Time to Surgery for Patients with Esophageal Cancer Undergoing Trimodal Therapy in Ontario: A Population-Based Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
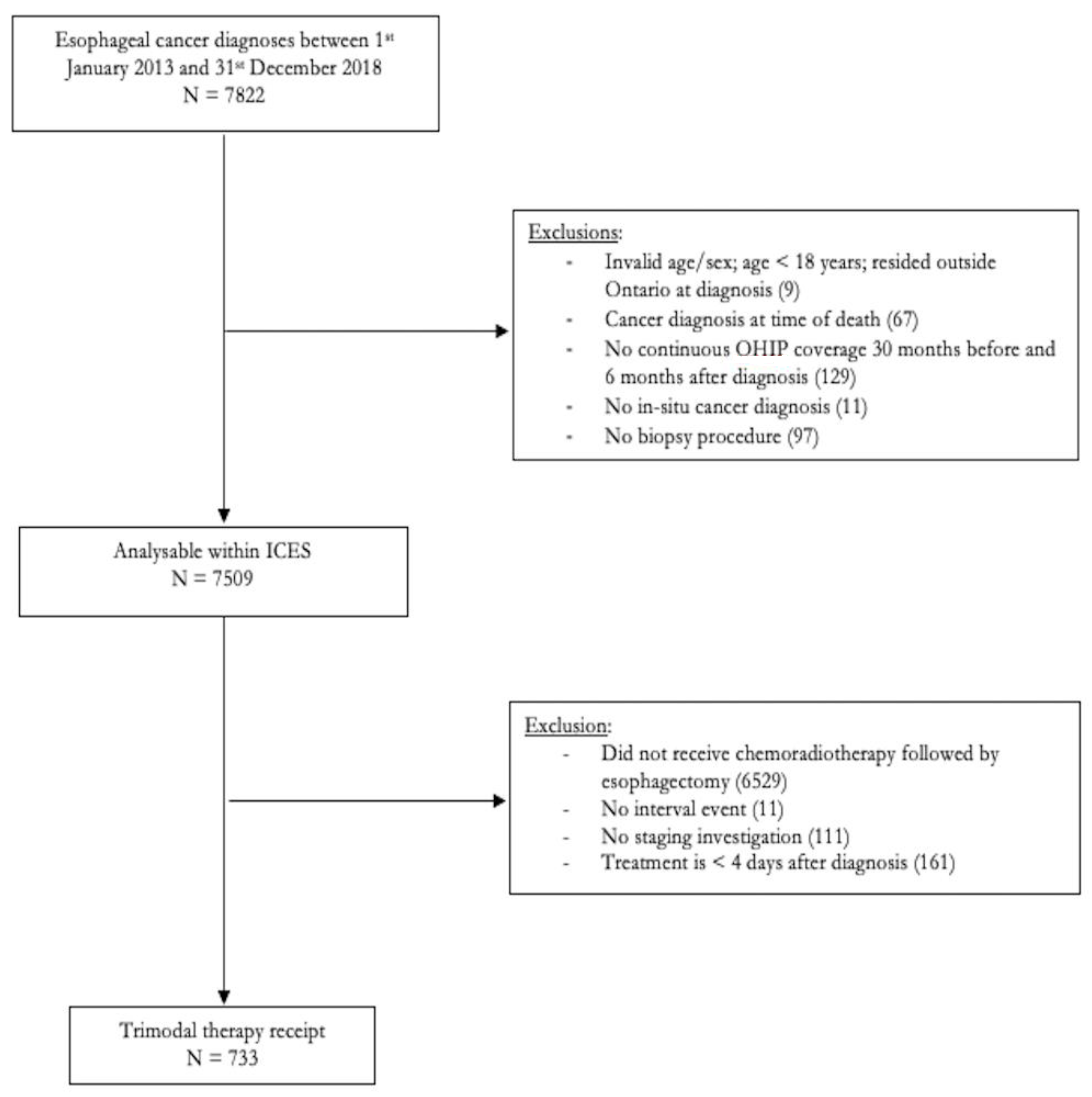
2.2. Study Population
2.3. Outcome Definitions
2.4. Covariates and Data Sources
2.5. Statistical Analyses
3. Results
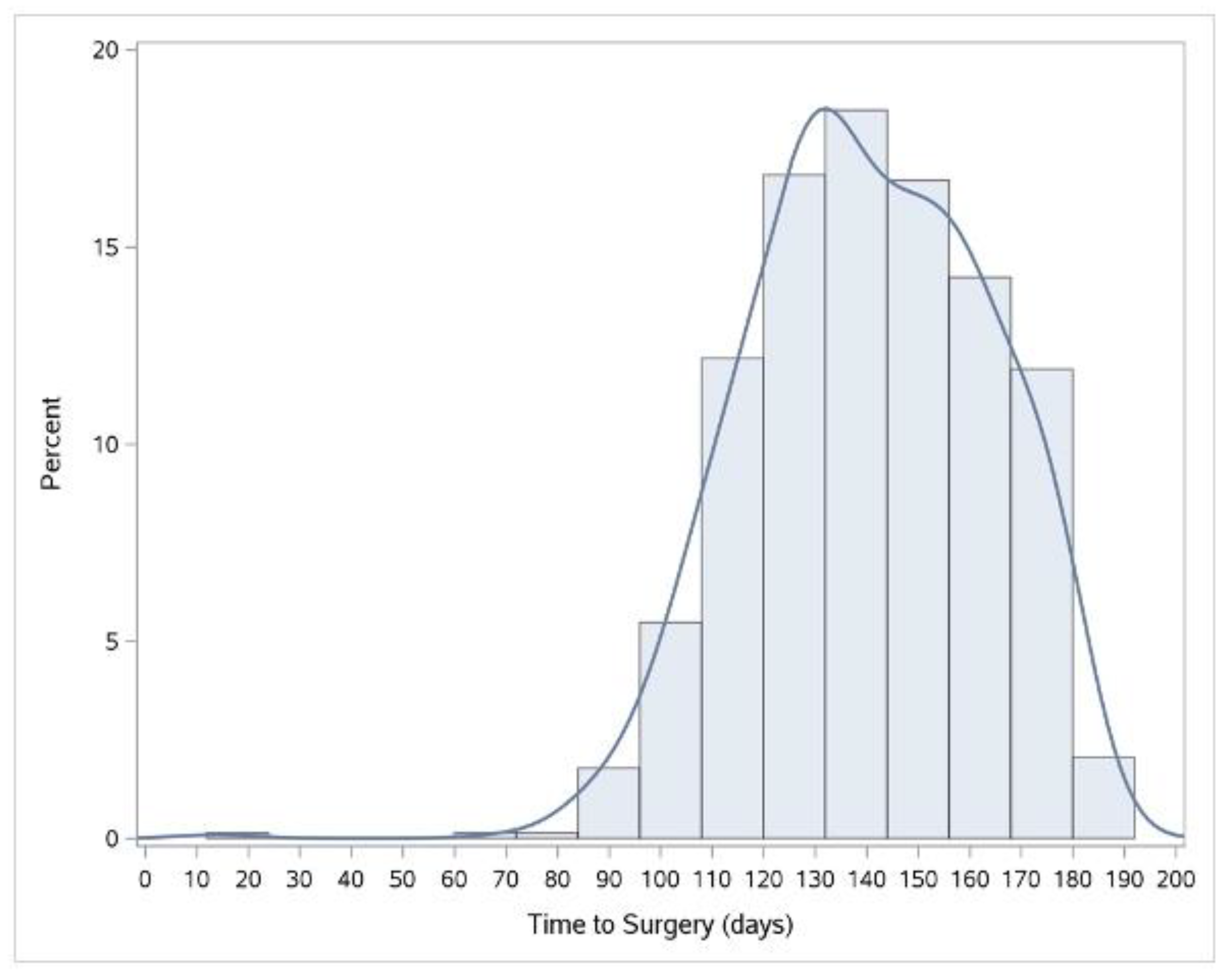
3.1. Length of Time Intervals
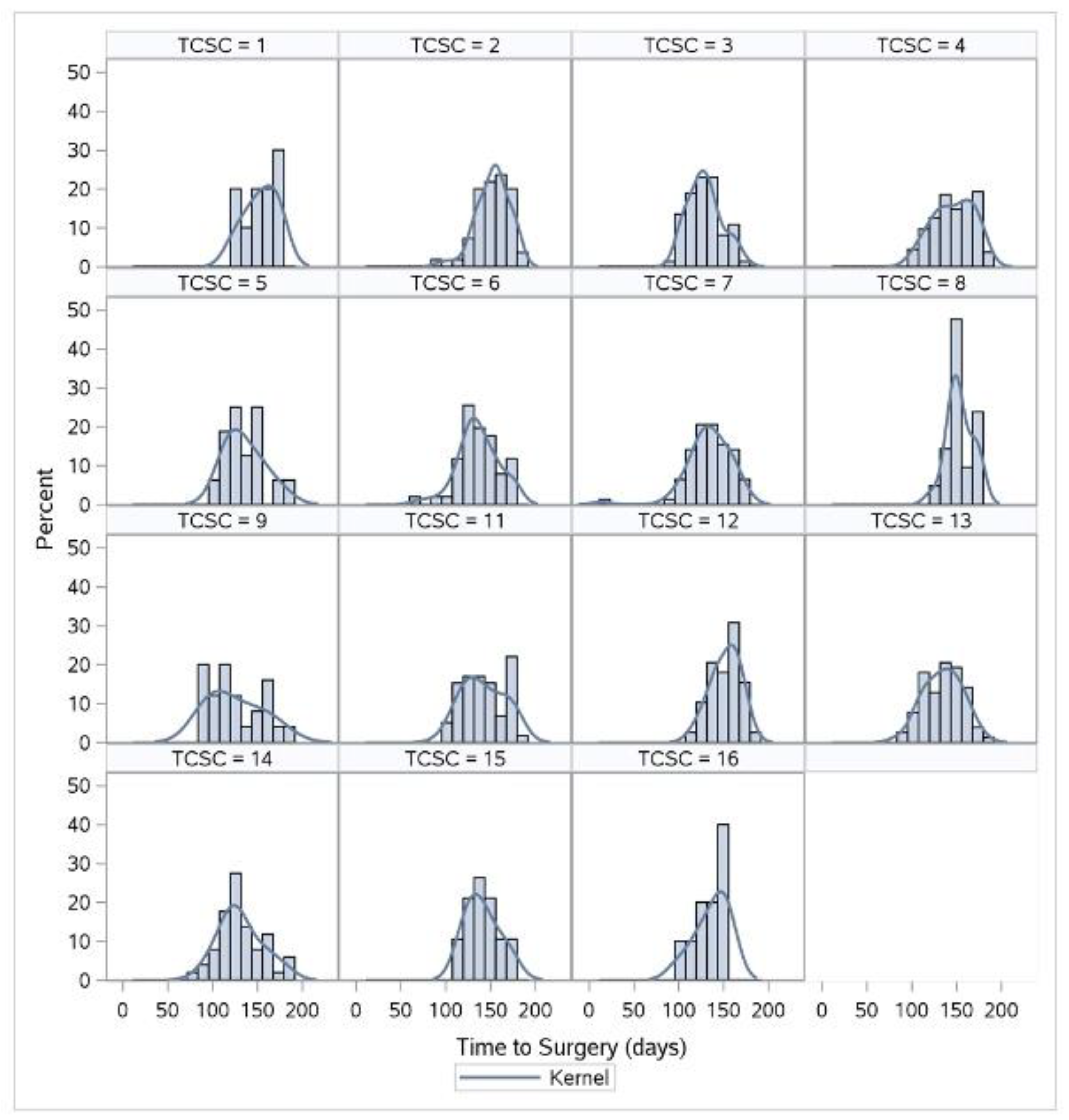
3.2. Changes in TTS and TTC Lengths by TCSC after Adjusting for Covariates
3.3. Bivariate Analysis of Factors Associated with TTS and TTC Lengths
3.4. Factors Associated with TTS and TTC
3.5. Sensitivity Analysis
4. Discussion
4.1. Time to Surgery in Ontario
4.2. Comparison between TCSCs
4.3. Factors Associated with TTS and TTC
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar-Danesh, N.; Shakeel, S.; Seow, H.; Akhtar-Danseh, G.G.; Finley, C. Trends in survival based on treatment modality for esophageal cancer: A population-based study. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Habbous, S.; Yermakhanova, O.; Forster, K.; Holloway, C.M.B.; Darling, G. Variation in Diagnosis, Treatment, and Outcome of Esophageal Cancer in a Regionalized Care System in Ontario, Canada. JAMA Netw. Open 2021, 4, e2126090. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl. S1), S92–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Hagen, P.; Lingsma, H.F.; Wijnhoven, B.P.; Biermann, K.; ten Kate, F.J.; Steyerberg, E.W.; van der Gaast, A.; van Lanschot, J.J. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann. Surg. 2014, 260, 807–813, discussion 813–814. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Leeftink, A.G.; van Rossum, P.S.N.; Siesling, S.; van Hillegersberg, R.; Ruurda, J.P. Waiting Time from Diagnosis to Treatment has no Impact on Survival in Patients with Esophageal Cancer. Ann. Surg. Oncol. 2016, 23, 2679–2689. [Google Scholar] [CrossRef] [Green Version]
- Visser, E.; van Rossum, P.S.N.; Leeftink, A.G.; Siesling, S.; van Hillegersberg, R.; Ruurda, J.P. Impact of diagnosis-to-treatment waiting time on survival in esophageal cancer patients—A population-based study in The Netherlands. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 461–470. [Google Scholar] [CrossRef]
- Raman, V.; Jawitz, O.K.; Voigt, S.L.; Yang, C.-F.J.; Wang, H.; Harpole, D.H.; D’Amico, T.A. Effect of time to surgery on outcomes in stage I esophageal adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2020, 159, 1626–1635.e21. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.R.; van Lanschot, J.J.; van der Velden, J.; Kloek, J.J.; Gouma, D.J.; Sprangers, M.A. Quality of life in newly diagnosed cancer patients waiting for surgery is seriously impaired. J. Surg. Oncol. 2006, 93, 571–577. [Google Scholar] [CrossRef]
- COO. Esophageal Cancer Treatment Pathway. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/EsophagealTreatmentPathway-2019-05.pdf (accessed on 1 March 2021).
- Sundaresan, S.; Langer, B.; Oliver, T.; Schwartz, F.; Brouwers, M.; Stern, H. Standards for thoracic surgical oncology in a single-payer healthcare system. Ann. Thorac. Surg. 2007, 84, 693–701. [Google Scholar] [CrossRef]
