Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology
Abstract
:1. Introduction
2. Diagnosis and Prognosis
2.1. How Can AML Present, How Is AML Diagnosed and What Are Important Initial Tests?
2.2. Assessing Prognosis in AML
3. Current Management of AML in the First-Line
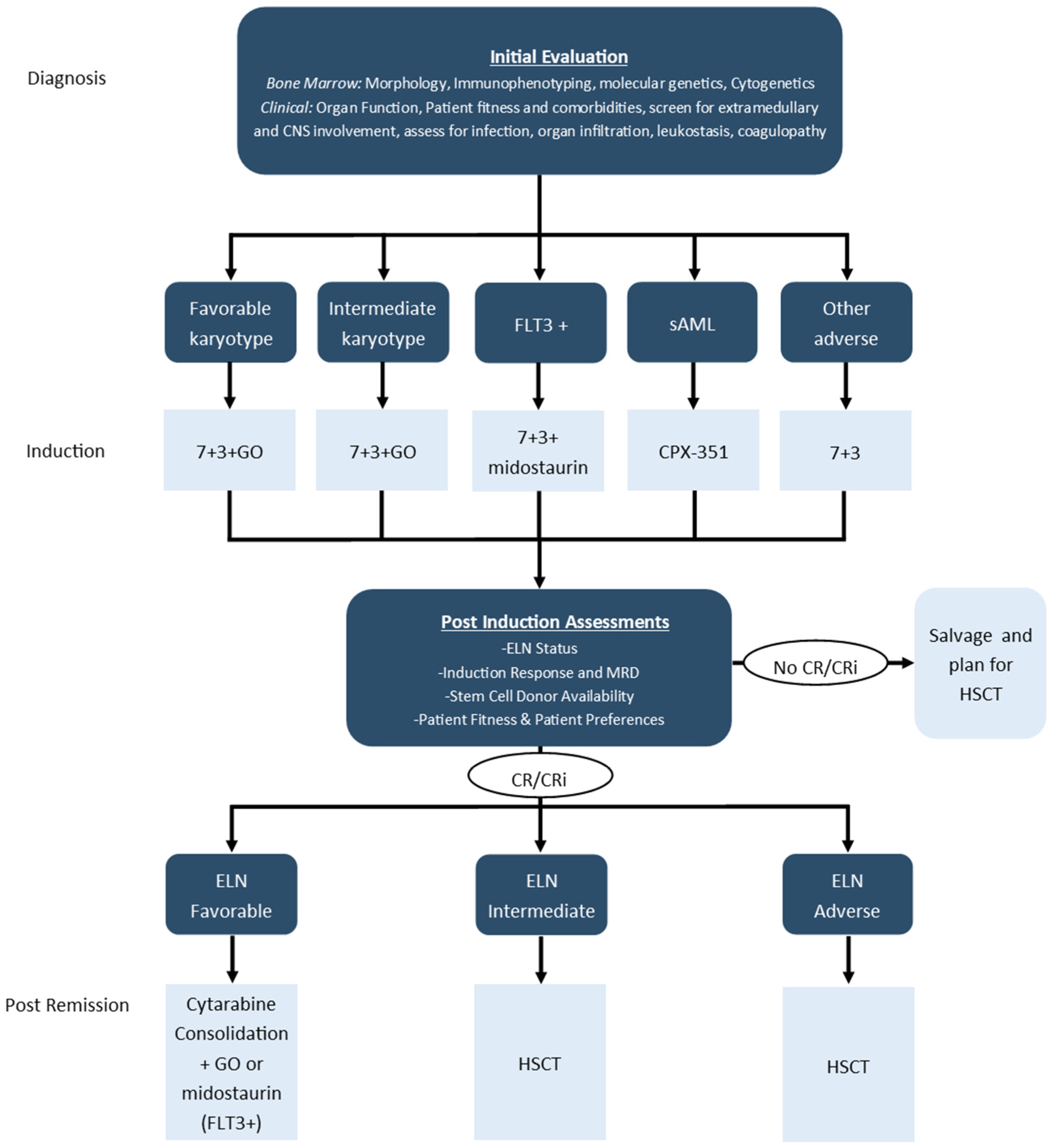
3.1. Frontline Intensive Treatment in Younger Fit Patients
3.2. Post-Remission Treatment in Younger Fit Patients
3.3. Non-Intensive Approaches in Older and Unfit Patients
4. Management of Relapsed and Refractory AML
4.1. Approach to Relapsed/Refractory Disease
4.2. The Role for Repeat FLT3 Testing and Gilteritinib
4.3. The Role for Inhibitors of Isocitrate Dehydrogenase (IDH) 1 and 2
4.4. The Role for Hypomethylating Agents with Venetoclax
4.5. The Role for HSCT
5. Palliative Care
5.1. Palliative Needs and Integrated Palliative Approaches in AML
5.2. Interdisciplinary Collaboration to Support Palliative Needs
5.3. Barriers to Palliative Integration and Community-Based End-of-Life Care
6. Summary
- Patients presenting with suspected acute leukemia should undergo a thorough assessment for associated complications along with an expedited diagnosis;
- Accurate diagnosis and risk stratification require cytogenetic and molecular genetic testing, and this information may guide initial treatment along with selection of post-remission therapy and use of HSCT;
- Repeat testing for FLT3-mutation is required for patients with R/R AML to guide appropriate therapy;
- Azacitidine and venetoclax is a new, more effective treatment for older patients with AML, although it is associated with increased myelosuppression requiring close monitoring and appropriate supportive care;
- Integration of palliative care during treatment can improve outcomes, symptom management, and facilitate discussions around goals of care and end-of-life planning.
Funding
Acknowledgments
Conflicts of Interest
References
- Stubbins, R.J.; Stamenkovic, M.; Roy, C.; Rodrigo, J.; Chung, S.; Kuchenbauer, F.C.; Hay, K.A.; White, J.; Abou Mourad, Y.; Power, M.M.; et al. Incidence and socioeconomic factors in older adults with acute myeloid leukaemia: Real-world outcomes from a population-based cohort. Eur. J. Haematol. 2022, 108, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 18 May 2022).
- Canadian Cancer Statistics Advisory Commitee. Canadian Cancer Statistics. 2019. Available online: cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 18 May 2022).
- Siegal, T.; Benouaich-Amiel, A.; Bairey, O. Neurologic complications of acute myeloid leukemia. Diagnostic approach and therapeutic modalities. Blood Rev. 2021, 53, 100910. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood 2022, 129, 424–447. [Google Scholar] [CrossRef]
- Zuckerman, T.; Ganzel, C.; Tallman, M.S.; Rowe, J.M. How I treat hematologic emergencies in adults with acute leukemia. Blood 2012, 120, 1993–2002. [Google Scholar] [CrossRef]
