, J.; Costa, S.D.; Jackisch, C.; et al. Neoadjuvant Vinorelbine-Capecitabine Versus Docetaxel-Doxorubicin-Cyclophosphamide in Early Nonresponsive Breast Cancer: Phase III Randomized GeparTrio Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 542–551. [Google Scholar] [CrossRef]
- Smith, I.C.; Heys, S.D.; Hutcheon, A.W.; Miller, I.D.; Payne, S.; Gilbert, F.J.; Ah-See, A.K.; Eremin, O.; Walker, L.G.; Sarkar, T.K.; et al. Neoadjuvant Chemotherapy in Breast Cancer: Significantly Enhanced Response with Docetaxel. J. Clin. Oncol. 2002, 20, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Blohmer, J.U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Eiermann, W.; Gerber, B.; Hanusch, C.; Hilfrich, J.; Huober, J.; et al. Response-Guided Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 2013, 31, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aspitia, A. Neoadjuvant Therapy in Early-Stage Breast Cancer. Crit. Rev. Oncol. Hematol. 2012, 82, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bear, H.D.; Tang, G.; Rastogi, P.; Geyer, C.E.; Robidoux, A.; Atkins, J.N.; Baez-Diaz, L.; Brufsky, A.M.; Mehta, R.S.; Fehrenbacher, L.; et al. Bevacizumab Added to Neoadjuvant Chemotherapy for Breast Cancer. N. Engl. J. Med. 2012, 366, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, M.; Ataseven, B.; Kümmel, S. Neoadjuvant Dose-Dense and Dose-Intensified Chemotherapy in Breast Cancer-Review of the Literature. Breast Care 2016, 11, 13–20. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Rezai, M.; Loibl, S.; Fasching, P.A.; Huober, J.; Tesch, H.; Bauerfeind, I.; Hilfrich, J.; Eidtmann, H.; Gerber, B.; et al. Capecitabine in Addition to Anthracycline- and Taxane-Based Neoadjuvant Treatment in Patients with Primary Breast Cancer: Phase III GeparQuattro Study. J. Clin. Oncol. 2010, 28, 2015–2023. [Google Scholar] [CrossRef]
- Bear, H.D.; Tang, G.; Rastogi, P.; Geyer, C.E.; Liu, Q.; Robidoux, A.; Baez-Diaz, L.; Brufsky, A.M.; Mehta, R.S.; Fehrenbacher, L.; et al. Neoadjuvant plus Adjuvant Bevacizumab in Early Breast Cancer (NSABP B-40 [NRG Oncology]): Secondary Outcomes of a Phase 3, Randomised Controlled Trial. Lancet Oncol. 2015, 16, 1037–1048. [Google Scholar] [CrossRef]
- Halloran, N.O.; McVeigh, T.; Martin, J.; Keane, M.; Lowery, A.; Kerin, M. Neoadjuvant Chemoradiation and Breast Reconstruction: The Potential for Improved Outcomes in the Treatment of Breast Cancer. Ir. J. Med. Sci. (1971-) 2018, 188, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Holmes, F.A.; Smith, T.L.; Buzdar, A.U.; Frye, D.K.; Fraschini, G.; Singletary, S.E.; Theriault, R.L.; McNeese, M.D.; Ames, F.; et al. The Use of Alternate, Non-Cross-Resistant Adjuvant Chemotherapy on the Basis of Pathologic Response to a Neoadjuvant Doxorubicin-Based Regimen in Women with Operable Breast Cancer: Long-Term Results From a Prospective Randomized Trial. J. Clin. Oncol. 2004, 22, 2294–2302. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Lei, X.; Alvarez, R.H.; Green, M.C.; Murray, J.L.; Valero, V.; Koenig, K.B.; Ibrahim, N.K.; Litton, J.K.; Nair, L.; et al. Phase II Randomized Study of Ixabepilone Versus Observation in Patients with Significant Residual Disease After Neoadjuvant Systemic Therapy for HER2-Negative Breast Cancer. Clin. Breast Cancer 2015, 15, 325–331. [Google Scholar] [CrossRef]