- Finley, C.J.; Bendzsak, A.; Tomlinson, G.; Keshavjee, S.; Urbach, D.R.; Darling, G.E. The effect of regionalization on outcome in pulmonary lobectomy: A Canadian national study. J. Thorac. Cardiovasc. Surg. 2010, 140, 757–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, C.J.; Jacks, L.; Keshavjee, S.; Darling, G. The effect of regionalization on outcome in esophagectomy: A Canadian national study. Ann. Thorac. Surg. 2011, 92, 485–490, discussion 490. [Google Scholar] [CrossRef] [PubMed]
- Bendzsak, A.M.; Baxter, N.N.; Darling, G.E.; Austin, P.C.; Urbach, D.R. Regionalization and Outcomes of Lung Cancer Surgery in Ontario, Canada. J. Clin. Oncol. 2017, 35, 2772–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, S.; Dhanoa, M.; Khan, O.; Dibajnia, P.; Akhtar-Danesh, N.; Behzadi, A. Wait times in the management of non–small cell lung carcinoma before, during and after regionalization of lung cancer care: A high-resolution analysis. Can. J. Surg. 2021, 64, E218–E227. [Google Scholar] [CrossRef]
- Robles, S.C.; Marrett, L.D.; Clarke, E.A.; Risch, H.A. An application of capture-recapture methods to the estimation of completeness of cancer registration. J. Clin. Epidemiol. 1988, 41, 495–501. [Google Scholar] [CrossRef]
- Ontario, C.C. Thoracic Cancer Surgery Centres List. Available online: https://www.cancercareontario.ca/en/find-cancer-services/designated-cancer-surgery-centres/thoracic-centres-list (accessed on 1 March 2021).
- Matheson, F.I.; Moineddin, R.; Glazier, R.H. The weight of place: A multilevel analysis of gender, neighborhood material deprivation, and body mass index among Canadian adults. Soc. Sci. Med. 2008, 66, 675–690. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Hao, L.N.D. Quantile Regression. 2007. Available online: https://methods.sagepub.com/book/quantile-regression (accessed on 3 December 2021).