- Eisfeld, A.K.; Kohlschmidt, J.; Mrozek, K.; Blachly, J.S.; Walker, C.J.; Nicolet, D.; Orwick, S.; Maharry, S.E.; Carroll, A.J.; Stone, R.M.; et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: An analysis of Alliance studies. Leukemia 2018, 32, 1338–1348. [Google Scholar] [CrossRef]
- Heuser, M. Therapy-related myeloid neoplasms: Does knowing the origin help to guide treatment? Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 24–32. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Buchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- Short, N.J.; Zhou, S.; Fu, C.; Berry, D.A.; Walter, R.B.; Freeman, S.D.; Hourigan, C.S.; Huang, X.; Nogueras Gonzalez, G.; Hwang, H.; et al. Association of Measurable Residual Disease With Survival Outcomes in Patients With Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1890–1899. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Dohner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Pautas, C.; Terre, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L.; et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef]
- Wei, A.H.; Dohner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C.; et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979–985. [Google Scholar] [CrossRef]
- Rollig, C.; Kramer, M.; Schliemann, C.; Mikesch, J.H.; Steffen, B.; Kramer, A.; Noppeney, R.; Schafer-Eckart, K.; Krause, S.W.; Hanel, M.; et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 2020, 136, 823–830. [Google Scholar] [CrossRef]
- Juliusson, G.; Hagberg, O.; Lazarevic, V.L.; Lehmann, S.; Hoglund, M. Impact of treatment delay in acute myeloid leukemia revisited. Blood Adv. 2021, 5, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Thol, F. What to use to treat AML: The role of emerging therapies. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, H.; Wood, B.L.; Walter, R.B.; Pagel, J.M.; Becker, P.S.; Sandhu, V.K.; Abkowitz, J.L.; Appelbaum, F.R.; Estey, E.H. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J. Clin. Oncol. 2015, 33, 1258–1264. [Google Scholar] [CrossRef]
- Danylesko, I.; Canaani, J.; Shimoni, A.; Fein, J.; Shem-Tov, N.; Yerushalmi, R.; Shouval, R.; Nagler, A. Complete Remission with Incomplete Blood Count Recovery Is a Strong Predictor of Nonrelapse Mortality in Acute Myeloid Leukemia Patients Undergoing Allogeneic Stem Cell Transplantation. Acta Haematol. 2021, 144, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J. Why is a 3-year NRM following allogeneic transplantation still stuck at approximately 20%? Best Pract. Res. Clin. Haematol. 2018, 31, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Schlenk, R.; Kopecky, K.J.; Honda, S.; Sierra, J.; Djulbegovic, B.J.; Wadleigh, M.; DeAngelo, D.J.; Stone, R.M.; Sakamaki, H.; et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA 2009, 301, 2349–2361. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Bixby, D.; Perl, A.; Bhatt, V.R.; Altman, J.K.; Appelbaum, F.R.; de Lima, M.; Fathi, A.T.; Foran, J.M.; Gojo, I.; et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 16–27. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- AbbVie Receives a Positive Reimbursement Recommendation from the Canadian Agencies for Drugs and Technology in Health (CADTH) Pan Canadian Oncology Drug Review Expert Review Committee (pERC) for VENCLEXTA® (venetoclax) in Combination with azacitidine for Acute Myeloid Leukemia (AML) Patients. Available online: https://www.abbvie.ca/content/dam/abbvie-dotcom/ca/en/documents/press-releases/PR-AML-Venclexta-pCODR-EN.pdf (accessed on 24 May 2022).
- Stubbins, R.J.; Maksakova, I.A.; Sanford, D.S.; Rouhi, A.; Kuchenbauer, F. Mitochondrial metabolism: Powering new directions in acute myeloid leukemia. Leuk. Lymphoma 2021, 62, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Jonas, B.A.; Pollyea, D.A. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 2019, 33, 2795–2804. [Google Scholar] [CrossRef]
- Ravandi, F.; Cortes, J.; Faderl, S.; O’Brien, S.; Garcia-Manero, G.; Verstovsek, S.; Santos, F.P.; Shan, J.; Brandt, M.; de Lima, M.; et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010, 116, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- de Lima, M.; Roboz, G.J.; Platzbecker, U.; Craddock, C.; Ossenkoppele, G. AML and the art of remission maintenance. Blood Rev. 2021, 49, 100829. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, G.J.; Janssen, J.J.; van de Loosdrecht, A.A. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica 2016, 101, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Breems, D.A.; Van Putten, W.L.; Huijgens, P.C.; Ossenkoppele, G.J.; Verhoef, G.E.; Verdonck, L.F.; Vellenga, E.; De Greef, G.E.; Jacky, E.; Van der Lelie, J.; et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 2005, 23, 1969–1978. [Google Scholar] [CrossRef]
- Frassoni, F.; Barrett, A.J.; Granena, A.; Ernst, P.; Garthon, G.; Kolb, H.J.; Prentice, H.G.; Vernant, J.P.; Zwaan, F.E.; Gratwohl, A. Relapse after allogeneic bone marrow transplantation for acute leukaemia: A survey by the E.B.M.T. of 117 cases. Br. J. Haematol. 1988, 70, 317–320. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Harrington, A.M.; Chaudhary, S.G.; Michaelis, L.C.; Carlson, K.B.; Abedin, S.; Runass, L.; Callander, N.S.; Fallon, M.J.; Juckett, M.; et al. Comparison of salvage chemotherapy regimens and prognostic significance of minimal residual disease in relapsed/refractory acute myeloid leukemia. Leuk. Lymphoma 2021, 62, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Girmenia, C.; Bruggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef]
- Rapaport, F.; Neelamraju, Y.; Baslan, T.; Hassane, D.; Gruszczynska, A.; Robert de Massy, M.; Farnoud, N.; Haddox, S.; Lee, T.; Medina-Martinez, J.; et al. Genomic and evolutionary portraits of disease relapse in acute myeloid leukemia. Leukemia 2021, 35, 2688–2692. [Google Scholar] [CrossRef] [PubMed]