- Kalra, M.; Tong, Y.; Jones, D.R.; Walsh, T.; Danso, M.A.; Ma, C.X.; Silverman, P.; King, M.-C.; Badve, S.S.; Perkins, S.M.; et al. Cisplatin +/− Rucaparib after Preoperative Chemotherapy in Patients with Triple-Negative or BRCA Mutated Breast Cancer. Npj Breast Cancer 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Rezai, M.; Tesch, H.; Huober, J.; Gerber, B.; Zahm, D.M.; Hilfrich, J.; Costa, S.D.; Dubsky, P.; Blohmer, J.U.; et al. Zoledronate for Patients with Invasive Residual Disease after Anthracyclines-Taxane-Based Chemotherapy for Early Breast Cancer\textendash The Phase III NeoAdjuvant Trial Add-oN (NaTaN) Study (GBG 36/ABCSG 29). Eur. J. Cancer 2016, 64, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Baselga, J. Trastuzumab-DM1: Building a Chemotherapy-Free Road in the Treatment of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J. Clin. Oncol. 2011, 29, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab Emtansine versus Capecitabine plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): A Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.-B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.-M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. Trastuzumab Emtansine versus Treatment of Physician’s Choice for Pretreated HER2-Positive Advanced Breast Cancer (TH3RESA): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.-B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.-M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab Emtansine versus Treatment of Physician’s Choice in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer (TH3RESA): Final Overall Survival Results from a Randomised Open-Label Phase 3 Trial. Lancet Oncol. 2017, 18, 743–754. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Can-cer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Medicine USNLo. Phase II Trial of Combination Immunotherapy with NeuVax and Trastuzumab in High-risk HER2+ Breast Cancer Patients (HER3+) (2014–2021). Available online: https://clinicaltrials.gov/ct2/show/NCT02297698 (accessed on 16 November 2021).
- Medicine USNLo. A Multicenter Phase II Study of Vaccines to Prevent Recurrence in Patients With HER-2Positive Breast Cancer (2018–2023). Available online: https://clinicaltrials.gov/ct2/history/NCT03384914?V_2=View (accessed on 12 October 2021).
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the addition of carboplatin and/or bevacizumab to Neoadjuvant Once per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete re-sponse rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP Inhibitor Veliparib plus Carboplatin or Carboplatin Alone to Standard Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2-Positive Early Breast Cancer (GeparSixto; GBG 66): A Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Ward, C.; Connor, C.S.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; et al. Efficacy of Neoadjuvant Carboplatin plus Docetaxel in Triple-Negative Breast Cancer: Combined Analysis of Two Cohorts. Clin. Cancer Res. 2017, 23, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Gluz, O.; Nitz, U.; Liedtke, C.; Prat, A.; Christgen, M.; Feuerhake, F.; Garke, M.; Grischke, E.-M.; Forstbauer, H.; Braun, M.; et al. Abstract GS5-06: No Survival Benefit of Chemotherapy Escalation in Patients with pCR and “High-Immune” Triple-Negative Early Breast Cancer in the Neoadjuvant WSG-ADAPT-TN Trial. Cancer Res. 2019, 79, GS5-06. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iwase, H. Clinicopathological Features and Treatment Strategy for Triple-Negative Breast Cancer. Int. J. Clin. Oncol. 2010, 15, 341–351. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Matthieu, M.-C.; Chollet, P.; Raoefils, I.; Abrial, C.; Dômont, J.; Spielmann, M.; Delaloge, S.; André, F.; Penault-Llorca, F. P53 Status and Efficacy of Primary Anthracyclines/Alkylating Agent-Based Regimen According to Breast Cancer Molecular Classes. Ann. Oncol. 2008, 19, 1261–1265. [Google Scholar] [CrossRef]
- Bergin, A.R.T.; Loi, S. Triple-Negative Breast Cancer: Recent Treatment Advances. F1000Research 2019, 8, 1342. [Google Scholar] [CrossRef]