- van Heijl, M.; van Lanschot, J.J.; Koppert, L.B.; van Berge Henegouwen, M.I.; Muller, K.; Steyerberg, E.W.; van Dekken, H.; Wijnhoven, B.P.; Tilanus, H.W.; Richel, D.J.; et al. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS). BMC Surg. 2008, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, S.; McLeod, R.; Irish, J.; Burns, J.; Hunter, A.; Meertens, E.; Langer, B.; Stern, H.; Sherar, M. Early Results After Regionalization of Thoracic Surgical Practice in a Single-Payer System. Ann. Thorac. Surg. 2013, 95, 472–479. [Google Scholar] [CrossRef]
- Darling, G.E. Regionalization in thoracic surgery: The importance of the team. J. Thorac. Cardiovasc. Surg. 2021, 161, 323–329. [Google Scholar] [CrossRef]
- De Hert, S.; Staender, S.; Fritsch, G.; Hinkelbein, J.; Afshari, A.; Bettelli, G.; Bock, M.; Chew, M.S.; Coburn, M.; De Robertis, E.; et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: Updated guideline from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2018, 35, 407–465. [Google Scholar] [CrossRef]
- Cordeiro, E.; Dixon, M.; Coburn, N.; Holloway, C.M. A Patient-Centered Approach to Wait Times in the Surgical Management of Breast Cancer in the Province of Ontario. Ann. Surg. Oncol. 2015, 22, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.; Elit, L.; Pond, G.; Seow, H. The wait time creep: Changes in the surgical wait time for women with uterine cancer in Ontario, Canada, during 2000-2009. Gynecol. Oncol. 2013, 131, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis, A.; Dixon, M.; Smith, A.; Law, C.; Coburn, N.G. A patient-centred approach toward surgical wait times for colon cancer: A population-based analysis. Can. J. Surg. 2014, 57, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, B. Role of volume outcome data in assuring quality in HPB surgery. HPB 2007, 9, 330–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, L.J.L.; Warburton, F.; Richards, M.A.; Ramirez, A.J. Risk factors for delay in symptomatic presentation: A survey of cancer patients. Br. J. Cancer 2014, 111, 581–588. [Google Scholar] [CrossRef]
- Lokanatha, D.; Hassan, S.A.; Jacob, L.A.; Suresh Babu, M.C.; Lokesh, K.N.; Rudresha, A.H.; Rajeev, L.K.; Saldanha, S.; Thottian, A.G.F. Socioeconomic and administrative factors associated with treatment delay of esophageal and gastric carcinoma: Prospective study from a tertiary care centre in a developing country. Cancer Epidemiol. 2020, 67, 101770. [Google Scholar] [CrossRef]
- Bardell, T.; Belliveau, P.; Kong, W.; Mackillop, W.J. Waiting Times for Cancer Surgery in Ontario: 1984–2000. Clin. Oncol. 2006, 18, 401–409. [Google Scholar] [CrossRef]
- Winget, M.; Turner, D.; Tonita, J.; King, C.; Nugent, Z.; Alvi, R.; Barss, R. Across-province standardization and comparative analysis of time-to-care intervals for cancer. BMC Cancer 2007, 7, 186. [Google Scholar] [CrossRef] [Green Version]
| Cohort Characteristics | Number of Patients (%) |
|---|---|
| Age Group (Years) | |
| 18–49 | 49 (6.7) |
| 50–59 | 200 (27.3) |
| 60–69 | 299 (40.8) |
| 70–79 | 166 (22.7) |
| 80+ | 19 (2.6) |
| Sex | |
| F | 151 (20.6) |
| M | 582 (79.4) |
| Sum of Minor ADGs | |
| 0 | 48 (6.6) |
| 1–2 | 161 (22.0) |
| 3–4 | 195 (26.6) |
| 5–6 | 168 (22.9) |
| 7+ | 161 (22.0) |
| Sum of Major ADGs | |
| 0 | 334 (45.6) |
| 1 | 222 (30.3) |
| 2 | 121 (16.5) |
| 3+ | 56 (7.6) |
| Total Number of ADGs | |
| 0 | 46 (6.3) |
| 1–3 | 202 (27.6) |
| 4–6 | 243 (33.2) |
| 7–9 | 162 (22.1) |
| 10+ | 80 (10.9) |
| Recent Immigration | |
| No | 688 (93.9) |
| Yes | 45 (6.1) |
| Material Deprivation | |
| Least Deprived | 154 (21.0) |
| 2 | 153 (20.9) |
| 3 | 138 (18.8) |
| 4 | 139 (19.0) |
| Most Deprived | 144 (19.7) |
| Unknown | <6 |
| Rurality | |
| Rural | 119 (16.2) |
| Urban | 611 (83.4) |
| Unknown | <6 |
| Calendar Year of Diagnosis | |
| 2013 | 87 (11.9) |
| 2014 | 115 (15.7) |
| 2015 | 118 (16.1) |
| 2016 | 123 (16.8) |
| 2017 | 137 (18.7) |
| 2018 | 153 (20.9) |