- Schmalbrock, L.K.; Dolnik, A.; Cocciardi, S.; Strang, E.; Theis, F.; Jahn, N.; Panina, E.; Blatte, T.J.; Herzig, J.; Skambraks, S.; et al. Clonal evolution of acute myeloid leukemia with FLT3-ITD mutation under treatment with midostaurin. Blood 2021, 137, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, B.; Li, J.; Liu, L.; Du, X.; Jiang, H.; Hu, J.; Yuan, M.; Sakatani, T.; Kadokura, T.; et al. Gilteritinib Versus Salvage Chemotherapy for Relapsed/Refractory FLT3-Mutated Acute Myeloid Leukemia: A Phase 3, Randomized, Multicenter, Open-Label Trial in Asia. Blood 2021, 138, 695. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Montesinos, P.S.; Schuh, A.C.; Papayannidis, C.; Vyas, P.; Wei, A.H.Z.; Zeidan, A.M.; Bluemmert, I.; Yu, X.; Hasan, M.; et al. Outcomes for Patients with Late-Stage Mutant-IDH2 (m IDH2) Relapsed/Refractory Acute Myeloid Leukemia (R/R AML) Treated with Enasidenib Vs Other Lower-Intensity Therapies in the Randomized, Phase 3 IDHentify Trial. Blood 2021, 138, 1243. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef]
- Stubbins, R.J.; Karsan, A. Differentiation therapy for myeloid malignancies: Beyond cytotoxicity. Blood Cancer J. 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Menghrajani, K.; Derkach, A.; Chan, A.; Xiao, W.; Glass, J.; King, A.C.; Daniyan, A.F.; Famulare, C.; Cuello, B.M.; et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021, 5, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Yang, D.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Mei, M.; Salhotra, A.; Khaled, S.; Nakamura, R.; et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 2018, 103, e404–e407. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Amit, O.; Zuckerman, T.; Gurion, R.; Raanani, P.; Bar-On, Y.; Avivi, I.; Wolach, O. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents-a multicenter historical prospective study. Ann. Hematol. 2019, 98, 1927–1932. [Google Scholar] [CrossRef]
- Tenold, M.E.; Moskoff, B.N.; Benjamin, D.J.; Hoeg, R.T.; Rosenberg, A.S.; Abedi, M.; Tuscano, J.M.; Jonas, B.A. Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center. Front. Oncol. 2021, 11, 649209. [Google Scholar] [CrossRef]
- Othus, M.; Appelbaum, F.R.; Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Nevill, T.; Brandwein, J.; Larson, R.A.; Stiff, P.J.; Walter, R.B.; et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol. Blood Marrow Transplant. 2015, 21, 559–564. [Google Scholar] [CrossRef]
- Chantry, A.D.; Snowden, J.A.; Craddock, C.; Peggs, K.; Roddie, C.; Craig, J.I.; Orchard, K.; Towlson, K.E.; Pearce, R.M.; Marks, D.I.; et al. Long-term outcomes of myeloablation and autologous transplantation of relapsed acute myeloid leukemia in second remission: A British Society of Blood and Marrow Transplantation registry study. Biol. Blood Marrow Transplant. 2006, 12, 1310–1317. [Google Scholar] [CrossRef]