- Arun, B.; Bayraktar, S.; Liu, D.D.; Barrera, A.M.G.; Atchley, D.; Pusztai, L.; Litton, J.K.; Valero, V.; Meric-Bernstam, F.; Hortobagyi, G.N.; et al. Response to Neoadjuvant Systemic Therapy for Breast Cancer in BRCA Mutation Carriers and Noncarriers: A Single-Institution Experience. J. Clin. Oncol. 2011, 29, 3739–3746. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Gronwald, J.; Zuziak, D.; Cybulski, C.; Kladny, J.; Gorski, B.; Lubinski, J.; Narod, S.A. Response to Neoadjuvant Therapy with Cisplatin in BRCA1-Positive Breast Cancer Patients. Breast Cancer Res. Treat. 2008, 115, 359–363. [Google Scholar] [CrossRef]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A.; et al. Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J. Clin. Oncol. 2010, 28, 1145–1153. [Google Scholar] [CrossRef]
- Telli, M.L.; Kurian, A.W.; Jensen, K.C.; Vinayak, S.; Flaherty, P.; Lipson, J.A.; Wapnir, I.; Daniel, B.; Carlson, R.W.; Mills, M.A.; et al. P3-14-08: A Phase II Study of Gemcitabine and Carboplatin (GC) Plus Iniparib (BSI-201) as Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer. Cancer Res. 2011, 71, P3-14-08. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Bottiglieri, L. Pathological Definition of Triple Negative Breast Cancer. Eur. J. Cancer 2009, 45, 5–10. [Google Scholar] [CrossRef]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; André, F.; Baselga, J.; et al. Tailoring Therapies-Improving the Management of Early Breast Cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, S.; Zang, L.; Li, J.; Li, J.; Kang, Y.; Ren, W. Combination of Shear Wave Elastography and Ki-67 Index as a Novel Predictive Modality for the Pathological Response to Neoadjuvant Chemotherapy in Patients with Invasive Breast Cancer. Eur. J. Cancer 2016, 69, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Galeana, P.; Muñoz-Montaño, W.; Lara-Medina, F.; Alvarado-Miranda, A.; Pérez-Sánchez, V.; Villarreal-Garza, C.; Quintero, R.M.; Porras-Reyes, F.; Bargallo-Rocha, E.; Carmen, I.D.; et al. Ki67 Changes Identify Worse Outcomes in Residual Breast Cancer Tumors After Neoadjuvant Chemotherapy. Oncologist 2018, 23, 670–678. [Google Scholar] [CrossRef]
- Muss, H.B.; Polley, M.C.; Berry, D.A.; Liu, H.; Cirrincione, C.T.; Theodoulou, M.; Mauer, A.M.; Kornblith, A.B.; Partridge, A.H.; Dressler, L.G.; et al. Randomized Trial of Standard Adjuvant Chemotherapy Regimens Versus Capecitabine in Older Women with Early Breast Cancer: 10-Year Update of the CALGB 49907 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2338–2348. [Google Scholar] [CrossRef]
- Lluch, A.; Gomez, H.; Ruiz-Borrego, M.; Bines, J.; Llombart, A.; Ramos, M.; Torres, R.; Martins-Segalla, J.G.; Torrecillas, L.; Barrios, C. Abstract P5-10-15: First Safety Data from a Randomised Phase III (CIBOMA 2004-01/GEICAM 2003-11) Trial Assessing Adjuvant Capecitabine Maintenance Therapy after Standard Chemotherapy for Triple-Negative Early Breast Cancer. Cancer Res. 2010, 70, P5-10-15. [Google Scholar] [CrossRef]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy Treatment for Triple Negative Breast Cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.A.; McArthur, H.L.; Kuemmel, S.; Bergh, J.C.S.; Park, Y.H.; Hui, R.; et al. Event-Free Survival by Residual Cancer Burden after Neoadjuvant Pembrolizumab + Chemotherapy versus Placebo + Chemotherapy for Early TNBC: Exploratory Analysis from KEYNOTE-522. J. Clin. Oncol. 2022, 40, 503. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.-S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Abstract GS3-04: Pathologic Complete Response (pCR) to Neoadjuvant Treatment with or without Atezolizumab in Triple Negative, Early High-Risk and Locally Advanced Breast Cancer. NeoTRIPaPDL1 Michelangelo Randomized Study. Cancer Res. 2020, 80, GS3-04. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Placebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Medicine USNLo. Testing MK-3475 (Pembrolizumab) as Adjuvant Therapy for Triple Receptor-Negative Breast Cancer (2016-2021). Available online: https://clinicaltrials.gov/ct2/show/NCT02954874 (accessed on 5 May 2022).