| Histology | |
| Adenocarcinoma | 578 (78.9) |
| Squamous Cell Carcinoma | 122 (6.6) |
| Other | 33 (4.5) |
| Tumor Site | |
| Cervical Esophagus | <6 |
| Upper Esophagus | <6 |
| Middle Esophagus | 64 (8.7) |
| Lower Esophagus | 423 (57.7) |
| Gastroesophageal Junction | 223 (30.4) |
| Other | 18 (2.5) |
| Stage | |
| I | 58 (7.9) |
| II | 165 (22.5) |
| III | 208 (28.4) |
| IV | 21 (2.9) |
| Unknown | 281 (38.3) |
| Diagnosing Physician Main Specialty | |
| Gastroenterology | 277 (37.8) |
| General Surgery | 270 (36.8) |
| Thoracic Surgery | 84 (11.5) |
| Other | 70 (9.6) |
| Unknown | 32 (4.4) |
| Diagnosing Physician Years in Practice | |
| 1–9 | 31 (4.2) |
| 10–14 | 115 (15.7) |
| 15–19 | 107 (14.6) |
| 20–24 | 61 (8.3) |
| 25–29 | 49 (6.7) |
| 30+ | 66 (9.0) |
| Unknown | 304 (41.5) |
| Diagnosing Physician Academic Affiliation | |
| 0 | 441 (60.2) |
| 1 | 159 (21.7) |
| Unknown | 133 (18.1) |
| TCSC Where Surgery Took Place | |
| 1 | 20 (2.7) |
| 2 | 55 (7.5) |
| 3 | 74 (10.1) |
| 4 | 135 (18.4) |
| 5 | 16 (2.2) |
| 6 | 51 (7.0) |
| 7 | 78 (10.6) |
| 8 | 21 (2.9) |
| 9 | 25 (3.4) |
| 10 | <6 |
| 11 | 59 (8.1) |
| 12 | 39 (5.3) |
| 13 | 78 (10.6) |
| 14 | 51 (7.0) |
| 15 | 19 (2.6) |
| 16 | 10 (1.4) |
| 17 | <6 |
| Emergency Department Visits | |
| 0 | 652 (89.2) |
| 1 | 69 (9.4) |
| >1 | 10 (1.4) |
| Hospital Admission | |
| 0 | 623 (85.2) |
| 1 | 96 (13.1) |
| >1 | 12 (1.6) |
| Variable | Time to Consult (days) | Time to Surgery (days) | ||
|---|---|---|---|---|
| Median (IQR) | 90th | Median (IQR) | 90th | |
| Whole Cohort | 14 (6, 26) | 41 | 140 (125, 158) | 171 |
| TCSC | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| 1 | 9 (2, 14) | 18 | 148 (137, 163) | 170 |
| 2 | 11 (3, 19) | 29 | 154 (141, 163) | 175 |
| 3 | 16 (8, 28) | 52 | 127 (116, 139) | 156 |
| 4 | 8 (2, 21) | 41 | 151 (131, 166) | 174 |
| 5 | 10 (1, 16) | 84 | 125 (119, 133) | 154 |
| 6 | 12 (4, 21) | 35 | 133 (122, 150) | 175 |
| 7 | 16 (7, 28) | 41 | 134 (119, 152) | 162 |
| 8 | 10 (0, 27) | 120 | 149 (141, 166) | 171 |
| 9 | 11 (4, 27) | 82 | 130 (108, 158) | 176 |
| 10 * | - | - | - | - |
| 11 | 17 (12, 23) | 35 | 139 (120, 161) | 176 |
| 12 | 27 (20, 36) | 119 | 156 (141, 164) | 171 |
| 13 | 27 (14, 35) | 44 | 138 (119, 150) | 161 |
| 14 | 10 (3, 18) | 27 | 126 (113, 139) | 162 |
| 15 | 13 (4, 20) | 24 | 139 (125, 147) | 169 |
| 16 | 23 (4, 88) | 148 | 143 (127, 153) | 155 |
| Age | p = 0.35 | p = 0.28 | p = 0.009 | p = 0.55 |
| 18–49 | 11 (5, 21) | 41 | 131 (118, 154) | 173 |
| 50–59 | 14 (6, 26) | 39 | 137 (125, 157) | 169 |
| 60–69 | 14 (6, 25) | 36 | 139 (124, 156) | 171 |
| 70+ | 16 (7, 27) | 47 | 144 (128, 160) | 173 |
| Sex | p = 0.10 | p = 0.07 | p = 1.00 | p = 0.70 |
| F | 17 (6, 27) | 52 | 141 (125, 160) | 173 |
| M | 14 (6, 26) | 39 | 139 (125, 156) | 171 |
| Sum of Minor ADGs | p = 0.31 | p = 0.42 | p = 0.70 | p = 0.37 |
| 0 | 18 (3, 34) | 52 | 135 (116, 154) | 173 |
| 1–2 | 16 (7, 26) | 41 | 141 (124, 161) | 172 |
| 3–4 | 13 (5, 21) | 36 | 138 (124, 154) | 169 |
| 5–6 | 16 (7, 27) | 36 | 143 (126, 158) | 167 |
| 7+ | 13 (3, 27) | 40 | 139 (127, 159) | 173 |
| Sum of Major ADGs | p = 0.10 | p = 0.92 | p = 0.08 | p = 0.78 |
| 0 | 14 (6, 26) | 42 | 135 (121, 154) | 169 |
| 1 | 14 (5, 23) | 42 | 141 (126, 160) | 171 |
| 2 | 14 (6, 23) | 36 | 143 (127, 159) | 172 |
| 3+ | 20 (5, 30) | 42 | 143 (126, 161) | 175 |
| Recent Immigration | p = 0.80 | p = 0.97 | p = 0.42 | p = 0.87 |
| 0 | 14 (6, 26) | 41 | 140 (125, 157) | 171 |
| 1 | 12 (0, 21) | 41 | 146 (120, 163) | 177 |
| Material Deprivation | p = 0.05 | p = 0.95 | p = 0.04 | p = 0.55 |
| Least Deprived | 13 (5, 25) | 42 | 134 (121, 154) | 173 |
| 2 | 14 (5, 25) | 36 | 142 (127, 155) | 168 |
| 3 | 11 (2, 24) | 37 | 139 (123, 156) | 171 |
| 4 | 14 (7, 24) | 38 | 143 (125, 158) | 172 |
| Most Deprived | 19 (7, 28) | 45 | 146 (127, 160) | 171 |
| Rurality | p = 0.38 | p = 0.44 | p = 0.03 | p = 0.19 |