- Wang, E.S.; Baron, J. Management of toxicities associated with targeted therapies for acute myeloid leukemia: When to push through and when to stop. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 57–66. [Google Scholar] [CrossRef]
- Boucher, N.A.; Johnson, K.S.; LeBlanc, T.W. Acute Leukemia Patients’ Needs: Qualitative Findings and Opportunities for Early Palliative Care. J. Pain Symptom Manag. 2018, 55, 433–439. [Google Scholar] [CrossRef]
- LeBlanc, T.W. Addressing End-of-Life Quality Gaps in Hematologic Cancers: The Importance of Early Concurrent Palliative Care. JAMA Intern. Med. 2016, 176, 265–266. [Google Scholar] [CrossRef]
- LeBlanc, T.W. Palliative care and hematologic malignancies: Old dog, new tricks? J. Oncol. Pract. 2014, 10, e404–e407. [Google Scholar] [CrossRef]
- Nickolich, M.; El-Jawahri, A.; LeBlanc, T.W. Palliative and End-of-Life Care in Myelodysplastic Syndromes. Curr. Hematol. Malig. Rep. 2016, 11, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Ariadne Labs. Serious Illness Care Program Reference Guide for Clinicians. Available online: https://divisionsbc.ca/sites/default/files/Divisions/Powell%20River/ClinicianReferenceGuide.pdf (accessed on 1 June 2022).
- Selvaggi, K.J.; Vick, J.B.; Jessell, S.A.; Lister, J.; Abrahm, J.L.; Bernacki, R. Bridging the gap: A palliative care consultation service in a hematological malignancy-bone marrow transplant unit. J. Community Support. Oncol. 2014, 12, 50–55. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, T.W.; El-Jawahri, A. When and why should patients with hematologic malignancies see a palliative care specialist? Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Back, A.L.; Park, E.R.; Greer, J.A.; Jackson, V.A.; Jacobsen, J.C.; Gallagher, E.R.; Temel, J.S. Clinician roles in early integrated palliative care for patients with advanced cancer: A qualitative study. J. Palliat. Med. 2014, 17, 1244–1248. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef]
- El-Jawahri, A.; LeBlanc, T.W.; Kavanaugh, A.; Webb, J.A.; Jackson, V.A.; Campbell, T.C.; O’Connor, N.; Luger, S.M.; Gafford, E.; Gustin, J.; et al. Effectiveness of Integrated Palliative and Oncology Care for Patients With Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 238–245. [Google Scholar] [CrossRef]
- Odejide, O.O.; Salas Coronado, D.Y.; Watts, C.D.; Wright, A.A.; Abel, G.A. End-of-life care for blood cancers: A series of focus groups with hematologic oncologists. J. Oncol. Pract. 2014, 10, e396–e403. [Google Scholar] [CrossRef]
- Odejide, O.O.; Cronin, A.M.; Condron, N.B.; Fletcher, S.A.; Earle, C.C.; Tulsky, J.A.; Abel, G.A. Barriers to Quality End-of-Life Care for Patients With Blood Cancers. J. Clin. Oncol. 2016, 34, 3126–3132. [Google Scholar] [CrossRef]
| Risk Category | Genetic Abnormality |
|---|---|
| Favorable | t (8;21) (q22;q22.1); RUNX1-RUNX1T1 inv (16) (p13.1q22) or t (16;16) (p13.1;q22); CBFB-MYH11 Mutated NPM1 without FLT3-ITD bZIP in-frame mutated CEBPA |
