Metastatic SDH-Deficient GIST Diagnosed during Pregnancy: Approach to a Complex Case
Abstract
:1. Introduction
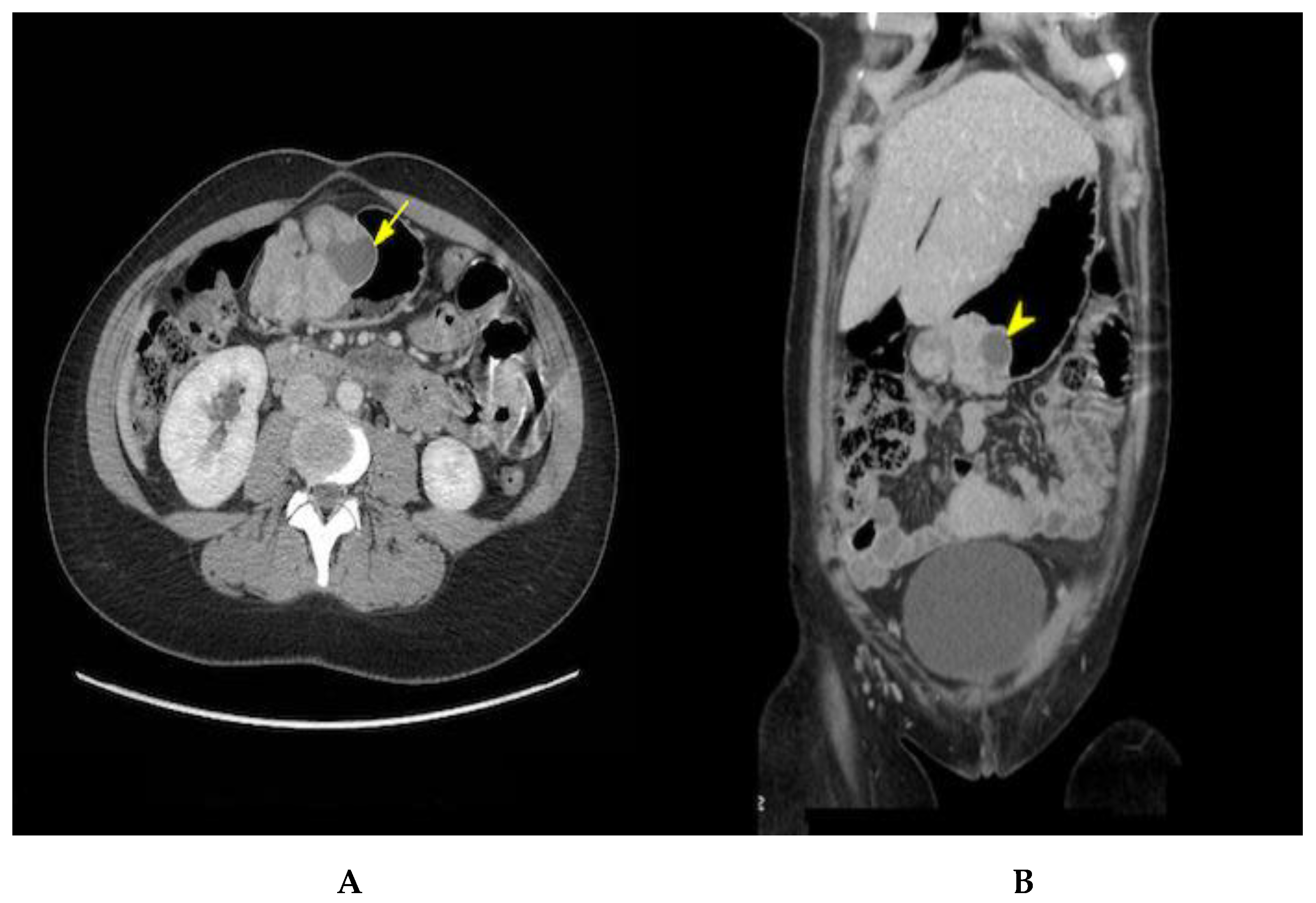
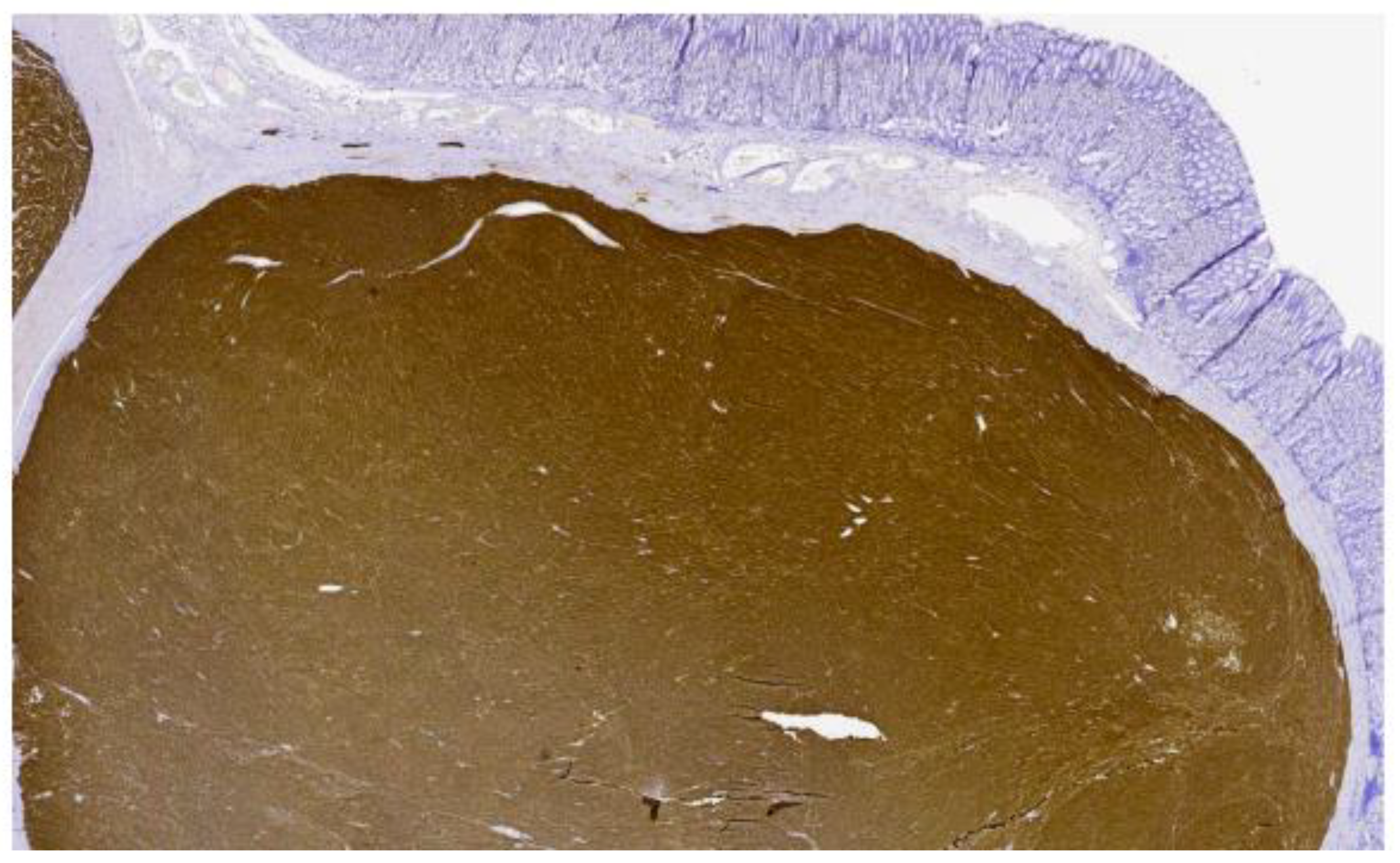
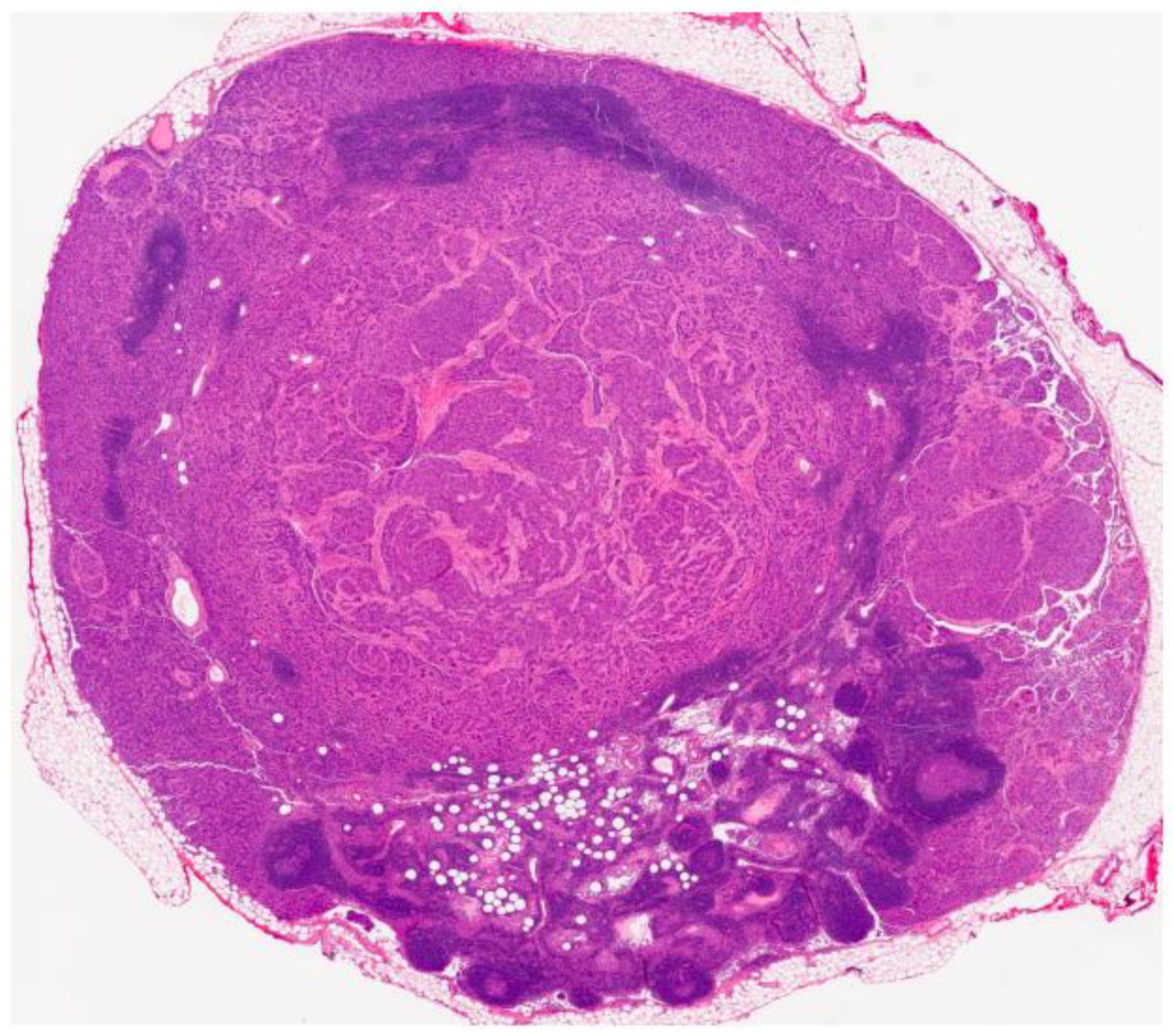
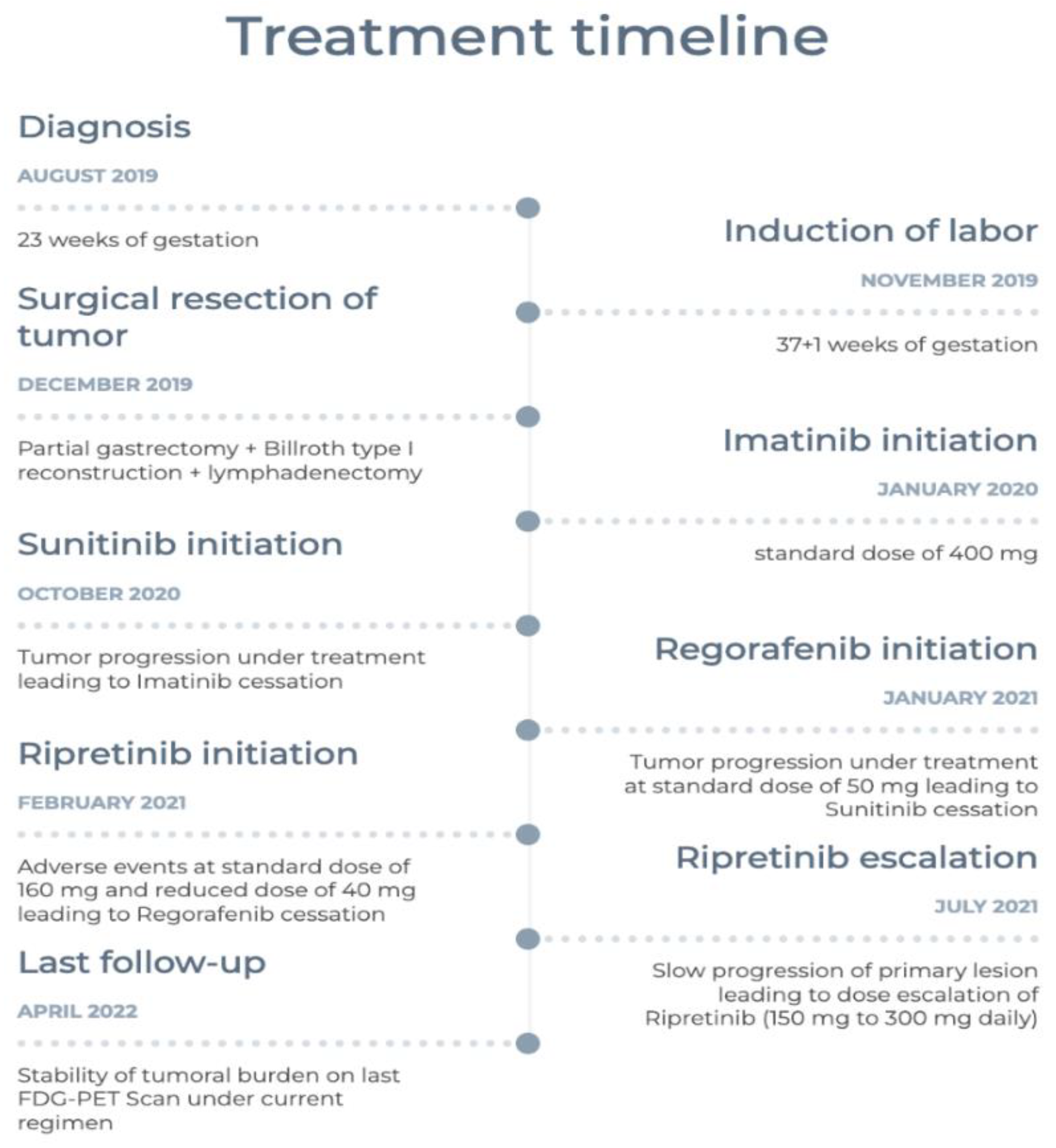
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.E.; Tzen, C.Y.; Wang, S.Y.; Yeh, C.N. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers 2019, 11, 679. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. Int. J. Pathol. Janv. 2001, 438, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.J.; Tuveson, D.J.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensuu, H.; Eriksson, M.; Hall, K.S.; Hartmann, J.T.; Pink, D.; Schütte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.-E.; et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012, 307, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Djerouni, M.; Dumont, S.N. Wild-type gastroinestinal stromal tumors. Bull Cancer 2020, 107, 499–505. [Google Scholar] [CrossRef]