| Rural | 16 (7, 29) | 47 | 145 (132, 160) | 174 |
| Urban | 14 (5, 25) | 40 | 138 (123, 156) | 171 |
| Calendar Year of Diagnosis | p = 0.26 | p = 0.91 | p < 0.0001 | p = 0.0001 |
| 2013 | 15 (4, 23) | 39 | 134 (124, 153) | 161 |
| 2014 | 13 (5, 21) | 38 | 131 (118,154) | 171 |
| 2015 | 15 (6, 27) | 44 | 143 (129, 161) | 169 |
| 2016 | 14 (2, 29) | 41 | 142 (125, 162) | 175 |
| 2017 | 18 (8, 28) | 47 | 150 (131, 163) | 175 |
| 2018 | 12 (6, 22) | 35 | 135 (120, 152) | 163 |
| Histology | p = 0.89 | p = 0.37 | p = 0.32 | p = 0.77 |
| Adenocarcinoma | 14 (6, 26) | 40 | 140 (125, 159) | 170 |
| Squamous Cell Carcinoma | 14 (2, 23) | 53 | 138 (123, 154) | 175 |
| Other | 14 (7, 28) | 61 | 139 (123, 161) | 175 |
| Tumor site | p = 0.30 | p = 0.08 | p = 0.74 | p = 0.85 |
| Middle Esophagus | 15 (2, 27) | 53 | 141 (123, 156) | 174 |
| Lower Esophagus | 14 (6, 26) | 40 | 141 (125, 156) | 170 |
| Gastroesophageal Junction | 15 (7, 28) | 44 | 139 (121, 159) | 171 |
| Other | 11 (4, 17) | 23 | 131 (128, 161) | 169 |
| Stage | p = 0.0002 | p = 1.00 | p = 0.59 | p = 0.75 |
| I | 16 (8, 21) | 30 | 140 (126, 161) | 170 |
| II | 16 (6, 28) | 39 | 143 (123, 158) | 174 |
| III | 13 (4, 23) | 41 | 137 (121, 154) | 165 |
| IV | 12 (0, 36) | 42 | 155 (128, 165) | 176 |
| Unknown | 14 (6, 26) | 41 | 139 (125, 160) | 171 |
| Specialty | p < 0.0001 | p = 0.0001 | p = 0.11 | p = 0.35 |
| Gastroenterology | 16 (7, 28) | 46 | 137 (124, 156) | 172 |
| General Surgery | 19 (11, 27) | 40 | 144 (128, 159) | 169 |
| Thoracic Surgery | −4 (−9, 0) | 11 | 133 (111, 149) | 166 |
| Other | 14 (6, 23) | 35 | 140 (120, 162) | 174 |
| Years in Practice | p = 0.19 | p = 0.95 | p = 0.26 | p = 0.76 |
| 1–9 | 10 (1, 18) | 23 | 131 (124, 153) | 166 |
| 10–14 | 12 (3, 22) | 39 | 133 (125, 155) | 173 |
| 15–19 | 15 (7, 28) | 52 | 144 (130, 159) | 167 |
| 20–24 | 17 (6, 34) | 49 | 135 (117, 157) | 171 |
| 25–29 | 13 (4, 29) | 52 | 138 (119, 161) | 169 |
| 30+ | 21 (14, 31) | 47 | 141 (130, 157) | 172 |
| Academic Affiliation | p < 0.0001 | p = 0.007 | p = 0.09 | p = 0.78 |
| No | 14 (5, 24) | 36 | 144 (125, 159) | 171 |
| Yes | 6 (-2, 19) | 29 | 134 (118, 155) | 172 |
| ED visit | p = 0.47 | p = 0.58 | p = 0.13 | p = 0.95 |
| 0 | 14 (5, 25) | 39 | 139 (124, 158) | 171 |
| 1 | 20 (8, 36) | 57 | 145 (131, 155) | 171 |
| >1 | 14 (11, 34) | 42 | 150 (138, 158) | 170 |
| Hospital Admission | p = 0.16 | p = 0.87 | p < 0.0001 | p = 0.74 |
| 0 | 15 (6, 26) | 40 | 138 (124, 155) | 170 |
| 1 | 13 (2, 26) | 44 | 153 (130, 163) | 172 |
| >1 | 9 (4, 18) | 37 | 151 (132, 169) | 174 |
| Variable | 50th Percentile | 90th Percentile | ||
|---|---|---|---|---|
| Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) | |
| Adjusted Intercept | 130 (120, 140) | 163 (151, 174) | ||
| Age Group (Years) | p = 0.005 | p = 0.01 | p = 0.54 | p = 0.35 |
| Unadjusted Intercept | 139 (136, 142) | 172 (169, 175) | ||
| 18–49 | −11 (−19, −3) | −11 (−19, −2) | −3 (−16, 10) | −8 (−22, 6) |
| 50–59 | −1 (−10, 8) | −1 (−6, 4) | −3 (−9, 3) | −4 (−10, 1) |
| 60–69 | Ref | Ref | Ref | Ref |
| 70+ | 5 (−1, 11) | 4 (−1, 9) | 1 (−4, 6) | −2 (−7, 2) |
| Sex | p = 1.00 | p = 0.38 | p = 0.69 | p = 0.78 |
| Unadjusted Intercept | 140 (134, 146) | 173 (169, 177) | ||
| Female | Ref | Ref | Ref | Ref |
| Male | 0 (−7, 7) | −2 (−6, 2) | −1 (−6, 4) | −1 (−7, 5) |
| Sum of Minor ADGs | p = 0.68 | p = 0.40 | p = 0.36 | p = 0.86 |
| Unadjusted Intercept | 140 (135, 145) | 174 (170, 178) | ||
| 0–2 | Ref | Ref | Ref | Ref |
| 3–4 | −2 (−9, 5) | −3 (−9, 2) | −5 (−11, 1) | −2 (−8, 4) |
| 5–6 | 3 (−5, 11) | 1 (−4, 6) | −4 (−11, 3) | −2 (−8, 4) |
| 7+ | −1 (−9, 7) | −2 (−8, 4) | −1 (−6, 4) | −2 (−8, 4) |
| Sum of Major ADGs | p = 0.09 | p = 0.41 | p = 0.79 | p = 0.61 |
| Unadjusted Intercept | 135 (131, 139) | 171 (168, 174) | ||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 6 (0, 12) | 1 (−4, 5) | 0 (−6, 6) | 3 (−2, 8) |
| 2 | 8 (0, 16) | 4 (−1, 9) | 3 (−3, 9) | 3 (−4, 9) |