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Pelizzari, G.; Gerratana, L.; Basile, D.; Fanotto, V.; Bartoletti, M.; Liguori, A.; Fontanella, C.; Spazzapan, S.; Puglisi, F. Post-Neoadjuvant Strategies in Breast Cancer: From Risk Assessment to Treatment Escalation. Cancer Treat. Rev. 2019, 72, 7–14. [Google Scholar] [CrossRef]
- Patel, S.A.; DeMichele, A. Adding Adjuvant Systemic Treatment after Neoadjuvant Therapy in Breast Cancer: Review of the Data. Curr. Oncol. Rep. 2017, 19, 56. [Google Scholar] [CrossRef]
- Labrosse, J.; Osdoit, M.; Hamy, A.-S.; Coussy, F.; Pierga, J.-Y.; Reyal, F.; Laas, E. Adjuvant Chemotherapy for Breast Cancer after Preoperative Chemotherapy: A Propensity Score Matched Analysis. PLoS ONE 2020, 15, e0234173. [Google Scholar] [CrossRef]
- Foldi, J.; Rozenblit, M.; Park, T.S.; Knowlton, C.A.; Golshan, M.; Moran, M.; Pusztai, L. Optimal Management for Residual Disease Following Neoadjuvant Systemic Therapy. Curr. Treat. Options Oncol. 2021, 22, 79. [Google Scholar] [CrossRef]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing Antibody-Drug Conjugates (ADCs) in HER2-Positive Breast Cancer: State of the Art and Future Directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef]
| Clinical Trial | Dual HER2 Therapy | pCR Rate with Single Anti-HER2 Agent (%) | pCR Rate with Dual Anti-HER2 Therapy (%) |
|---|---|---|---|
| NeoALTTO [38] | L + T | 30 (95% CI, 22.4–37.5) with T 25 (95% CI, 18.1–32.3) with L | 51 (95% CI, 43.1–59.5; p = 0.0001) |
| NeoSphere [39] | T + P | 29 (95% CI, 20.6–38.5) with T 24 (95% CI, 15.8–33.7) with P | 46 (95% CI, 36.1–55.7; p = 0.0141) |
| CALGB 40601 [40] | L + T | 40 (95% CI, 32–49) with T 32 (95% CI, 22–44) with L | 51 (95% CI, 42–60; p = 0.11) |
| NSABP B-41 [41] | L + T | 53 (95% CI, 44.9–59.5) with T 53 (95% CI, 44.4–60.3) with L | 62 (95% CI, 54.3–68.8; p = 0.095) |
| TRYPHAENA [36] | T + P | NA | 57–66 |
| Treatment | Pathologic Complete Response (pCR) Rates (%) |
|---|---|
| Anthracycline [70,71] | 14–47 |
| Anthracycline + taxane [72] | 17–39 |
| Anthracycline + cyclophosphamide followed by taxanes [65,66,73] | 30–44 |
| Carboplatin to a backbone of anthracycline/taxanes [65,66,67,68] | 52–57 |
| Platinum monotherapy (carboplatin, cisplatin) [74,75] | 23–90 * |
| Gemcitabine + carboplatin + iniparib [76] | 36 |
| Pembrolizumab + standard chemotherapy [77] | 64.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Razeq, H.; Khalil, H.; Assi, H.I.; Dargham, T.B. Treatment Strategies for Residual Disease following Neoadjuvant Chemotherapy in Patients with Early-Stage Breast Cancer. Curr. Oncol. 2022, 29, 5810-5822. https://doi.org/10.3390/curroncol29080458
Abdel-Razeq H, Khalil H, Assi HI, Dargham TB. Treatment Strategies for Residual Disease following Neoadjuvant Chemotherapy in Patients with Early-Stage Breast Cancer. Current Oncology. 2022; 29(8):5810-5822. https://doi.org/10.3390/curroncol29080458
Chicago/Turabian StyleAbdel-Razeq, Hikmat, Hanan Khalil, Hazem I. Assi, and Tarek Bou Dargham. 2022. "Treatment Strategies for Residual Disease following Neoadjuvant Chemotherapy in Patients with Early-Stage Breast Cancer" Current Oncology 29, no. 8: 5810-5822. https://doi.org/10.3390/curroncol29080458