- Ibrahim, A.; Chopra, S. Succinate Dehydrogenase–Deficient Gastrointestinal Stromal Tumors. Arch. Pathol. Lab. Med. 2020, 144, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; Killian, J.K.; Wang, Z.F.; Lasota, J.; Lau, C.; Jones, L.; Walker, R.; Pineda, M.; Zhu, Y.J.; Kim, S.Y.; et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am. J. Surg. Pathol. 2013, 37, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Mason, E.F.; Hornick, J.L. Conventional Risk Stratification Fails to Predict Progression of Succinate Dehydrogenase-deficient Gastrointestinal Stromal Tumors: A Clinicopathologic Study of 76 Cases. Am. J. Surg. Pathol. 2016, 40, 1616–1621. [Google Scholar] [CrossRef]
- Weldon, C.B.; Madenci, A.L.; Boikos, S.A.; Janeway, K.A.; George, S.; von Mehren, M.; Pappo, A.S.; Schiffman, J.D.; Wright, J.; Trent, J.C.; et al. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J. Clin. Oncol. 2016, 35, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Wu, C.E.; Lai, C.C.; Chen, J.S.; Tsai, C.Y.; Cheng, C.T.; Yeh, T.S.; Yeh, C.N. Prospective Evaluation of Neoadjuvant Imatinib Use in Locally Advanced Gastrointestinal Stromal Tumors: Emphasis on the Optimal Duration of Neoadjuvant Imatinib Use, Safety, and Oncological Outcome. Cancers 2019, 11, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, M.; Balachandran, V.P.; Li, G.Z.; Bertagnolli, M.M.; Antonescu, C.; Tap, W.; Singer, S.; DeMatteo, R.P.; Raut, C.P. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann. Surg. 2018, 268, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Neppala, P.; Banerjee, S.; Fanta, P.T.; Yerba, M.; Porras, K.A.; Burgoyne, A.M.; Sicklick, J.K. Current management of succinate dehydrogenase-deficient gastrointestinal stromal tumors. Cancer Metastasis Rev. 2019, 38, 525–535. [Google Scholar] [CrossRef]
- Jean-Yves, B.; Yoon-Koo, K.; Toshiroo, N.; Margaret, v.M. Gastrointestinal stromal tumours (primer). Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrichet, M.C.; Morganal, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Ricci, R.; Martini, M.; Ravegnini, G.; Cenci, T.; Milione, M.; Lanza, P.; Pierconti, F.; Santini, D.; Angelini, S.; Biondi, A.; et al. Preferential MGMT methylation could predispose a subset of KIT/PDGFRA-WT GISTs, including SDH-deficient ones, to respond to alkylating agents. Clin. Epigenet. 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Vadakara, J.; von Mehren, M. Gastrointestinal stromal tumors: Management of metastatic disease and emerging therapies. Hematol. Oncol. Clin. N. Am. 2013, 27, 905–920. [Google Scholar] [CrossRef] [Green Version]
- McWhinney, S.R.; Pasini, B.; Stratakis, C.A. Familial Gastrointestinal Stromal Tumors and Germ-Line Mutations. N. Engl. J. Med. 2007, 357, 1054–1056. [Google Scholar] [CrossRef]
- Wong, M.Y.; Andrews, K.A.; Challis, B.G.; Park, S.-M.; Acerini, C.L.; Maher, E.R.; Casey, R.T. Clinical Practice Guidance: Surveillance for phaeochromocytoma and paraganglioma in paediatric succinate dehydrogenase gene mutation carriers. Clin. Endocrinol. 2019, 90, 499–505. [Google Scholar] [CrossRef]
- Zarkavelis, G.; Petrakis, D.; Pavlidis, N. Gastrointestinal stromal tumors during pregnancy: A systematic review of an uncommon but treatable malignancy. Clin. Transl. Oncol. 2015, 17, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Ford, J.M. Imatinib treatment: Specific issues related to safety, fertility, and pregnancy. Semin. Hematol. 2003, 40, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Pye, S.M.; Cortes, J.; Ault, P.; Hatfield, A.; Kantarjian, H.; Pilot, R.; Rosti, G.; Apperley, J.F. The effects of imatinib on pregnancy outcome. Blood 2008, 111, 5505–5508. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chennouf, A.; Zeidan, E.; Borduas, M.; Noël-Lamy, M.; Kremastiotis, J.; Beaudoin, A. Metastatic SDH-Deficient GIST Diagnosed during Pregnancy: Approach to a Complex Case. Curr. Oncol. 2022, 29, 5933-5941. https://doi.org/10.3390/curroncol29080468
Chennouf A, Zeidan E, Borduas M, Noël-Lamy M, Kremastiotis J, Beaudoin A. Metastatic SDH-Deficient GIST Diagnosed during Pregnancy: Approach to a Complex Case. Current Oncology. 2022; 29(8):5933-5941. https://doi.org/10.3390/curroncol29080468
Chicago/Turabian StyleChennouf, Anas, Elie Zeidan, Martin Borduas, Maxime Noël-Lamy, John Kremastiotis, and Annie Beaudoin. 2022. "Metastatic SDH-Deficient GIST Diagnosed during Pregnancy: Approach to a Complex Case" Current Oncology 29, no. 8: 5933-5941. https://doi.org/10.3390/curroncol29080468
APA StyleChennouf, A., Zeidan, E., Borduas, M., Noël-Lamy, M., Kremastiotis, J., & Beaudoin, A. (2022). Metastatic SDH-Deficient GIST Diagnosed during Pregnancy: Approach to a Complex Case. Current Oncology, 29(8), 5933-5941. https://doi.org/10.3390/curroncol29080468