| 3+ | 8 (−2, 18) | 4 (−4, 11) | 2 (−6, 10) | 3 (−5, 12) |
| Material Deprivation | p = 0.04 | p = 0.32 | p = 0.56 | p = 0.21 |
| Unadjusted Intercept | 133 (128, 138) | 173 (169, 177) | ||
| Least Deprived | Ref | Ref | Ref | Ref |
| 2 | 10 (3, 17) | 5 (−1, 12) | −5 (−12, 2) | −1 (−7, 5) |
| 3 | 5 (−3, 13) | 4 (−2, 10) | −2 (−8, 4) | 1 (−5, 7) |
| 4 | 10 (2, 18) | 7 (1, 14) | 0 (−6, 6) | 1 (−6, 7) |
| Most Deprived | 8 (−1, 17) | 7 (0, 14) | 1 (−6, 8) | 2 (−5, 9) |
| Rurality | p = 0.05 | p = 0.68 | p = 0.17 | p = 0.25 |
| Unadjusted Intercept | 138 (135, 141) | 171 (169, 173) | ||
| Urban | Ref | Ref | Ref | Ref |
| Rural | 7 (0, 14) | 3 (−3, 8) | 4 (−2, 10) | 3 (−2, 9) |
| Recent Immigration | p = 0.42 | p = 0.19 | p = 0.88 | p = 0.92 |
| Unadjusted Intercept | 140 (138, 142) | 172 (170, 174) | ||
| No | Ref | Ref | Ref | Ref |
| Yes | −6 (−21, 9) | 2 (−8, 12) | 1 (−12, 14) | 8 (−6, 22) |
| Histology | p = 0.30 | p = 0.06 | p = 0.76 | p = 0.72 |
| Unadjusted Intercept | 140 (137, 143) | 171 (168, 174) | ||
| Adenocarcinoma | Ref | Ref | Ref | Ref |
| Squamous Cell Carcinoma | −4 (−10, 2) | −5 (−11, 1) | 2 (−6, 10) | 3 (−4, 9) |
| Other | 4 (−7, 15) | 10 (−3, 22) | 4 (−10, 18) | 2 (−12, 17) |
| Tumor location | p = 0.77 | p = 0.68 | p = 0.86 | p = 0.93 |
| Unadjusted Intercept | 139 (133, 145) | 173 (169, 177) | ||
| Middle Esophagus | 1 (−11, 13) | 4 (−6, 15) | 0 (−8, 8) | 1 (−8, 10) |
| Lower Esophagus | 1 (−5, 7) | 0 (−4, 4) | −2 (−7, 3) | 0 (−5, 4) |
| Gastroesophageal Junction | Ref | Ref | Ref | Ref |
| Other | −8 (−27, 11) | −6 (−22, 10) | −5 (−27, 17) | −4 (−20, 12) |
| TCSC | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.003 |
| Unadjusted Intercept | 135 (129, 141) | 163 (156, 170) | ||
| 01 | 19 (4, 34) | 19 (3, 35) | 11 (−5, 27) | 13 (−6, 32) |
| 02 | 19 (12, 26) | 19 (11, 28) | 13 (5, 21) | 13 (3, 22) |
| 03 | −8 (−16, 0) | −5 (−12, 3) | −3 (−14, 8) | −4 (−16, 8) |
| 04 | 14 (4, 24) | 14 (5, 22) | 12 (5, 19) | 13 (5, 21) |
| 05 | −3 (−22, 16) | −8 (−28, 12) | 7 (−28, 43) | 7 (−19, 33) |
| 06 | −2 (−10, 6) | 5 (−4, 14) | 8 (−8, 24) | 7 (−8, 23) |
| 07 | 0 (−9, 9) | 3 (−5, 11) | −1 (−10, 8) | 1 (−10, 11) |
| 08 | 17 (8, 26) | 16 (5, 28) | 11 (−14, 36) | 11 (−10, 32) |
| 09 | −22 (−44, 1) | −13 (−33, 7) | 4 (−20, 28) | 4 (−22, 30) |
| 10 * | - | - | - | - |
| 11 | 4 (−8, 16) | 4 (−8, 16) | 13 (6, 20) | 12 (3, 21) |
| 12 | 20 (10, 30) | 19 (10, 27) | 10 (−2, 22) | 10 (−1, 21) |
| 13 | Ref | Ref | Ref | Ref |
| 14 | −8 (−16, 0) | −4 (−13, 4) | 0 (−18, 18) | 0 (−17, 17) |
| 15 | 4 (−9, 17) | 7 (−7, 21) | 6 (−17, 29) | 9 (−13, 31) |
| Non−TCSC | 8 (−14, 30) | 7 (−12, 26) | −8 (−88, 72) | −9 (−64, 47) |
| 50th Percentile | 90th Percentile | |||
|---|---|---|---|---|
| Variable | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) |
| Adjusted Intercept | 29 (24, 35) | 54 (35, 72) | ||
| Age | p = 0.36 | p = 0.54 | p = 0.35 | p = 0.56 |
| Unadjusted intercept | 14 (12, 16) | 35 (30, 40) | ||
| 18–49 | −3 (−8, 2) | −3 (−7, 1) | 5 (−24, 34) | −7 (−27, 14) |
| 50–59 | 0 (−3, 3) | 0 (−3, 3) | 4 (−3, 11) | 1 (−7, 8) |
| 60–69 | Ref | Ref | Ref | Ref |
| 70+ | 2 (−2, 6) | 0 (−3, 2) | 12 (−1, 25) | 6 (−4, 16) |
| Sex | p = 0.11 | p = 0.09 | p = 0.08 | p = 0.93 |
| Unadjusted Intercept | 17 (14, 20) | 52 (38, 66) | ||
| Female | Ref | Ref | Ref | Ref |
| Male | −3 (−7, 1) | −2 (−5, 0) | −13 (−28, 2) | 0 (−9, 10) |
| Sum of Minor ADGs | p = 0.31 | p = 0.10 | p = 0.38 | p = 0.19 |
| Unadjusted Intercept | 15 (12, 18) | 47 (34, 60) | ||
| 0–2 | Ref | Ref | Ref | Ref |
| 3–4 | −2 (−7, 3) | 1 (−2, 4) | −12 (−26, 2) | −8 (−16, 1) |
| 5–6 | 1 (−3, 5) | 2 (−1, 5) | −6 (−27, 15) | −6 (−16, 5) |
| 7+ | −2 (−5, 1) | −2 (−5, 2) | −6 (−20, 8) | −10 (−20, 0) |
| Sum of Major ADGs | p = 0.13 | p = 0.09 | p = 0.93 | p = 0.06 |
| Unadjusted Intercept | 14 (12, 16) | 39 (32, 46) | ||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 0 (−3, 3) | −1 (−3, 2) | 2 (−9, 13) | 6 (−3, 14) |