| Intermediate | Mutated NPM1 with FLT3-ITD Wild-type NPM1 with FLT3-ITD t (9;11) (p21.3;q23.3); MLLT3-KMT2A Cytogenetic abnormalities not classified as favorable or adverse |
| Adverse | t (6;9) (p23;q34.1); DEK-NUP214 t (v;11q23.3); KMT2A rearranged t (9;22) (q34.1;q11.2); BCR-ABL1 inv(3) (q21.3q26.2) or t (3;3) (q21.3;q26.2); GATA2, MECOM(EVI1) t (3q26.2;v); MECOM (EVI1)-rearranged −5 or del (5q); −7; −17/abn (17p) Complex karyotype, monosomal karyotype Mutated ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2 Mutated TP53 |
| Treatment | Indication | Median OS Exp. vs. Ctrl | Selected Toxicities | Approval Status a | Ref. |
|---|---|---|---|---|---|
| Midostaurin | FLT3+ Frontline with intensive chemotherapy | 74.7 vs. 25.6 months | GI (nausea, vomiting, diarrhea), infection, skin rash, pulmonary toxicities, QT prolongation | HC/FDA Approved (Frontline) | [12] |
| Gilteritinib | FLT3 + R/R | 9.3 months vs. 5.6 months | GI (nausea, vomiting, diarrhea), infection, transaminitis, increased CK, myelosuppression, QT Prolongation, differentiation syndrome | HC/FDA Approved (R/R) | [15] |
| Gemtuzumab-ozogamicin | Favorable/Intermediate/Unknown cytogenetics Frontline with intensive chemotherapy | 27.5 vs. 21.8 months (NS) | Infection, myelosuppression and delayed platelet recovery, hepatic toxicity and VOD, infusion reactions | HC/FDA Approved (Frontline) | [14] |
| CPX-351 | Secondary AML Frontline | 9.56 vs. 5.95 months | Infection, myelosuppression, bleeding | HC/FDA Approved (Frontline) | [13] |
| Oral Azacitidine (CC-486) | Maintenance following intensive chemotherapy, HSCT ineligible | 24.7 vs. 14.8 months | GI (nausea, vomiting, diarrhea), infection, myelosuppression | HC/FDA Approved (Post-induction maintenance) | [17] |
| Venetoclax | Elderly/Unfit Frontline with azacitidine | 14.7 vs. 9.6 months | Infection, myelosuppression, tumor lysis syndrome | HC/FDA Approved (Frontline, induction ineligible) | [18] |
| Considerations | |
|---|---|
| Tumor Lysis Prophylaxis |
|
| Antimicrobial Prophylaxis |
|
| Cytopenias |
|
| Disease Assessment |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stubbins, R.J.; Francis, A.; Kuchenbauer, F.; Sanford, D. Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 6245-6259. https://doi.org/10.3390/curroncol29090491
Stubbins RJ, Francis A, Kuchenbauer F, Sanford D. Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology. Current Oncology. 2022; 29(9):6245-6259. https://doi.org/10.3390/curroncol29090491
Chicago/Turabian StyleStubbins, Ryan J., Annabel Francis, Florian Kuchenbauer, and David Sanford. 2022. "Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology" Current Oncology 29, no. 9: 6245-6259. https://doi.org/10.3390/curroncol29090491
APA StyleStubbins, R. J., Francis, A., Kuchenbauer, F., & Sanford, D. (2022). Management of Acute Myeloid Leukemia: A Review for General Practitioners in Oncology. Current Oncology, 29(9), 6245-6259. https://doi.org/10.3390/curroncol29090491