| 2 | −1 (−4, 2) | 0 (−3, 4) | −3 (−17, 11) | −4 (−13, 4) |
| 3+ | 7 (0, 14) | 6 (0, 12) | 4 (−55, 63) | 13 (−3, 29) |
| Recent Immigration | p = 0.79 | p = 0.64 | p = 0.97 | p = 0.75 |
| Unadjusted Intercept | 14 (13, 15) | 40 (35, 45) | ||
| No | Ref | Ref | Ref | Ref |
| Yes | −1 (−8, 6) | −2 (−8, 5) | −1 (−54, 52) | −3 (−24, 17) |
| Material Deprivation | p = 0.04 | p = 0.002 | p = 0.94 | p = 0.09 |
| Unadjusted Intercept | 13 (11, 15) | 35 (26, 44) | ||
| 1 | Ref | Ref | Ref | Ref |
| 2 | 1 (−3, 5) | −2 (−6, 1) | 1 (−15, 17) | −3 (−12, 5) |
| 3 | 0 (−4, 4) | −2 (−6, 2) | 2 (−12, 16) | 1 (−10, 12) |
| 4 | 1 (−3, 5) | 0 (−3, 4) | 4 (−11, 19) | 8 (−3, 19) |
| 5 | 5 (1, 9) | 4 (1, 7) | 8 (−11, 27) | 10 (−2, 21) |
| Rurality | p = 0.38 | p = 0.43 | p = 0.44 | p = 0.61 |
| Unadjusted Intercept | 14 (13, 15) | 39 (34, 44) | ||
| Urban | Ref | Ref | Ref | Ref |
| Rural | 2 (−2, 6) | 1 (−2, 5) | 5 (−8, 18) | 3 (−13, 8) |
| Histology | p = 0.89 | p = 0.76 | p = 0.35 | p = 0.41 |
| Unadjusted Intercept | 14 (12, 16) | 39 (34, 44) | ||
| Adenocarcinoma | Ref | Ref | Ref | Ref |
| Squamous Cell Carcinoma | 1 (−3, 5) | 0 (−4, 5) | 19 (8, 46) | 9 (−5, 23) |
| Other | 0 (−6, 6) | −2 (−8, 4) | 10 (−27, 47) | 5 (−20, 30) |
| Tumor Site | p = 0.30 | p = 0.10 | p = 0.08 | p = 0.36 |
| Unadjusted Intercept | 15 (13, 17) | 43 (34, 52) | ||
| Middle Esophagus | 1 (−5, 7) | 0 (−7, 7) | 41 (−2, 84) | −7 (−28, 14) |
| Lower Esophagus | −1 (−4, 2) | −1 (−3, 2) | −4 (−13, 5) | −4 (−12, 3) |
| Gastroesophageal Junction | Ref | Ref | Ref | Ref |
| Other | −7 (−15, 1) | −5 (−9, −1) | −20 (−57, 17) | −19 (−42, −5) |
| TCSC | p < 0.0001 | p < 0.0001 | p = 0.02 | p < 0.0001 |
| Unadjusted Intercept | 27 (23, 31) | 49 (33, 65) | ||
| 01 | −17 (−23, −11) | −17 (−23, −11) | −31 (−70, 8) | −23 (−50, 5) |
| 02 | −14 (−21, −7) | −17 (−23, −11) | −20 (−41, 1) | −21 (−38, −3) |
| 03 | −10 (−16, −4) | −11 (−17, −5) | 3 (−42, 48) | 7 (−27, 42) |
| 04 | −18 (−23, −13) | −17 (−22, 13) | −10 (−27, 7) | −17 (−31, −2) |
| 05 | −13 (−23, −3) | −12 (−23, −1) | −29 (−149, 91) | −29 (−114, 55) |
| 06 | −18 (−24, −12) | −16 (−21, −11) | −20 (−68, 28) | −17 (−52, 18) |
| 07 | −10 (−16, −4) | −10 (−15, −4) | −8 (−27, 11) | −14 (−29, 2) |
| 08 | −10 (−25, 5) | −14 (−30, 2) | 98 (28, 168) | 98 (43, 154) |
| 09 | −7 (−20, 6) | −11 (−21, −2) | 36 (−12, 84) | 41 (−2, 84) |
| 10 * | - | - | - | - |
| 11 | −10 (−15, −5) | −10 (−15, −6) | −13 (−32, 6) | −16 (−33, 2) |
| 12 | −6 (−16, 5) | −8 (−17, 2) | 21 (−67, 109) | −1 (−75, 74) |
| 13 | Ref | Ref | Ref | Ref |
| 14 | −16 (−22, −10) | −16 (−21, −11) | −22 (−43, −1) | −27 (−43, −11) |
| 15 | −14 (−22, −6) | −13 (−21, −6) | −25 (−99, 49) | −31 (−76, 14) |
| Non−TCSC | −9 (−65, 47) | −13 (−54, 29) | 99 (−262, 460) | 79 (−77, 234) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, N.M.; Nguyen, P.; Chung, W.; Groome, P.A. Time to Surgery for Patients with Esophageal Cancer Undergoing Trimodal Therapy in Ontario: A Population-Based Cross-Sectional Study. Curr. Oncol. 2022, 29, 5901-5918. https://doi.org/10.3390/curroncol29080466
Hanna NM, Nguyen P, Chung W, Groome PA. Time to Surgery for Patients with Esophageal Cancer Undergoing Trimodal Therapy in Ontario: A Population-Based Cross-Sectional Study. Current Oncology. 2022; 29(8):5901-5918. https://doi.org/10.3390/curroncol29080466
Chicago/Turabian StyleHanna, Nader M., Paul Nguyen, Wiley Chung, and Patti A. Groome. 2022. "Time to Surgery for Patients with Esophageal Cancer Undergoing Trimodal Therapy in Ontario: A Population-Based Cross-Sectional Study" Current Oncology 29, no. 8: 5901-5918. https://doi.org/10.3390/curroncol29080466
APA StyleHanna, N. M., Nguyen, P., Chung, W., & Groome, P. A. (2022). Time to Surgery for Patients with Esophageal Cancer Undergoing Trimodal Therapy in Ontario: A Population-Based Cross-Sectional Study. Current Oncology, 29(8), 5901-5918. https://doi.org/10.3390/curroncol29080466





